Abstract
The aim of this study was to design and evaluate of mucoadhesive gel formulations for the vaginal application of clomiphene citrate (CLM) for local treatment of human papilloma virus (HPV) infections. Chitosan (CHI) and polycarbophil (PC) were covalently modified using the thioglycolic acid and L-cysteine, respectively. The formation of thiol conjugates of chitosan (CHI-TG) and polycarbophil (PC-CYS) were confirmed by FT-IR analysis and PC-CYS and CHI-TG were found to have 148.42 ± 4.16 and 41.17 ± 2.34 μmol of thiol groups per gram of polymer, respectively. One percent CLM gels were prepared by combination of various concentrations of PC and CHI with thiolated conjugates of these polymers. Hardness, compressibility, elasticity, adhesiveness and cohesiveness of the gels were measured by Texture profile analysis and the vaginal mucoadhesion was investigated by mucoadhesion test. The increasing in the amount of the thiol conjugates was found to enhance the elasticity, cohesiveness, adhesiveness and mucoadhesion of the gel formulations but not their hardness and compressibility when compared to gels prepared using their respective parent formulations. Slower release rate of CLM from gels was achieved when the polymer concentrations were increased in the gel formulations. PC and its thiol conjugate were found to prolong the release of CLM longer than 70 h unlike gel formulations prepared using CHI and its thiol conjugate which were able to release CLM up to 12 h. Stability of CLM was preserved during the 3 month stability analysis under controlled room temperature and accelerated conditions.
Keywords: chitosan–thioglycolic acid, clomiphene citrate, gel, human papilloma virus, polycarbophil–cysteine, texture profile analysis, vaginal mucoadhesion
INTRODUCTION
Genital HPV infection is a sexually transmitted disease that is caused by human papilloma virus (HPV). HPV is a large family of viruses consisting of more than 100 strains and subtypes. Approximately 20 million people are currently infected with HPV (1,2). Infected patients usually display no symptoms. In some cases, visible venereal warts named as Condylomata acuminata or pre-cancerous changes in the cervix and vulva are observed (3). HPV can alter the genetic structure of cells and causes the proliferation of cells in a rapid and uncontrolled manner. Approximately 10 of the 30 identified genital HPV types can lead, in rare cases, to development of life-threatening cervical cancer and up to 87% of cervical carcinomas contain HPV DNA (4,5). In addition, HPV can be passed on from the pregnant woman to her baby. In very rare cases, the baby can develop warts in the throats or vocal cords (4).
Clomiphene citrate (CLM) is commonly used for infertility treatment due to its ability to initiate ovulation (6). Since HPV binds favourably to estrogen receptors, CLM has also found application in the treatment of HPV infection by competing with HPV for binding to the estrogen receptors (4). Hence, it is hypothesised that effective HPV treatment could be achieved locally by using mucoadhesive vaginal formulations of CLM.
Mucoadhesion is defined as the binding ability of a synthetic or natural polymer to the biological tissue (7). Thus far, mucoadhesive formulations have been prepared for various routes of administration such as rectal (8,9), vaginal (10–12), oral (13), buccal (14–16), nasal (17,18), and ocular (19,20) routes. Mucoadhesive drug delivery systems are capable of increasing the bioavailability of the active compounds by extending their retention time and subsequently controlling the release of the drug in the desired region (21,22). Importantly, a tight contact between the mucoadhesive material and biological tissue is crucial for mucoadhesion to occur. Due to the humidifying effect of the intermediate surface, polymer swells causing mutual interpenetration and formation of low energy chemical bonds between the mucus and the polymeric material (23,24). Examples of mucoadhesive polymers used in vaginal drug delivery systems include CHI, starch, collagen, gelatine, cellulose derivatives, sodium alginate and polyacrylic acids. Recently, polymers with abilities for covalently binding to mucus have been developed. The thiol conjugates of the polymers have an increased ability for interacting with mucus glycoproteins (25–27).
The aim of this study was to prepare CLM gel formulations with improved mechanical, mucoadhesion and sustained release properties for the local treatment of HPV infections. In this study, the anionic structured PC and cationic structured CHI and the thiol conjugates of these polymers were used to prepare the gel formulations. The formation of thiol conjugates were confirmed by FT-IR analysis and the amount of thiol groups conjugated per gram of polymer were calculated. Various experiments were performed to evaluate the mechanical properties (hardness, compressibility, elasticity, adhesiveness and cohesiveness) and the mucoadhesive ability of the prepared gel formulations. The release rate of CLM from gel formulations was also investigated via in vitro dissolution tests at an acidic pH (pH 5.0) corresponding to the pH of the vaginal mucosa. The stability of the drug in the gel formulations was determined on the basis of measuring the amount of drug in the defined storage conditions. It is hoped that by developing a sustained release CLM formulation for vaginal delivery, a safe and effective treatment of HPV infection could be realised.
