Abstract
The purpose of this study was to design a ‘Traveller Friendly Drug Delivery System’ for PM-HCl. Conventional promethazine (PM-HCl) tablets are bitter, need to be taken 1 h before symptoms and water is also needed. Taste-masked granules were produced with Eudragit® E100 by extrusion, and analyzed with FTIR, DSC, and XRD. Tablets formulated from granules by direct compression using Ac-Di-Sol, Polyplasdone®-XL, Primojel® and ion-exchanger Tulsion®339 and evaluated for mass uniformity, friability, tensile strength, drug content uniformity, water absorption ratio, in-vitro and in-vivo disintegration time and in-vitro dissolution studies. The observed drug-polymer interactions and reduced crystallinity may be reasons for increased dissolution rates. The formulated tablets were disintegrated within 15 s. Tablets (25 mg PM-HCl) with Ac-Di-Sol (4%) showed complete release within 1 min, while marketed conventional tablets (Phenergan®; Rhone-Poulec) release 25% during the same period. A preliminary stability studies for the prepared tablets carried at 30 ± 2°C/60 ± 5% RH, and 40 ± 2°C/75 ± 5%RH for 3 months showed no significant changes in the tablets quality at 30 ± 2°C/60 ± 5% RH. However, at 40 ± 2°C/75 ± 5%RH marked increase in in-vitro disintegration time, tensile strength and decrease in friability and water absorption ratio was found. The present studies indicate the abilities of Eudragit® E 100 for taste masking and improving the dissolution profile of PM-HCl after complexation. In addition, by employing cost effective direct compression method, fast-dissolving tablets of 400 mg total weight with an acceptable quality could be prepared.
Key words: Eudragit® E 100, Eudragit® RD 100, promethazine hydrochloride, rapidly disintegrating tablet, stability study, superdisintegrants
INTRODUCTION
Although various novel and advanced drug delivery systems have been introduced for therapeutic use, the popularity of oral dosage forms, particularly tablets have not been eclipsed, because tablets still have numerous advantages, besides others an economical production. However, one important drawback of tablets as a dosage form is the need to swallow. Dysphasia or general difficulties in swallowing of tablets may be a problem for geriatric, pediatric (1), or traveling patients, if the latter do not have access to water. Dysphasia is also pertinent with the number of medical conditions including strokes, Parkinson’s disease, AIDS, thyroidectomy, head and neck radiation therapy and other neurological disorders including cerebral palsy. Thus, the melt-in-mouth drug delivery system (DDS) is fast dissolving /dispersing, and dissolves in the patient’s mouth within a matter of seconds without need of water or chewing (2). It may therefore be the best solution for patient suffering from dysphasia. This DDS is also suitable for traveling patients suffering from motion sickness and not having access to water.
PM-HCl, the active material used in the present study, is a H1 receptor antagonist of the phenothiazine class, and serves as one of the most important prophylactics against motion sickness. In addition to the general disadvantages of tablets named above, a major drawback of the PM-HCl therapy, in the form of conventional tablets, is the necessity of drug administration more than 1 h before the commencement of the actual embark (3). This may in some cases cause unnecessary medication, as motion sickness is not absolute, or give rise to therapy failure.
Therefore, the purpose of the present study was to develop a fast disintegrating tablet of promethazine hydrochloride (PM-HCl) by direct compression and to mask the intensely bitter taste of the PM-HCl. Such tablet should disintegrate rapidly in the saliva without need of water (‘Traveller Friendly Drug Delivery System’), release the drug instantly for immediate therapeutic effect, and be of acceptable taste.
MATERIALS AND METHODS
Materials
Promethazine HCl (Harika Drug Pvt. Ltd. Hyderabad, India), Ac-Di-Sol (FMC International, Germany), Primojel® (DMV International, Netherland) and Polyplasdone®-XL (ISP Chemical, USA), Mannitol (Getech Ltd., UK) were donation samples from Unimark India Ltd., while Emcure PharmaceuticalLtd., Pune, donated Eudragit® E 100, Eudragit® RD 100 and Aerosil® (Röhm Degussa, Germany). Aristo pharmaceuticals Ltd., Pune (India) donated Avicel® PH 102 (FMC Biopolymer, Belgium). Also Tulsion® 339 (Polacrilin potassium) was kindly supplied by Thermax India Ltd. Magnesium stearate and vanilla flavors of analytical grade were purchased from Qualigen Ltd., Mumbai (India).
Determination of Threshold Value of Bitterness for PM-HCl
Degree of bitterness of PM-HCl was calculated by comparing bitterness values of PM-HCl with that of quinine hydrochloride (QH) as per procedure described in Ph. Eur. 2004. The bitterness value is the reciprocal of the dilution of a compound that still has a bitter taste. The bitterness value of QH is set to 200,000 (4). The bitterness value of PM-HCl was determined as follows: Initially the stock solution containing 0.1 g/100 ml of QH was prepared and subjected for serial dilutions as per procedure described in Ph. Eur. After rinsing the mouth with purified water, 10 ml of the most dilute solution of QH was tasted by keeping in the mouth, mainly near the base of tongue for 30 s. If the bitter sensation was no longer felt in the mouth after 30 s, the solution was spitted out and the mouth was rinsed with purified water. The next dilution in the order of increasing concentration was tasted only after 10 min had passed. The dilution with the lowest concentration to provoke a bitter sensation that continued after 30 s was noted and the respective correction factor κ for each panel member calculated from the following formula:
 |
1 |
Where, η = number of millilitres of the stock solution in the dilution of lowest concentration that is judged to be bitter.
