INTRODUCTION
Phenobarbital is called a barbiturate that acts by slowing down the activity of the brain.
Phenobarbital has sedative and hypnotic properties, which will help patients to relax before surgery or help to sleep. It also reduces or controls seizures or convulsions, except for absence (petit mal) seizures (1,2). Generic Phenobarbital oral elixir is available containing a high amount of alcohol which can increase possible unpleasant effects.
The pharmaceutical industry supplies oral solid dosage forms that are generally inadequate for pediatric needs. This obliges pharmacies to prepare capsules with doses corresponding to the age and weight of the children. A suspension may be considered, but has some practical disadvantages (3). To reduce individualized preparations, oral liquids have been developed. In the case of Phenobarbital an elixir containing a high amount of alcohol is available which is not recommended for children (4).
Phenobarbital has poor water solubility (1 mg/ml) but it is freely soluble in ethanol (100 mg/ml). Its solubility usually can be increased by addition of water–miscible solvents. It has also been shown that the stability of Phenobarbital in solution formulations is promoted by using a mixed solvent of water and generic solvents such as alcohol, propylene glycol, glycerin or polyethylene glycol. Therefore, for this reason alcohol is often used in Phenobarbital solutions. For neonates there is need for an easy to administer liquid oral dosage form of Phenobarbital without alcohol. In order to increase the solubility of Phenobarbital, a co-solvent system without alcohol should be created using mixtures of various oral co-solvents. Cosolvents are defined as water–miscible organic solvents that are used in liquid drug formulations to increase the solubility of poorly water-soluble substances (5).
Ethanol was the most commonly employed solvent in oral preparations because of its excellent solvent properties for many non polar drugs as well as its favorable taste. Its use is often undesirable, however, in oral preparations intended for pediatric patients. Ethanol may also accentuate the saline taste of ionic solutes (6). Sorbitol, glycerin, propylene glycol, and several polyethylene glycol polymers are cosolvents that are both useful and acceptable in the formulation of oral liquids. Cosolvents are employed not only to affect solubility of the drug, but also to improve the solubility of volatile constituents used to impart a desirable flavor and odor to the product (7). The biggest limitation of cosolvency is the toxicity of most water miscible solvents that have a high potential for increasing drug solubility. The toxicological properties of a solvent that may limit or eliminate its use in drug formulations include its general toxicity, target organ toxicity, or tissue irritation. Even if found to be relatively nontoxic, a cosolvent can rarely be administered as a neat or 100% solvent because of its poor taste or objectionable odor. Although a cosolvent may increase the solubility of the drug, it may also affect the solubility of other polar or ionic components of the formulation such as buffer materials. Early formulation work using cosolvency involved an empirical approach for choosing the type and amount of cosolvent for a liquid vehicle. An improvement in the purely empirical approach was the introduction of allegation methods that could be used to reformulate vehicles based upon experimental formulation or solubility data (7,8).
This process is known as co-solvency, and the solvents used in combination to increase the solubility of the solute are known as cosolvents. The mechanism responsible for solubility enhancement through co-solvency is not clearly understood. It has been proposed that a co-solvent system works by reducing the interfacial tension between the predominately aqueous solutions and the hydrophobic solute (8). The solubility of substance in a blend of solvents is usually not equal to the value predicted on the basis of its solubility in the pure solvents. The ratio of solvents, as well as pH, can alter the solubility of Phenobarbital. Phenobarbital is stable in air but decomposes by hydrolysis methods to ureide and diamide (9). These degradation products alter the color of the solution from colorless to yellow. A common method for minimizing Phenobarbital degradation in solution dosage forms is to dissolve the drug in a mixed solvent of water and organic solvents (10,11).
Therefore, the purpose of the present investigation was to prepare Phenobarbital oral solutions free of alcohol covering the usual pediatric and neonatal doses. The present research also determines the shelf life of alcohol-free oral solution of Phenobarbital.