MATERIALS AND METHODS
Materials
Clomiphene citrate was purchased from Koçak Pharma (Turkey), 1-ethyl-3-(3-dimethylamino propyl) carbodiimide (EDCI), ethylenediamine tetraacetic acid (EDTA), l-cysteine hydrochloride monohydrate and thioglycolic acid from Sigma (USA), polycarbophil (Noveon AA1) from BF Goodrich (USA), chitosan (medium molecular weight, 200–800 cP, deacetylation degree >75%) from Aldrich (Germany), hydrochloric acid (37%), 0.1 mM iodine solution, lactic acid from Merck (Germany). All chemicals used were of pharmaceutical grade and used without further purification.
Preparation of Conjugates
Polycarbophil–cysteine (PC-CYS)
PC-CYS conjugates were synthesized and purified according to previously described methods (25,27). These conjugates were prepared by formation of amide bonds between the carboxylic acid groups of the PC and the primary amino group of the CYS.
Firstly, PC was neutralised prior to the preparation of conjugates. 10 g of the polymer was slowly added to 4% methanolic sodium hydroxide solution (100 mL) with continuous stirring until neutralisation was achieved. Upon neutralisation, the polymers were precipitated and separated by filtration, washed with methanol until the pH 5 was reached and then dried in a ventilated oven at 35°C.
Two grams of neutralised PC were left to swell in 500 mL distilled water at room temperature for 24 h in order to stabilise the polymer dispersion. With the aim of activating the carboxylic acid moieties of PC, EDCI was added into the PC solution to form a final concentration of 50 mM. The pH value of the resultant solution was adjusted to pH 5 with 5N HCl under a stream of nitrogen gas for 15 min to prevent the oxidation of sulphydryl groups with atmospheric oxygen. 1 g l-cysteine hydrochloride monohydrate was added to the solution and dissolved by mixing. The conjugates formed were incubated under a stream of nitrogen gas for 3 h. The solution was dialysed in the dialysis tubing (MW cut-off 12–14 kDa, Medicell International Ltd., UK) protected from light at 10°C against 1 mN HCl containing 2 μM EDTA, twice against 1 mN HCl containing 1% NaCl and finally against 0.5 mN HCl to remove EDCI and CYS from the conjugate solution. The pH value of the dialysed solutions was adjusted to pH 5 with 2 N NaOH. The solution was lyophilised and the conjugates kept at 4°C until needed for the preparation of gels.
Chitosan–thioglycolic acid (CHI-TG)
The conjugates were prepared by covalent modification of the CHI via formation of amide bonds between primary amino groups of the CHI with the carboxylic acid group of the TGA (28).
Briefly, 0.5 g CHI was hydrated in 4 mL of 1 N HCl for 2 h and diluted with distilled water to obtain a 1% CHI solution and kept at room temperature for 24 h to stabilise the polymer dispersion. EDCI in a final concentration of 50 mM and 0.5 g TGA was added into the solution and the pH value of the solution adjusted to 5 with 5 N HCl. The reaction mixtures were incubated for 3 h with permanent stirring. The resultant solution was dialysed twice in a dialysis tubing (MW cut-off 12–14 kDa) protected from light at 10°C against 5 mN HCl containing 1% NaCl and finally against 1 mN HCl to remove EDCI and TGA from the conjugate solution. The solution was lyophilised and the conjugates kept at 4°C until needed for the preparation of gels.
Characterisation of Conjugates
Fourier Transform Infrared (FT-IR) Analysis
The FT-IR spectrums of conjugates were measured in potassium bromide disks using a Perkin Elmer Model 1600 FT-IR spectrometer (USA) operating in transmission mode at a resolution of 4 cm−1 in the range of 4,000–650 cm−1. Twenty scans were co-added for each spectrum.
Amount of Thiol Groups
The amount of thiol groups on the conjugates was determined by iodometric microtitration (25). Three milligrams conjugate was hydrated in 1 mL of distilled water. The pH value was adjusted to pH 3 with 1 N HCl and 500 μL of 1% m/v starch solution was added. The solution was titrated with 1 mM iodine solution until the solution has turned into a clear light blue colour. The same process was repeated with the unmodified parent polymers as control groups. The amount of thiol groups in the conjugates determined using the equation below (Eq. 1):
 |
1 |
whereby M: The molarity of iodine solution used for titration; V: The volume of 1 mM iodine solution used for titration (mL) and 33 mg thiol group is equivalent to 1 mmol thiol.