In addition, the stock solution containing 1 g/100 ml of PM-HCl was prepared and subjected for serial dilutions as per procedure described in Ph. Eur. Starting with the lowest concentration; each panel member determined the dilution, which still had a bitter taste as described above. This solution was assigned as D. The dilution factor of solution D was taken as Y. The number of milliliters of solution D after diluting to 10, 0 ml with water, still having bitter taste was determined (X). The bitterness value for each panel member was calculated by using following formula:
 |
2 |
Preparation of the Taste Masked Granules
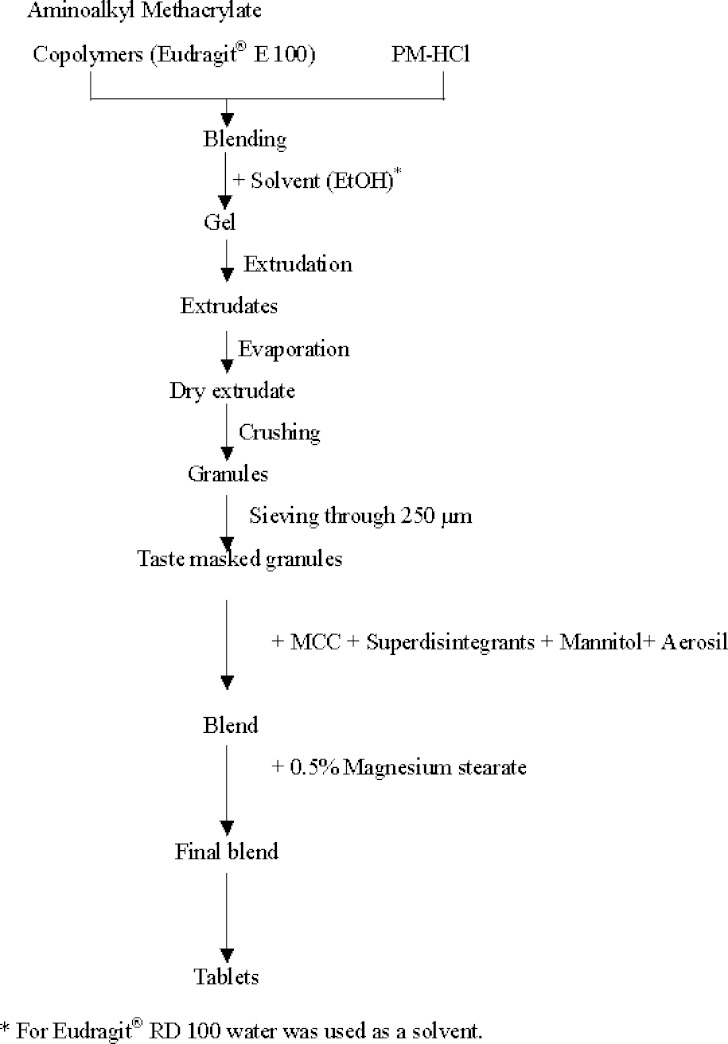
Physical mixtures of PM-HCl: Eudragit® E 100 were prepared in various ratios from 1:2 to 1:16. The recommended solvent for Eudragit® E 100 and Eudragit® RD 100 are ethanol and water respectively (Röhm GmbH, Darmstadt, Germany) (5). A gel containing PM-HCl and Eudragit® E 100 was prepared by gradual addition of 10% ethanol using a mechanical stirrer in a glass beaker. The gel was manually extrudated through a syringe. The ethanol was evaporated, by keeping the extrudates overnight at room temperature. The solidified gel in the shape of strings was crushed and sieved through a sieve sized 255 μm to make the granules (6). Taste-masked granules using Eudragit® RD 100 were prepared accordingly using water as a solvent. The scheme of preparation of the taste-masked granules is shown in Fig. 1.
Fig. 1.
Scheme of preparation of taste masked granules and melting tablets
Selection of Eudragits for the Taste Masking of PM-HCl
A simplified dissolution test was performed to determine the optimum fraction of polymer for taste masking of PM-HCl. This is an in vitro test to evaluate the degree of masking the bitter taste of the fine granules, under the assumption that the fine granules would be held in the mouth together with 10 ml of distilled water, with weak mixing by the tongue for 30 s (7). The method was as follows: The fine granules containing 25 mg of PM-HCl were mixed with 10.0 ml of distilled water in a 10.0 ml syringe by revolving the syringe end to end five times within 30 s. Thereafter, the concentrations of PM-HCl dissolved in the filtrate (D30s), filtered through a membrane filter 0.45 μm were determined spectrophotometrically at 250.2 nm.
Characterization of PM-HCl and Eudragit® E 100 Complex
Thermal Analysis
Differential scanning calorimetry (DSC) was performed using a Mettler TA 4000 apparatus equipped with a DSC 25 cell (Mettler Toledo, USA). The drug, the polymer, and the drug-polymer complex were subjected to the DSC study. 300–340 mg samples were heated at a scanning rate of 20 K/min from 40°C to 300°C under nitrogen.
Fourier Transform Infrared Spectroscopy (FTIR)
The drug, polymer and drug polymer complex were subjected to IR spectroscopy to check the drug polymer interaction using FT-IR (SHIMADZU 8400 S) and the KBr disk method.
X-Ray Diffraction (XRD) Studies
X-Ray Diffraction analysis was carried out to evaluate the degree of crystallinity. The pure PM-HCl, pure Eudragit® E 100, and the PM-HCl-Eudragit® E 100 complex (1:10) were subjected to powder XRD (P.W. 1729, X-Ray Generator, Philips, Netherland) at 2θ angles between 2° and 38° in increments of 0.4°.
Characterization of Powder Flow Properties
The powder flow properties were determined using Carr consolidation index.
Bulk and tapped densities were determined according to Ph. Eur. 2004 with three repetitions (Erweka® Tapped Volumeter, Heusenstamm, Germany). The Carr consolidation index (C.I.) was calculated as:
 |
3 |
Where, ρt—Tapped density, ρb—Bulk density
The interpretation of the values follows commonly accepted suggestions (8).