MATERIALS AND METHODS
Materials
Phenobarbital, propylene glycol, glycerin, sucrose, sodium saccharin, boric acid, hydrochloric acid, sodium hydroxide, sodium acetate trihydrate, acetic acid, methanol, Dichloromethane, anhydrous sodium sulfate and butyl paraben were used. All of the mentioned chemicals were obtained from Merck (Darmstadt, Germany). All chemicals and solvents were of analytical grade.
Preparation of Oral Solution
Phenobarbital oral solution was prepared using simple USP syrup. To prepare USP syrup the sucrose (850 g) was added to purified water and heated until solution is clear, and then sufficient purified water was added to make 1000 ml (5).
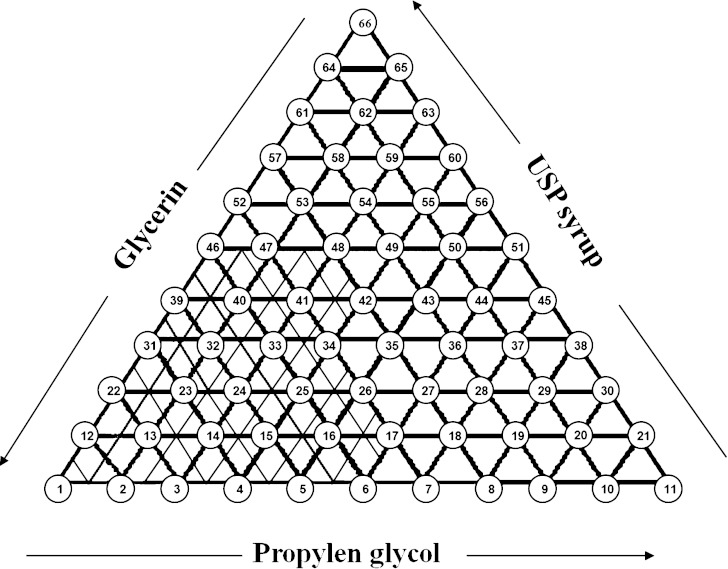
In order to investigate the effect of cosolvents on solubility of Phenobarbital, a ternary phase diagram was used (Fig. 1). Figure 1 shows a ternary system of USP syrup, propylene glycol, and glycerin; one component at each of the apexes. The units may be volume or mass or moles. In the present study, since all co-solvents are liquids, so, volume percent was used. Preliminary studies showed that if the concentrations of propylene glycol, glycerin and USP syrup were in the range 6–26%, 14–34% and 0–20%, respectively, Phenobarbital could be dissolved easily. However, various proportions of cosolvents were examined for finding of maximum solubility of Phenobarbital in cosolvents as shown in Fig. 1. In each formulation, the amount of phenobarbital was kept constant at a concentration of 400 mg/100 ml and the volumes of the three components (co-solvents) were changed to determine the optimum concentration of each solvent. For example, at point no.14 in Fig. 1 the concentration of glycerin was 28%, USP syrup 2%, and propylene glycol 10%. Sodium acetate trihydrate buffer pH 4.5 was used as vehicle providing pH of products at about 3.3–3.8. Among all formulations studied in the present work, four combinations of cosolvents were able to dissolve Phenobarbital easily. Their compositions are listed in Table I.
Fig. 1.
Co-solvents components used in ternary diagram development of alcohol-free solutions
Table I.
Solvents Components of Phenobarbital Solutions
| Formulation code in the diagram | Cosolvents | ||
|---|---|---|---|
| Propylen glycol (v/v %) | Glycerin (v/v %) | USP syrup (v/v %) | |
| F1 (14) | 10 | 28 | 2 |
| F2 (15) | 12 | 26 | 2 |
| F 3 (24) | 10 | 26 | 4 |
| F4 (33) | 10 | 24 | 6 |
At all of the formulations have used Phenobarbital (400 mg/100 ml), sucrose 30 (w/w %), saccharin sodium 0.35 (w/w %), butyl paraben 0.1 (w/w %), strawberry oil 5 (w/w %) and buffer pH 4.5 almost 25 (%v/v).