Preparation of Gel Formulations
Various gel formulations containing CLM were prepared and the composition of these gel formulations was given in Table I. Both PC and PC-CYS conjugates were left to swell in half of the pH 5 lactate buffer overnight to prepare the PC (PC1–3) and PC-CYS (PC-CYS 1–6) gel formulations, respectively. CLM was dissolved in the remaining half of the lactate buffer by mechanical shaking at 3,000 rpm for 3 h. Then, this solution was added onto polymer dispersion, mixed homogenously and pH value of the gel adjusted to pH 4.5 ± 0.1 with 0.1 N NaOH.
Table I.
Formulation of Polycarbophil–cystein and Chitosan–thioglycolic Acid Gels
| Formulation | PC1 | PC2 | PC3 | PC-CYS1 | PC-CYS2 | PC-CYS3 | PC-CYS4 | PC-CYS5 | PC-CYS6 | CHI1 | CHI2 | CHI3 | CHI-TG1 | CHI-TG2 | CHI-TG3 | CHI-TG4 | CHI-TG5 | CHI-TG6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clomiphene citrate (g) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Polycarbophil® (g) | 2 | 3 | 4 | 1.8 | 2.8 | 3.8 | 1.5 | 2.5 | 3.5 | – | – | – | – | – | – | – | – | – |
| Chitosan (g) | – | – | – | – | – | – | – | – | – | 2 | 2.5 | 3 | 1.8 | 2.3 | 2.8 | 1.5 | 2.0 | 2.5 |
| Polycarbophil®–cysteine conjugate (g) | – | – | – | 0.2 | 0.2 | 0.2 | 0.5 | 0.5 | 0.5 | – | – | – | – | – | – | – | – | – |
| Chitosan–thioglycolic acid conjugate (g) | – | – | – | – | – | – | - | – | – | – | – | – | 0.2 | 0.2 | 0.2 | 0.5 | 0.5 | 0.5 |
| 0.1 N NaOH | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. |
| Lactate buffer pH 5 (g) | 97 | 96 | 95 | 97 | 96 | 95 | 97 | 96 | 95 | 57 | 56.5 | 56 | 57 | 56.5 | 56 | 57 | 56.5 | 56 |
| 0.2 N HCl (g) | – | – | – | – | – | – | – | – | – | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
In order to prepare CHI gel formulations (CHI 1–3), CHI was left to swell in 0.2 N HCl overnight. CLM was added into the CHI dispersion and mixed homogenously in pH 5 lactate buffer by mechanical shaking at 3,000 rpm for 3 h. Then, the gel was adjusted to pH 4.5 ± 0.1 with 0.1 N NaOH. To prepare the gel formulations containing CHI-TG conjugates (CHI-TG 1–6), the polymer and the conjugate of the same polymer were swollen with 0.2 N HCl overnight and the gel formulations were prepared according to the above mentioned procedure.
Texture Profile Analysis of Gel Formulations
The mechanical properties of all the gel formulations were determined using a software-controlled penetrometer, TA-XTPlus Texture analyzer (Stable Micro Systems, UK), with a 5 kg load cell at 37 ± 0.5°C. Each formulation was transferred into a universal bottle (25 mL) to a fixed height of 8 cm and kept in the ultrasonic water bath to remove air bubbles for 20 min and the temperature was adjusted to 37°C. The Perspex probe of 10 mm diameter was twice compressed into each formulation at a defined rate of 2 mm s−1 to a depth of 15 mm. A delay period of 15 s was allowed between the two compressions. Data collection and calculation were performed using the Texture Exponent 4.0.4.0 software package of the instrument. From the resultant force-time plot, mechanical parameters such as hardness, compressibility, adhesiveness, cohesiveness and elasticity of the gel formulations were defined (29).
Hardness was defined as the force required to attain a given deformation or as the maximum peak force during the first compression cycle.
Compressibility was defined as the work required to deform the product during the first compression of probe.
Adhesiveness was defined as the negative force area for the first compression cycle and represents the work required to overcome the attractive forces between the surface of the gel and the surface of the probe.
Cohesiveness was defined as the ratio of the area under the force-time curve produced on the second compression cycle to that produced on the first compression cycle, where successive compressions are separated by a defined recovery period.
Elasticity was defined as the ratio of the time required to achieve maximum structural deformation on the second compression cycle to that on the first compression cycle, where successive compressions are separated by a defined recovery period.
Each experiment was repeated six times.