Tablet Manufacturing
For the final blend, mannitol, aerosil, vanillin dry flavor and magnesium sterate were added in fractions of 2.75%, 2%, 1%, and 0.5% of the tablet mass, respectively. Mannitol was used to impart cooling sensation in mouth. Aerosil was used as it acts as both glidant and tablet disintegrating agent. The concentration of superdisintegrants such as Polyplasdone®-XL, Ac-Di-Sol, Primojel® and Tulsion® 339 was between 2–5%. The concentration of filler-binder such as Avicel®PH 102 was varied between 20–25%, depending on the concentration of superdisintegrants. Avicel®PH 102 was preferred over PH 101 to produce a tablet of an optimum tensile strength: Due to the smaller particle size of Avicel®PH 101compared to PH 102, it would produce tablets of higher tensile strength, which would hamper rapid tablet disintegration. A control formulation was made without a disintegrant. All ingredients were passed through mesh 250 μm. The ingredients were mixed according to Table I and blended in a lab-scale free-flow mixer for 15 min. Magnesium sterate was added in a final step and blended for another 5 min. The tablets were manufactured by direct compression using a 16-station rotary tablet press with 13-mm flat-faced punches (CIP Machineries, Ahmadabad, India). The tablet mass was set to 400 mg per tablet, corresponding to 25 mg PM-HCl per tablet. For each formulation, a batch of 80 tablets was produced.
Table I.
Tablet Formulations
| Ingredients | Amount of ingredients | ||||
|---|---|---|---|---|---|
| No disintegrants (Control) | 2% disintegrant (A1/B1/C1/D1)b | 3% disintegrant (A2/B2/C2/D2) | 4% disintegrant (A3/B3/C3/D3) | 5% disintegrant (A4/B4/D4) | |
| Granules of promethazine HCl and Eudragit E 100 (1:10) | 68.75% | 68.75% | 68.75% | 68.75% | 68.75% |
| Polyplasdone®-XL Ac-Di-Sol Tulsion® 339 | – | 2% | 3% | 4% | 5% |
| Primojel® | – | 2%a | 3%a | 4%a | – |
| Micro crystalline cellulose (Avicel®PH 102) | 25% | 23% | 22% | 21% | 20% |
| Mannitol | 2.75% | 2.75% | 2.75% | 2.75% | 2.75% |
| Vanillin dry flavor | 1% | 1% | 1% | 1% | 1% |
| Colloidial silicon dioxid (Aerosil®) | 2% | 2% | 2% | 2% | 2% |
| Mg stearate | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% |
| Total | 400 mg | 400 mg | 400 mg | 400 mg | 400 mg |
aIndicates formulations with primojel only
bA, B, C and D indicates formulations with Polyplasdone®-XL, Ac-Di-Sol, Primojel® and Tulsion® 339 as superdisintegrants respectively
Characterization of Tablet Properties
Uniformity of Mass
The test was performed according to specifications given in the Ph. Eur., 2004 (9) on 20 tablets. The maximum acceptable limit is ±5% deviation of an individual mass from average mass.
Measurement of Tablet Friability
Tablet friability was measured using the Roche Friabilator according to Ph. Eur., 2004 (10), on ten tablets each. The friability was determined as the mass loss in percent according to Eq. 4:
 |
4 |
Where f—Friability, WA—Initial weight (g), WB—Final weight (g)
Tablets of friabilities under 1% are acceptable (8).
Measurement of Tablet Tensile Strength
The crushing strength of tablets was measured by a Monsanto Hardness Tester (Sheetal Scientific Industries, Mumbai, India) and tensile strength calculated by Eq. 5 (11):
 |
5 |
Where, F is the crushing force in N, D diameter, and t thickness of tablet in mm.
Uniformity of Drug Content
The test is obligatory for tablets containing less than 10 mg or less than 10% w/w of the active ingredient (12). This test was performed as per Indian Pharmacopoeia, 1996. A tablet was crushed and dissolved in 1 ml of dilute hydrochloric acid and 30.0 ml of distilled water. This solution was shaken for 15 min. The volume of this solution was made up to 50.0 ml with distilled water and centrifuged. Five milliliters of the clear supernatant was mixed with 10 ml of 0.1 M hydrochloric acid, and made up to 100.0 ml with distilled water. The absorption of the solution was determined spectrophotometrically at 250.2 nm. The same procedure was followed for another nine tablets.
Water Absorption Ratio
A piece of tissue paper folded twice was placed in a small petri dish containing 6 ml of water. A tablet was put on the paper and was allowed for complete wetting. The wetted tablet was then weighed. Water absorption ratio, R, was determined using following equation (13):
 |
6 |
Where, Wb—Weight of tablet before water absorption, Wa—Weight of tablet after water absorption.
In Vitro Disintegration Time
Disintegration times were measured as per Ph. Eur. specifications, 2004 (14) in water of 37 ± 0.5°C using discs. Tablets that disintegrated within 1 min were considered acceptable.
In Vivo Disintegration Time
Six healthy volunteers, from whom informed consent was first obtained, randomly took one tablet containing PM-HCl and the time required for complete disintegration of the tablet in the mouth without biting and without drinking water was observed.
In Vitro Dissolution Study
PM-HCl tablet test conditions for the dissolution rate studies were used according USP specifications (15) using USP 24, type I apparatus. The dissolution medium was 900 ml of 0.1N HCl. The temperature of the dissolution medium and the rate of agitation were maintained at 37 ± 0.5°C and 100 rpm, respectively.
Aliquots of 10.0 ml of the dissolution medium were withdrawn at specific time intervals and the volume replaced by fresh dissolution medium, pre-warmed to 37 ± 0.5°C. The drug concentration was determined spectrophotometrically at 250.2 nm using UV spectrophotometer (Shimadzu UV 1601, Japan).
Preliminary Evaluation of Stability
Preliminary stability studies were carried out at conditions at 30 ± 2°C/60 ± 5% relative humidity and 40 ± 2°C/75 ± 5%RH for a period of 3 months (16). The effect on tablets in high-density polyethylene bottles regarding the bitter taste of taste-masked granules of PM-HCl: Eudragit® E 100 (1:10) on various mechanical tablet properties such as friability, water absorption ratio, disintegration time and tensile strength was studied.
Data Analysis
Values are expressed as mean ± standard deviation of at least three independent experiments was calculated using Origin® software. Statistical analyses were performed using a Student t test at p < 0.05.