Assay of Phenobarbital
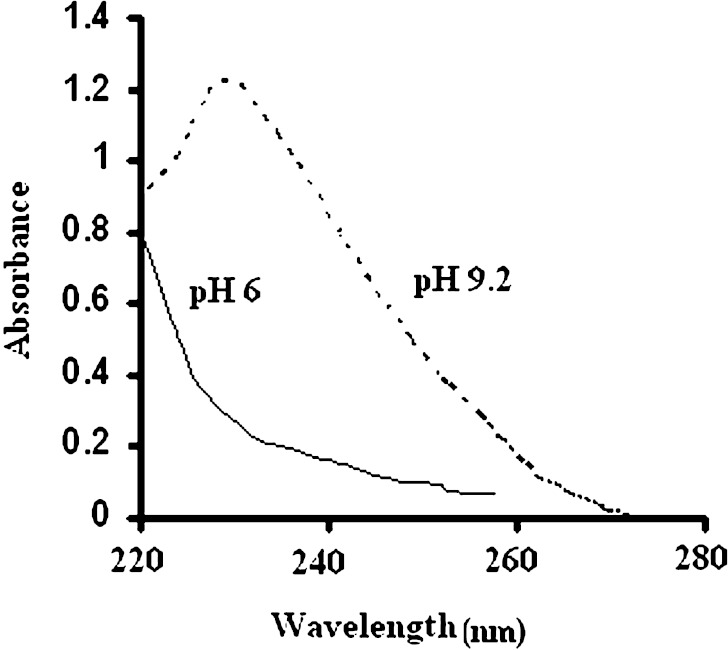
Ultraviolet spectrum of pure Phenobarbital (obtained from extraction) did not show any interference with degradation products of Phenobarbital. Therefore, the drug concentration was analyzed by means of ultraviolet (UV) spectrophotometer. The spectrum of the Phenobarbital solution at pH 4.5 did not show any peak, when the pH of the extracted solution of Phenobarbital was adjusted to 9.2 by borax a peak was appeared at 239 nm which was used for determination of Phenobarbital concentration (Fig. 2).
Fig. 2.
Comparison of Phenobarbital spectra at pH 6 and at pH 9.2 (borax buffer)
In order to determine the standard calibration curve of Phenobarbital, a stock solution of 100 mg/ml Phenobarbital at pH 9.2 (borax buffer) was prepared. Then, various dilutions were made to prepare a series of solutions containing Phenobarbital with different concentrations in the range of 4 to 24 µg/ml. The absorbance of Phenobarbital solutions was measured at a wavelength of 239 nm by UV spectrophotometer.
Measurement of Viscosity
A Brookfield rotational digital viscometer DVLV-II was used to measure the viscosity (Pa S) of solutions formulations at 20–25 °C. Spindle number 1 was rotated at 100 rpm.
Chemical Stability Tests and Prediction of Shelf Life
In order to determine the shelf life of Phenobarbital solutions, the accelerated stability test (Arrhenius method) at high temperatures was used (12). According to the accelerated technique, the rates of the decomposition of a drug in solution at various elevated temperatures are obtained by plotting some functions of concentration against time. The logarithms of specific rates of decomposition are then plotted against the reciprocals of the absolute temperatures and resulting line is extrapolated to room temperature. The k25˚ is used to obtain a measure of the stability of the drug under ordinary shelf conditions. To this end, Phenobarbital solutions were stored at different temperatures of 40, 50, 60 and 70 °C. In order to determine the chemical stability of Phenobarbital solutions, at different time intervals (0, 7, 15, 30, 45, 60, 90 and 120 days) 1 ml samples of Phenobarbital solution containing 4 mg active ingredient was taken and transferred to a small decantation funnel. Then 0.2 ml hydrochloric acid (3 N) was added to the solution.