RESULTS AND DISCUSSION
Taste Masking of PM-HCl by Aminoalkyl Methacrylate Copolymers
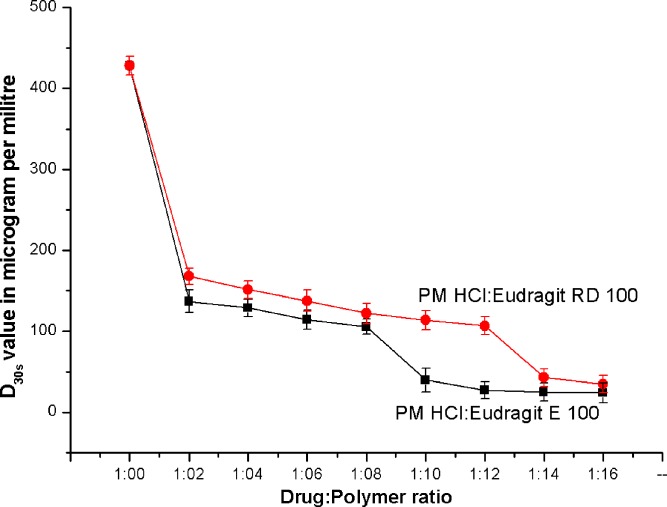
Initially, the most diluted solutions of QH and PM-HCl of bitter taste after keeping in the mouth for 30 s was noted. The test persons experienced from 6.40 ml solution of QH of concentration 0.036 mg/10 ml, and respectively 5.0 ml solution of PM-HCl of concentration 0.1 mg/10 ml as bitter after 30 s. From these values, the bitterness value of PM-HCl was found to be 7,200 units in comparison with QH, the bitterness value of which is set to 200,000 units (4). These results indicated that PM-HCl was 27.8 times less bitter than QH. The concentration 0.1 mg/10 ml was used as the ‘threshold concentration’ to determine the optimum concentration of polymers required to mask the bitter taste of PM-HCl. A simplified dissolution test was performed, to determine the degree of masking the bitter taste of PM-HCl by Eudragit® E 100 and Eudragit® RD 100. It was found that the amount of PM-HCl dissolved from the coated fine granules within 30 s (D30s) decreased with increased proportions of both types of Eudragit® (Fig. 2). However, the respective proportion of polymer yielding D30s values below 100 μg/ml of PM-HCl was considered the optimum amount for the taste masking. The D30s value of uncomplexed PM-HCl was found to be 428.01 μg/ml. The PM-HCl complexed with the Eudragit® E 100 and Eudragit® RD 100 in proportions of 1:10 and 1:14, respectively, showed D30s values below 100 μg/ml. Thus, it was concluded that Eudragit® E 100 and Eudragit® RD 100 in proportions of 1:10 and 1:14 respectively were optimum with respect to masking the bitter taste of PM-HCl.
Fig. 2.
Comparison of D 30s values of Promethazine HCl from Eudragit®E 100 and Eudragit ®RD 100 (±S.D. n = 3)
Characterization of Promethazine Hydrochloride and Eudragit® E100 Complex
The DSC thermograms of PM-HCl, Eudragit® E 100, and PM-HCl: Eudragit® E 100 complex are shown in Fig. 3. The DSC thermogram of PM-HCl exhibits an endothermic peak at 216.4°C, corresponding to its melting point. The thermogram of PM-HCl: Eudragit® E 100 complex shows the melting peak of PM-HCl at a slightly lower temperature (210.1°C), indicating that there is a weak drug-polymer interaction.
Fig. 3.
DSC thermogram of PM-HCl, Eudragit® E100 and PM-HCl: Eudragit® E 100 complex
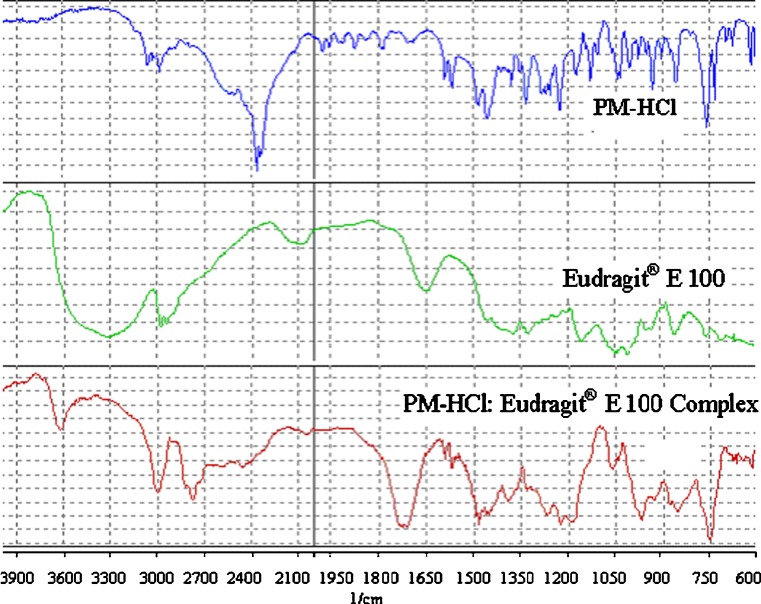
Such interaction between the drug and the carrier may lead to changes in the infrared (IR) spectrum of the solid materials. Hence, PM-HCl, Eudragit® E 100 and PM-HCl–Eudragit® E 100 complex were subjected to FTIR analysis (Fig. 4). When the IR spectrum of HCl–Eudragit® E 100complex was compared with the standard spectrum of PM-HCl, several changes of characteristic bands were noted: The spectrum of pure PM-HCl showed characteristic signals at 750, 3,000, 1,470, 1,600 cm−1 that are assigned to C–S, aromatic C–S, C–N, and aromatic C–C stretchings respectively, while 2,350 cm−1 assigned to the tertiary amine group. The spectra of PM-HCl: Eudragit® E 100 complex showed the characteristic bands of both PM-HCl and Eudragit® E 100, with the exception of the peak at 2,350 cm−1 being much smaller and shifted to longer wavelengths. This indicates the presence of interaction between the drug (tertiary amino group) and the polymer.
Fig. 4.
FTIR spectra of PM-HCl, Eudragit® E 100 and PM-HCl: Eudragit® E 100 complex
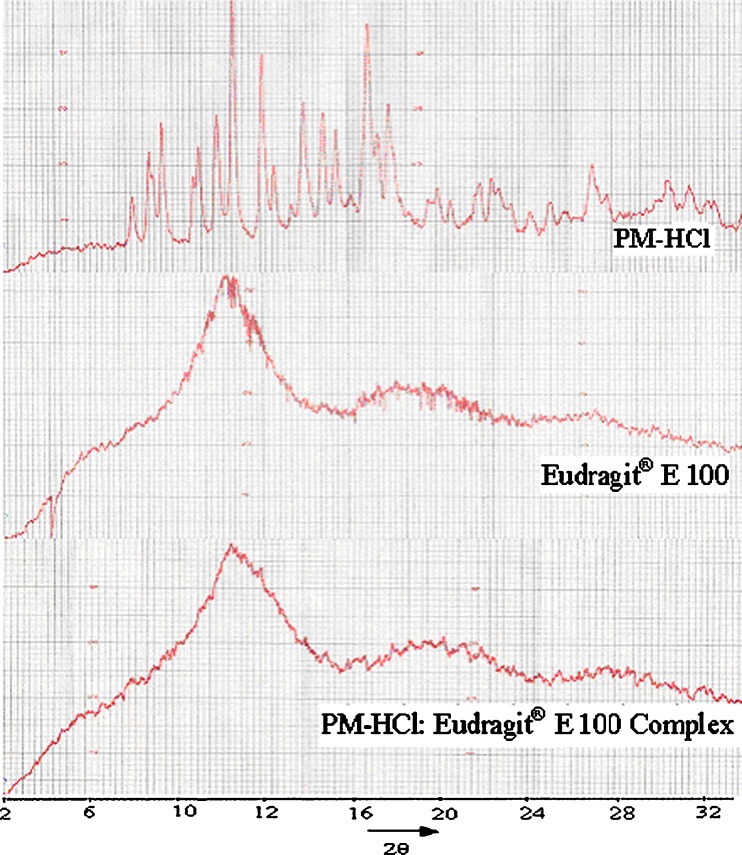
The X-ray diffraction (XRD) pattern of PM-HCl (Fig. 5) shows sharp peaks indicating that the drug is of crystalline nature, while that of Eudragit® E 100 shows blunt peaks indicating its amorphous nature. When crystalline PM-HCl forms a complex with amorphous Eudragit® E 100, the sharp peaks of PM-HCl disappear. This indicates that the drug forms an apparent amorphous state. The change in crystallinity may be the reason for increased dissolution rates of PM-HCl when complexed with Eudragit® E 100, as described above.
Fig. 5.
X-Ray diffraction pattern of PM-HCl, Eudragit® E 100 and PM-HCl: Eudragit® E 100 complex
Characterization of Powder Flow Properties
During tablet production, the die-filling process is based on continuous and uniform flow of the powder from the hopper to the feed frame and into the die cavity. Hence, to determine the suitability of the powder blend for direct compression, all formulations were characterized for various powder properties. The results are shown in Tables II and III. All the studied materials showed Carr consolidation indexes within 11–15. These values are commonly regarded as good flowability (8).
Table II.
Powder Properties of Control Formulation and Powder Containing Polyplasdone®-XL and Ac-Di-Sol as Disintegrant (Mean ± SD, n = 3)
| Sr. No | Formulation Properties | Control | A1 | A2 | A3 | A4 | B1 | B2 | B3 | B4 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bulk density (g/cm3) | 0.492 ± 0.02 | 0.560 ± 0.04 | 0.538 ± 0.02 | 0.531 ± 0.01 | 0.515 ± 0.01 | 0.585 ± 0.03 | 0.583 ± 0.03 | 0.508 ± 0.02 | 0.507 ± 0.04 |
| 2 | Tapped density (g/cm3) | 0.553 ± 0.04 | 0.635 ± 0.04 | 0.616 ± 0.01 | 0.611 ± 0.01 | 0.605 ± 0.01 | 0.668 ± 0.04 | 0.672 ± 0.02 | 0.591 ± 0.02 | 0.596 ± 0.04 |
| 3 | Carr consolidation index | 10.98 ± 0.04 | 11.17 ± 0.01 | 12.66 ± 0.01 | 13.11 ± 0.03 | 14.81 ± 0.03 | 12.5 ± 0.01 | 13.23 ± 0.03 | 14.08 ± 0.05 | 14.98 ± 0.06 |
| 4 | Flowability | Excellent | Good | Good | Good | Good | Good | Good | Good | Good |
Table III.
Powder Properties of Formulation Containing Primojel® and Tulsion® 339 as Disintegrant (Mean ± SD, n = 3)
| Sr. No. | Formulation Properties | Control | C1 | C2 | C3 | D1 | D2 | D3 | D4 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Bulk density (g/cm3) | 0.492 ± 0.02 | 0.488 ± 0.01 | 0.477 ± 0.02 | 0.545 ± 0.2 | 0.493 ± 0.01 | 0.474 ± 0.01 | 0.469 ± 0.02 | 0.474 ± 0.02 |
| 2 | Tapped density (g/cm3) | 0.553 ± 0.04 | 0.560 ± 0.03 | 0.548 ± 0.02 | 0.623 ± 0.03 | 0.554 ± 0.03 | 0.541 ± 0.04 | 0.550 ± 0.02 | 0.549 ± 0.02 |
| 3 | Carr consolidation index | 10.98 ± 0.04 | 12.76 ± 0.02 | 12.92 ± 0.04 | 13.44 ± 0.04 | 11.14 ± 0.02 | 12.30 ± 0.03 | 14.75 ± 0.02 | 13.69 ± 0.01 |
| 4 | Flowability | Excellent | Good | Good | Good | Good | Good | Good | Good |
Characterization of the Tablet Properties
It was found that all the tablets prepared by the direct compression method described above, independently of their composition, passed the weight variation test and the uniformity of content test according to Ph.Eur.2004 specifications (Tables IV and V). The tensile strengths of all the tablets containing superdisintegrants were found in the range of 0.372–0.598 N/mm2, which is less than the control tablet, with the exception of the tablets containing Primojel®. This decrease in tensile strength may be attributed to lower amounts of microcrystalline cellulose (MCC) as a binder in the blends. MCC is known as a potent dry binder as the particles have a large number of free hydroxyl groups and thus the interaction forces at contact points between particles may be strong hydrogen bonds between hydroxyl groups, causing increased tablet hardness (13). The friability was observed to be below 1%, which is regarded to be good mechanical resistance, except for the tablet prepared with Tulsion® 339 as superdisintegrant (formulations D1, D2, D3, D4), which had slightly higher friability (more than 1%).