After that the drug was extracted by 2 ml dichloromethane three times. The dichloromethane phase was transferred to the test tube and dried under nitrogen gas. The dried samples were dissolved in 2 ml methanol. The solution was diluted twice with distilled water followed by five times dilutions with borax buffer. Absorption of the samples was determined by UV spectrophotometer at a wavelength of 239 nm (12,13). After determination of Phenobarbital concentration in the taken samples, the log percent of drug remaining in the solutions is plotted against time in days, and the slope of the resultant line is calculated. The slopes at the different temperatures are then plotted against 1/T, and the time at 25 °C gives the shelf life of product in days (13–15).
The shelf life or t90% can also be calculated using the following Eq. 12.
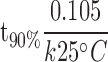 |
1 |
According to FDA rules, if the product is kept at 40 ± 2 °C for 3 months and no reduction is observed in the amount of active ingredient, the shelf life of the product will be 2 years (16).
Statistical Analysis
Where appropriate, results were evaluated using a one-way analysis of variance (using SPSS version 13), Where p < 0.05 was taken to represent a statistically significant difference.
RESULTS AND DISCUSSION
According of Fig. 1, Phenobarbital solubility area was shown for 20 samples (hatched area). The samples prepared and stored for 2 months at 4 °C in refrigerator to investigate the crystal growth of the drug. The degradation rate varies with temperature. In general, the rate decreases with decreasing temperature. The results showed that 15 out of 20 formulations did not show any crystal growth and were selected for further studies. To the selected samples 30% or 40% sucrose was added and the crystal growth and any changes in pH of the solutions were recorded. The results showed that formulations F1, F2, F3 and F4 (these formulations are numbers 14, 15, 24 and 33, respectively, on the ternary diagram in Fig. 1) did not show any crystal growth or changes in color or pH of the solutions and were selected for chemical stability