Table IV.
Tableting Properties of Control and Tablet with Polyplasdone®-XL and Ac-Di-Sol as Disintegrant (Mean ± SD, n = 3)
| Sr. No | Formulation Properties | Control | A1 | A2 | A3 | A4 | B1 | B2 | B3 | B4 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Weight variationa (± %) | 1.32 ± 0.02 | 1.64 ± 0.01 | 1.62 ± 0.04 | 2.68 ± 0.03 | 2.39 ± 0.03 | 1.80 ± 0.06 | 1.23 ± 0.05 | 1.53 ± 0.05 | 1.28 ± 0.04 |
| 2 | Tensile strength (N/mm2) | 0.592 ± 0.11 | 0.559 ± 0.17 | 0.538 ± 0.17 | 0.538 ± 0.12 | 0.538 ± 0.12 | 0.538 ± 0.13 | 0.538 ± 0.11 | 0.484 ± 0.11 | 0.484 ± 0.16 |
| 3 | Friability (%) | 0.68 ± 0.02 | 0.75 ± 0.12 | 0.89 ± 0.06 | 0.89 ± 0.07 | 0.90 ± 0.05 | 0.67 ± 0.02 | 0.68 ± 0.02 | 0.99 ± 0.13 | 0.99 ± 0.05 |
| 4 | Uniformity of contentb | Passed | Passed | Passed | Passed | Passed | Passed | Passed | Passed | Passed |
| 5 | Water absorption ratio | 76.07 ± 0.20 | 91.41 ± 0.31 | 95.64 ± 0.20 | 96.45 ± 0.13 | 100.75 ± 0.12 | 143.32 ± 0.41 | 158.69 ± 0.62 | 198.6 ± 0.52 | 186.2 ± 0.63 |
| 6 | Disintegration time (s) | |||||||||
| In vitro | 59.66 ± 0.58 | 33.33 ± 1.15 | 28 ± 1.12 | 25.66 ± 1.15 | 21.33 ± 2.31 | 13.33 ± 0.57 | 12.00 ± 1.28 | 11.33 ± 1.53 | 52.33 ± 0.58 | |
| In vivo (s) | 64.66 ± 0.51 | 36.66 ± 1.15 | 31.33 ± 1.05 | 27.66 ± 1.15 | 28.66 ± 1.21 | 18.66 ± 1.52 | 15.66 ± 0.58 | 13.66 ± 1.53 | 60.21 ± 1.25 | |
aWeight variation is the percentage of deviation from the mean value
bUniformity of content <5%
Table V.
Tableting Properties of Tablet with Primojel® and Tulsion® as Disintegrant (Mean ± SD, n = 3)
| Sr. No | Formulation Properties | Control | C1 | C2 | C3 | D1 | D2 | D3 | D4 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Weight variationa (± %) | 1.32 ± 0.02 | 1.99 ± 0.03 | 1.46 ± 0.04 | 1.57 ± 0.05 | 1.11 ± 0.01 | 1.91 ± 0.02 | 2.99 ± 0.02 | 1.97 ± 0.01 |
| 2 | Tensile strength (N/mm2) | 0.592 ± 0.11 | 0.593 ± 0.11 | 0.593 ± 0.12 | 0.593 ± 0.15 | 0.376 ± 0.13 | 0.376 ± 0.12 | 0.376 ± 0.15 | 0.376 ± 0.14 |
| 3 | Friability (%) | 0.68 ± 0.02 | 0.67 ± 0.02 | 0.67 ± 0.02 | 0.87 ± 0.02 | 1.26 ± 0.02 | 1.26 ± 0.03 | 1.25 ± 0.03 | 1.26 ± 0.01 |
| 4 | Uniformity of contentb | Passed | Passed | Passed | Passed | Passed | Passed | Passed | Passed |
| 5 | Water absorption ratio | 76.07 ± 0.20 | 122.47 ± 0.70 | 147.28 ± 0.52 | 176.84 ± 0.83 | 69.77 ± 0.13 | 84.17 ± 0.23 | 89.05 ± 0.20 | 81.13 ± 0.23 |
| 6 | Disintegration time(s) | ||||||||
| In vitro | 59.66 ± 0.57 | 30.33 ± 0.12 | 71.00 ± 0.57 | 75.66 ± 0.57 | 98.66 ± 1.15 | 46.34 ± 1.12 | 10.25 ± 0.99 | 11.11 ± 0.26 | |
| In vivo | 64.66 ± 0.51 | 34.66 ± 1.52 | 76.00 ± 1.53 | 81.00 ± 0.28 | 108.9 ± 0.68 | 55.12 ± 0.91 | 14.63 ± 0.99 | 16.8 ± 1.21 | |
aWeight variation is the percentage of deviation from the mean value
bUniformity of content <5%
Water absorption is one of the important steps in the disintegration process: disintegration of the tablet was observed to be fast both in vivo and in vitro, for the formulations of high water absorption ratio (Tables IV and V). The water absorption ratio of all the tablets containing superdisintegrants was found to be larger than for the control tablets. Furthermore, the tablets containing Polyplasdone®-XL, Ac-Di-Sol and Tulsion® 339 showed both in vitro and in vivo disintegration times shorter than the control tablet. In addition, the tablet containing 2% Primojel® disintegrated faster than the control tablet; however, the tablets prepared with more than 2% Primojel® needed longer time to disintegrate than the control tablet.
Increased fractions of the Polyplasdone®-XL improved in the water uptake and reduced the disintegration time compared to the control tablet. The reason may be the highly porous structure of crospovidone, allowing it to draw large amounts of water, by a water wicking mechanism, into the porous network of the tablet. Due to this, though, crospovidone swells little, yet rapidly absorbs water into its network (17).
The tablets containing up to 4% Ac-Di-Sol as a superdisintegrant showed highest water absorption ratio and least disintegration times, however, exactly opposite results were observed with fractions higher than 4% of Ac-Di-Sol (Table IV). Ac-Di-Sol is a ‘superdisintegrants’ of excellent disintegration ability. It swells to a larger extent when in contact with water. The fibrous nature of Ac-Di-Sol allows intra-particulate as well as extra-particulate wicking of water even at lower concentrations. However, Ac-Di-Sol is made by cross -linking (etherification) of sodium carboxymethylcellulose, which greatly reduces its water solubility, while permitting the material to swell and absorb water in amounts of several times its own mass without loosing its fibrous structure. Such hydration makes Ac-Di-Sol more viscous and adhesive, when added in the higher concentrations (18). This can be the possible reason for the reduction of the water absorption ratio and the increase of disintegration time of the tablets containing more than 4% of Ac-Di-Sol.
It was also observed that the water absorption ratio of the tablets was directly proportional to the concentration of Primojel®, but both in vivo and in vitro disintegration time were increased with increase in fraction of Primojel®. The ‘superdisintegrant’ action of Primojel® is governed by its extensive swelling, which increases with increased proportions of Primojel®. Contact of water with Primojel® leads to the formation of viscous plugs (17). Due to the increase in viscosity, further uptake of water may be retarded; and the tablets break into large floccules instead of disintegrating into smaller particles. This might be the reason for increased disintegration times with increased amounts of Primojel®.
The water absorption ratio of tablets containing Tulsion® 339 is higher than for control tablets but less than for tablets containing Polyplasdone®-XL, Ac-Di-Sol, or Primojel® as superdisintegrant (Table V). Tulsion® 339 is a swellable ion exchanger (19). The tablets were formulated with 2–5% of Tulsion® 339, and this amount seems not to be optimum for a rapid swelling.
In all 16 formulations, formulation B3 (containing 4% Ac-Di-Sol as a superdisintegrant) and formulation D3 (containing 4% Tulsion® 339 as a superdisintegrant) showed least disintegration time. When the disintegration times of both formulations were analyzed by t-test, no significant difference was observed.
Dissolution Profile
The release of PM-HCl from the control tablet was immediate as compared to pure PM-HCl, and faster than from the marketed conventional tablet (Phenergan®), reaching 100% dissolution within less than 10 min. The total drug release for Phenergan® was found within 40 min (Fig. 6). Each tablet of Phenergan® contains 25 mg of PM-HCl as an active ingredient while the inactive ingredients are lactose, magnesium stearate and microcrystalline cellulose (20). It is supposed that the reason for fast dissolution of the control tablet is the conversion of the crystalline form of PM-HCl to an amorphous complex with the Eudragit® E 100 as revealed from the XRD studies.
Fig. 6.
Comparison of dissolution profile of Pure PM-HCl, control tablet and conventional tablet of PM-HCl (Phenergan®) (±S.D. n = 3)
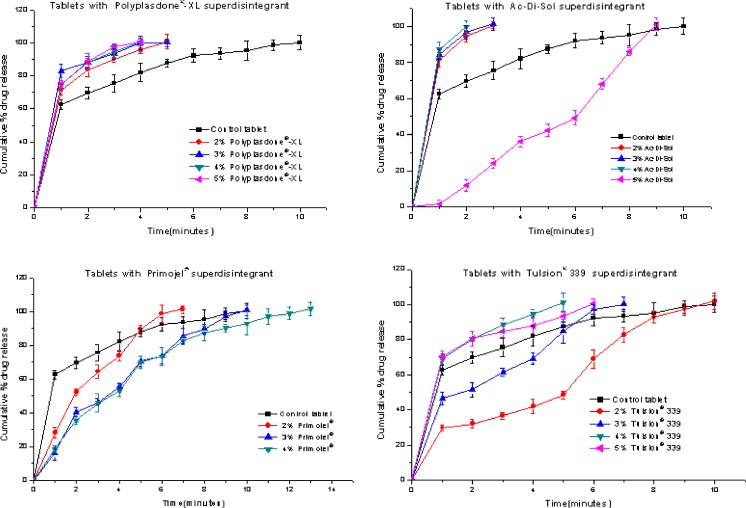
The tablets containing disintegrants showed even faster release of PM-HCl than the control tablet: tablets prepared with Polyplasdone®-XL, Ac-Di-Sol, Primojel® and Tulsion® 339 released 100% drug within 4 to 5, 1–3, 7–13 and 6–10 min respectively (Fig. 7). The overall release rate of PM-HCl from the tablets containing 4% Ac-Di-Sol was the highest. Erosion of the tablets is probably an important mechanism of drug release, since very rapid disintegration was noticed with this tablet by visual inspection during dissolution and disintegration studies. The tablets containing more than 4% of Ac-Di-Sol took more time for the complete release of PM-HCl. This might be due to higher viscosity and adhesiveness in cases where Ac-Di-Sol is added in the higher fractions (18). Probably, due to similar reasons the tablets with more than 2% of Primojel® showed slower release. These tablets showed rapid ability to hydrate with the formation of a swollen sponge-like arrangement, which might create a barrier for drug diffusion.
Fig. 7.
Dissolution profiles for tablet prepared by direct compression technology using Polyplasdone®-XL, Ac-Di-Sol, Primojel® and Tulsion® 339 as superdisintegrant
Preliminary Stability Studies
After production, the taste-masked granules showed a D30s value of 40.0 μg/ml, which is considerably lower than the threshold concentration of the bitter taste of PM-HCl. The D30s of the granules after storage in the high-density polyethylene bottle at 30 ± 2°C/60 ± 5% RH, and 40 ± 2°C/75 ± 5%RH for 3 month were closely similar. These results showed that the masking characteristics of the granules are extremely stable and accord quite well with those of the sensory test. No bitter taste was recognized for at least 30 s after being held in the mouth with 10 ml of water from the granules kept under any storage condition.