test. Phenobarbital is stable in air but decomposes by hydrolysis methods to ureide and diamide (9). These degradation products alter the color of the solution from clear to yellow color. A common method for minimizing Phenobarbital degradation in solution dosage forms is to dissolve the drug in a mixed solvent of water and organic solvents.
After determining the optimum level of solvents and syrup, other materials were added to oral solution formulations, which are listed in Table Ι. For masking the unpleasant taste 0.35% g sodium saccharine and 5% strawberry oil were added to the solutions. Butyl paraben was added as preservative to the solutions (5,13).
Before the solutions were kept in the oven, their closures were closed under nitrogen gas in order to expel the oxygen out of the bottle.
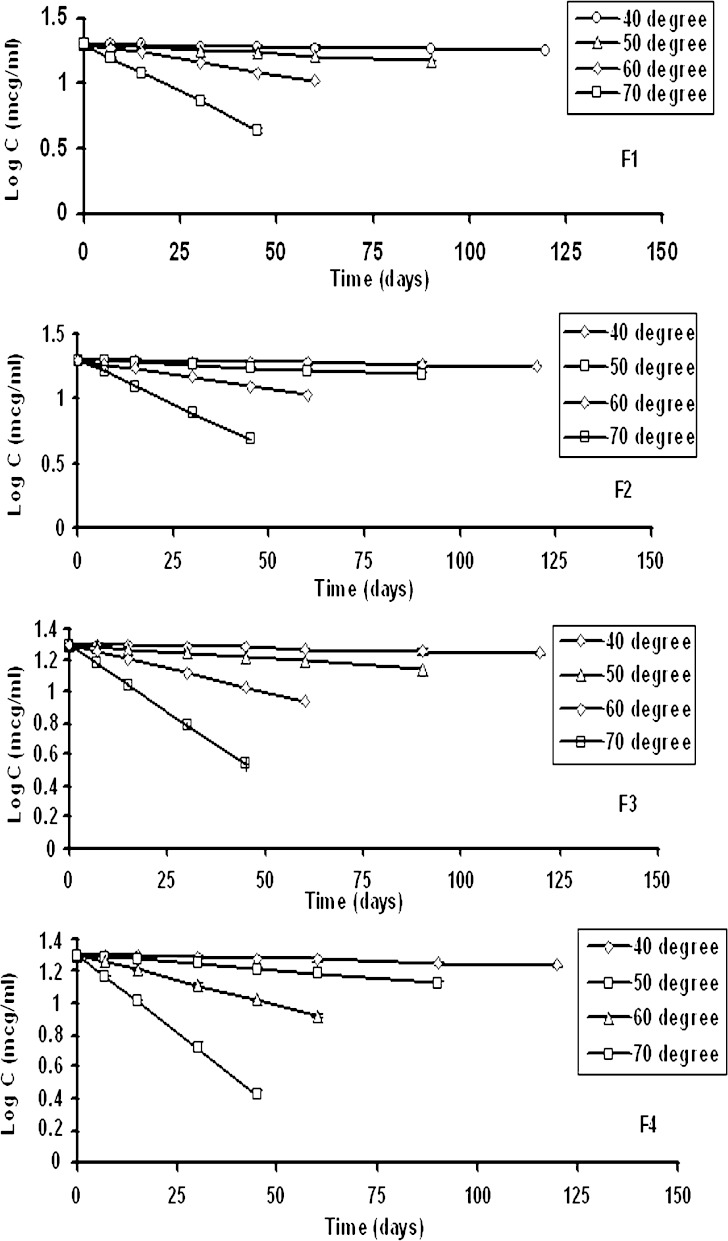
At certain time intervals, Phenobarbital concentration was measured in the solutions, using the UV method described (9). The results are given in Fig. 3. Log concentration of Phenobarbital remained in the solutions was plotted as a function of time. Linearity was observed, indicating that Phenobarbital degradation followed a first order reaction (12,17).
Fig. 3.
Linear relationship between log drug concentrations remained in the samples versus time at different temperatures
From these figures, degradation constants, K, were calculated for different temperatures and formulations. The time at which 10% Phenobarbital degraded was also calculated and the results were given in Table II.
Table II.
Regression Equations Concern to Plots Log K VS. 1/T × 1000 and Shelf-Life Formulations
| Formulation code | Slope (°K) | Intercept (°K) | R 2 | K25 °C × 10 4 | t 90% (years) |
|---|---|---|---|---|---|
| F1 | −5.48 ± 0.19 | 14.51 ± 0.55 | 0.9999 | 1.22 | 2.41 ± 0.40 |
| F2 | −5.50 ± 0.07 | 13.53 ± 0.2 | 0.9999 | 1.11 | 2.59 ± 0.19 |
| F3 | −5.53 ± 0.01 | 14.73 ± 0.027 | 0.9995 | 1.39 | 2.07 ± 0.01 |
| F4 | −5.51 ± 0.16 | 14.72 ± 0.48 | 0.9999 | 1.57 | 1.85 ± 0.27 |
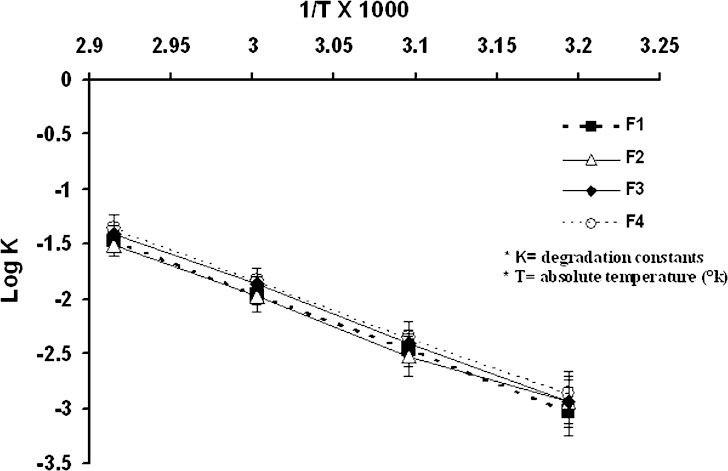
In Fig. 4, an Arrhenius plot of the Phenobarbital degradation in the solutions is given.