The result of stability testing indicate that there is no significant change in the disintegration time at 30 ± 2°C/60 ± 5% relative humidity, but a marked increase in in-vitro disintegration time at 40 ± 2°C/75 ± 5%RH was observed. The tensile strength was found to increase to a small extent at 40 ± 2°C/75 ± 5%RH with no changes at 30 ± 2°C/60 ± 5% relative humidity were observed. The friability and water absorption ratio was found to decrease at 40 ± 2°C/75 ± 5%RH. Only a moderate change in the drug content of PM-HCl at both temperatures was observed (data not shown). A probable reason for the marked changes in tablet properties at 40 ± 2°C/75 ± 5%RH is that above 30°C, Eudragit® form clumps. Also at the higher humidity conditions, water may be adsorbed by the external phase (Avicel®PH 102 and superdisintegrant). These reasons might be responsible for the increase in tensile strength, in vitro disintegration time, and decrease in friability and water absorption ratio.
CONCLUSION
Results of the present study indicate that complexation of PM-HCl with Eudragit® E 100 cannot only mask its bitter taste significantly but also improves its dissolution profile. By employing a cost effective direct compression method, fast-dissolving tablets of 400 mg total weight with a taste and texture acceptable to patients and sufficient structural integrity could be prepared. From all the superdisintegrants studied, tablets containing 4% Ac-Di-Sol gave the highest improvement in disintegration and dissolution rate of PM-HCl. In addition, from preliminary stability studies, it can be concluded that below 30 ± 2°C/60 ± 5% relative humidity; no substantial change in the quality of the tablets during storage is to be expected. However, in the view of the potential utility of the formulation, stability studies should be carried out at recently changed ICH conditions at 30 ± 2°C/65 ± 5% and 40 ± 2°C/75 ± 5%RH for 6 months (21).
References
- 1.Chang R.-K., Guo X., Burnside B. A., Couch R. A. Fast-dissolving tablets. Pharm. Technol. 2000;24(6):52, 54, 56, 58. [Google Scholar]
- 2.Guidance for Industry Orally Disintegrating Tablet. http://www.fda.gov/cder/guidance/5909dft.htm. Accessed March 3, 2008.
- 3.Delgado J. N. Histaminics and Antihistaminics. In: Grisvold W., editor. In Text Book Of Organic Medical and Pharmaceutical Chemistry. 11. Philadelphia: Lippincott; 1998. pp. 710–711. [Google Scholar]
- 4.Bitterness value . European Pharmacopoeia. 5. Strasbourg: Council of Europe; 2004. pp. 221–223. [Google Scholar]
- 5.GmbH R. Eudragit®: Polymethacrylate for phatmaceutical use, Technical literature. Darmstadt, Germany.
- 6.Ishikawa T., Watanabe Y., Utoguchi N., Matsumoto M. Preparation and evaluation of tablets rapidly disintegrating in saliva containing bitter-taste-masked granules by the compression method. Chem. Pharm. Bull. (Tokyo) 1999;47(10):1451–1454. doi: 10.1248/cpb.47.1451. [DOI] [PubMed] [Google Scholar]
- 7.Shirai Y., Sogo K., Fujioka H., Nakamura Y. Influence of heat treatment on dissolution and masking degree of bitter taste for a novel fine granule system. Chem. Pharm. Bul. 1996;44(2):399–402. [Google Scholar]
- 8.W. A. Ritschel, and A. Bauer-Brandl. Herstellung verpreßbarer tablettiermessen. Die Tablette Handbuch der Entwicklung, Herstellung und Qualitätssicherung. Editio Cantor, Aulendorf, 2002, p. 355.
- 9.Uniformity of mass of single dose preparations. European pharmacopoeia, 233 (2004).
- 10.Friability of uncoated tablets. European Pharmacopoeia. 5th ed. Council of Europe, Strasbourg, 2004, p. 3103.
- 11.Newton J. M., Rowley G., Fell J. T., Peacock D. G., Ridgway K. Computer analysis of the relation between tablet strength and compaction pressure. J. Pharm. Pharmacol. 1971;23(Suppl.):195S–201S. doi: 10.1111/j.2042-7158.1971.tb08789.x. [DOI] [PubMed] [Google Scholar]
- 12.Uniformity of drug content. Indian Pharmacopoeia, vol 2. 4th ed. Controller of Publication, Government of India, New-Delhi, 1996, pp. 734–736.
- 13.Bi Y., Sunada H., Yonezawa Y., Danjo K., Otsuka A., Iida K. Preparation and evaluation of a compressed tablet rapidly disintegrating in the oral cavity. Chem. Pharm. Bull. (Tokyo) 1996;44(11):2121–2127. doi: 10.1248/cpb.44.2121. [DOI] [PubMed] [Google Scholar]
- 14.Disintegration of tablets and capsules. European Pharmacopoeia. 5th ed. Council of Europe, Strasbourg, 2004, pp. 3351–3353.
- 15.The United States Pharmacopoeia-27/National Formulary-22. Asia Edition ed. Rockville MD, 2000, pp. 1572–1573.
- 16.Matthews B. R. Regulatory aspects of stability testing in Europe. Drug Dev. Ind. Pharm. 1999;25(7):831–856. doi: 10.1081/DDC-100102245. [DOI] [PubMed] [Google Scholar]
- 17.Augsburger L. B. A., Shah U., Hahm H. Super disintegrants: Characterization and function. In: Swarbrick J., Boylan J. C., editors. Encyclopedia of Pharmaceutical Technology, vol 20. New York: Marcel Dekker; 2001. pp. 269–290. [Google Scholar]
- 18.Bi Y. X., Sunada H., Yonezawa Y., Danjo K. Evaluation of rapidly disintegrating tablets prepared by a direct compression method. Drug Dev. Ind. Pharm. 1999;25(5):571–581. doi: 10.1081/DDC-100102211. [DOI] [PubMed] [Google Scholar]
- 19.Kibbe A. H. Handbook of Pharmaceutical Excipients. 3. Washington: Pharmaceutical; 2000. [Google Scholar]
- 20.Phenergan. http://www.rxlist.com/cgi/generic/prometh.htm Accessed March 3, 2007.
- 21.International Conference on Harmonisation of Technical Requirement for Registration of Pharmaceuticals for Human Use. http://www.ich.org/LOB/media/MEDIA419.pdf. Accessed March 3, 2007.