Fig. 4.
Linear relationship between log degradation constant and reciprocal of the temperature
In this plot, ln degradation constant (ln K) is given as a function of the reciprocal of the Temperature (12,13). The Phenobarbital degradation seemed to follow the Arrhenius equation pretty well. From this equation the stability of Phenobarbital in the solution at a determined temperature could be estimated.
According to Table ΙΙ, the best formulations were F1, F2 and F3. Shelf lives of F1 and F2 formulations were more than 2 years (2.41 and 2.59 year respectively, p > 0.05) and for F3 formulation was nearly 2 years (2.07 year, p < 0.05). F4 formulation had minimum stability for oral solution (less than 2 years, p < 0.05).
F1, F2 and F3 formulations viscosity were nearly 10–14 Pa S and F4 formulation had lower viscosity (7 Pa S). Also concentration of water in formulation F4 was more than other formulations. Results showed that the temperature relationship with the degradation constant from the Arrhenius equation, it can be seen that the higher the value for the heat of activation, the more the stability is temperature-dependent (Fig. 4). Therefore, the utility of the temperature-dependency relationship rests on the controlling mechanisms of degradation (first-order). If first-order degradation describes phenobarbital degradation is constant with time, 3.3 half-lives must pass before the amount of phenobarbital remaining is reduced to 10% of its original amount, 6.6 half-lives to reduce it to 1%, and ten half-lives to reduce it to 0.1% of the original amount. The amount of degradation needed depends upon the initial amount applied, the toxicity of the product, and the manner preparation (12,13,17).
The following Figs. (3 and 4) illustrate the shape of these first-order degradation curves for half-life values of 120 days. The curves show the amount of phenobarbital remaining at different time’s physical instability of liquid formulations involves the formation of precipitates, less soluble polymorphs, adsorption of the drug substances onto container surfaces, microbial growth, and product appearance (17,18). The results showed that only formulations containing 10–12% propylene glycol, 26–28% glycerin and 2–6% USP syrup had no crystal growth. Chemical stability (shelf-life) of the selected formulations was investigated using Arrhenius accelerated stability test. The calculated stable period was over 2 years (Table II).
SUMMARY AND CONCLUSION
Cosolvency technique used to increase the solubility phenobarbital. Our results showed that Phenobarbital can be dissolved easily in the mixtures of glycerin, propylene glycol and water without addition of alcohol. Formulations containing 10–12% propylene glycol, 26–28% glycerin and 2–6% USP syrup had no crystal growth. These solvents enhanced shelf-life and chemical stability of alcohol-free formulations of Phenobarbital. Degradation kinetic of phenobarbital is first-order. Chemical stability (shelf-life) of the selected formulations was investigated using Arrhenius accelerated stability test. Shelf-life of the selected formulations was over 2 years.
Acknowledgments
The financial support from the research council of Tabriz University of Medical Sciences is greatly acknowledged.
References
- 1.Colquhoun-Flannery W., Wheeler R. Treating neonatal jaundice with phenobarbitone: the inadvertent administration of significant doses of ethyl alcohol. Arch. Dis. Child. 1992;67:152. doi: 10.1136/adc.67.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Touw D. J., Graafland O. G., Cranendonk A., Vermeulen R. J., Weissenbruch M. M. Clinical pharmacokinetics of Phenobarbital in neonates. Eur. J. Pharm. Sci. 2000;12:111–116. doi: 10.1016/S0928-0987(00)00145-7. [DOI] [PubMed] [Google Scholar]
- 3.Li W., Da-Yao Z., Zong-HAO Z., Chi-hua Z., Yuan Z., Yuan Z. Trail of antiepilepsirine (AES) in children with epilepsy. Brain Develop. 1990;21:36–40. doi: 10.1016/s0387-7604(98)00066-7. [DOI] [PubMed] [Google Scholar]
- 4.Haim B., Yoram B., Eilon S., Itai B., Paul F., Loren L. neonatal seizures: Dilemmas in workup and management. Ped. Neurol. 2008;38:415–421. doi: 10.1016/j.pediatrneurol.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 5.The United States Pharmacopoeia XXIX, fifth Supplement. Easton Mack Printing Co. 2006; p. 1530–1531, 3447.
- 6.Amirjahed A. K., Blythe R. H. Product formulation and stability prediction. J. Pharm. Sci. 1977;66:785. doi: 10.1002/jps.2600660610. [DOI] [PubMed] [Google Scholar]
- 7.M. Pernarwski, A. Osol, J. E. Hoover. Solution, Emulsions, Suspensions and Extractives. In: Remington’s Pharmaceutical Sciences. 14th ed., Mack, Easton, 2006, pp. 1070–1071, 1963.
- 8.Boylan J. C., Lachman L., Lieberman H. A., Kanig J. L. Liquids. In: Lachman L., Lieberman H. A., Kanig J. L., editors. The theory and practice of industrial pharmacy. 3. Philadelphia: Lea & Febiger; 1986. pp. 457–478. [Google Scholar]
- 9.Gupta Das V. Effect of ethanol, glycerol, and propylene glycol on the stability of phenobarbital sodium. J. Pharm. Sci. 2006;73(11):1661–1662. doi: 10.1002/jps.2600731149. [DOI] [PubMed] [Google Scholar]
- 10.Loyd V., Allen J. R., Martin A., Erickson I. I. I. Stability of bethanechol chloride, pyrazinamide, quinidine sulfate, rifampin, and tetracycline hydrochloride in extemporaneously compounded oral liquids. Am. J. Health–Syst. Pharm. 1998;55:1804–1808. doi: 10.1093/ajhp/55.17.1804. [DOI] [PubMed] [Google Scholar]
- 11.Loyd V., Allen J. R., Martin A., Erickson I. I. I. Stability of alprazolam, chloroquine phosphate, cisapride, enalapril maleate, andhydralazinehydrochloride in extemporaneouslycompounded oral liquids. Am. J. Health–Syst. Pharm. 1998;55:1915–1920. doi: 10.1093/ajhp/55.18.1915. [DOI] [PubMed] [Google Scholar]
- 12.Martin A., Bustamante P., Chun A. H. C. Physical Pharmacy. 4. Philadelphia: Lea and Febiger; 1993. p. 314. [Google Scholar]
- 13.Garrett E. R., Bean H. S., Beckett A. H., Careless J. E. Kinetics and mechanisms in stability of drugs, in Advances in Pharmaceutical Sciences. Vol. 2. New York: Academic Press; 1967. pp. 74–84. [PubMed] [Google Scholar]
- 14.Santoro M. I., Hackmann E. R., Borges V. M. Stability of phenobarbital sodium in liquid pharmaceutical preparations. Boll. Chim. Farm. 1992;131(6):226–229. [PubMed] [Google Scholar]
- 15.Yashoda P., Gupts V. D. Preformulation studies of spironolactone: effect of pH, two buffer species, ionic strength, and temperature on stability. J. Pharm. Sci. 1991;80(6):551–553. doi: 10.1002/jps.2600800611. [DOI] [PubMed] [Google Scholar]
- 16.Lioyd A. V. J. R. Chloramphenicol 0.5% ophthalmic solution. Int. J. Pharm. 2003;7:468. [Google Scholar]
- 17.Jithan A. V., Mohan C. K., Vimaladevi M. Development and evaluation of a chloramphenicol hypertonic ophthalmic solution. Ind. J. Pharm. Sci. 2008;70:66–70. doi: 10.4103/0250-474X.40334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haijian J. Z., Kathy M., Alison F. Preformulation studies for an ultra short acting neuromuscular blocking agent GW280430a. I. Buffer and cosolvent effects on the solution stability. Drug Dev. Ind. Pharm. 2002;28(2):135–142. doi: 10.1081/DDC-120002446. [DOI] [PubMed] [Google Scholar]