Mucoadhesion Studies
TA-XTPlus Texture analyzer equipped with a 5 kg load cell was used for mucoadhesion studies (30,31). Freshly excised bovine vaginal mucosa was frozen at −30°C. A section of 2 mm thickness was taken from the inner part of the surface of the frozen vaginal mucosa and attached to the lower end of the probe (P0.5 Perspex, θ: 12.5 mm) of the instrument with cyanoacrylate glue. The mucosa was dipped into the cow vaginal mucus (frozen at −30°C just after the excision and adjusted to 37°C during the experiment) and kept for 10 min prior to the commencement of the experiment.
The gels were packed into 30 mm diameter tubes and centrifuged at 20,000 rpm for 10 min to remove the air bubbles from the gels and to ensure a smooth contact between the gels and the vaginal mucosa. The mucoadhesion studies were perfomed at 37°C. The probe holding the vaginal mucosa was lowered onto the surface of the gel with a constant speed of 0.1 mm s−1 and contact force of 0.5 N applied. After a contact period of 120 s, the probe was then moved vertically upwards at a constant speed of 0.1 mm s−1. The area under the curve (AUC) was calculated from force–distance plot as the work of mucoadhesion. The formulation given below was used to calculate the work of mucoadhesion per square centimeter (mJ cm−2; Eq. 2). Each experiment was carried out in triplicate.
 |
2 |
whereby πr2: Surface area of the vaginal mucosa which is in contact with the gel formulations.
In vitro Drug Release Studies
Five hundred milliliters pH 5 lactate buffer at 37 ± 0.5°C was used as the dissolution medium for the in vitro drug release studies. Five grams gel was put into a cylindrical vessel which has 3.8 cm diameter and 1 cm height. A 4 cm diameter sieve (θ: 30 mesh) was attached on the edge of the cylindrical vessel and located on the bottom of the dissolution vessel providing the edge remained at the upper surface (32). The study was held at 100 rpm using USP 24 paddle rotation method. Samples were taken at certain time intervals and the same amount of pH 5 lactate buffer was added to the medium. The absorbance of the solutions was read spectrophotometrically at 292 nm and the concentrations were calculated using the standard curve. The test was conducted in triplicate.
Stability of CLM in Gel Formulations
The gel formulations were filled into aluminium tubes and placed in the stability cabinets. The stability of CLM in gels was observed at 25 ± 2°C, 60 ± 5% RH; 30 ± 2°C, 65 ± 5% RH and 40 ± 2°C, 75 ± 5% RH for 3 months. The samples taken after 0, 15, 30, 60, 90 days were weighed to control the loss of weight during the incubation periods and the CLM assay was performed using a high-performance liquid chromatography method described in USP 24. The experiment was performed in triplicate for each gel formulation.
Statistical Evaluation
Results of the mechanical and mucoadhesion properties of the formulations and in vitro drug release data were statistically evaluated using one-way analysis of variance (ANOVA) followed by Newman–Keuls multiple comparisons test. p < 0.05 was considered to be indicative of significant difference between the chosen formulations.
RESULTS AND DISCUSSION
Characterisation of PC-CYS and CHI-TG Conjugates
PC-CYS and CHI-TG conjugates were obtained by the covalent modification of the PC and CHI, respectively, via the formation of amide bonds. The suitable amount of EDCI for the activation of the carboxyl groups, the appropriate pH for the conjugate preparation and the reaction periods were selected according to previous studies.(25,27,32) FT-IR spectrums confirmed the formation of PC-CYS and CHI-TG conjugates (Fig. 1). According to the FT-IR spectrum of the PC-CYS conjugate (Fig. 1a), –SH stretching peak at 2,569 cm−1 was related to the –SH groups of CYS moiety; aliphatic carboxyl group at 1,698 cm−1 indicated the presence of free carboxyl groups of PC. The amide band C=O stretching peak at 1,670 cm−1 and amide band N–H incline at 1,633 cm−1 confirmed the formation of amide bond in the PC-CYS conjugates. According to spectrum of the CHI-TG conjugate (Fig. 1b), –OH stretching peak at 3,519 cm−1 indicated the presence of hydroxyl groups of CHI; –SH stretching peak at 2,601 cm−1 was related to –SH groups of TG. The amide band C=O stretching peak at 1,689 cm−1 and amide band N–H incline at 1,636 cm−1 confirmed the formation of amide bond in the CHI-TG conjugates.
Fig. 1.
FT-IR spectrums of a polycarbophil and polycarbophil–cystein conjugate and b chitosan and chitosan–thioglycolic acid conjugate
According to the determination of thiol groups of conjugates using the iodometric microtitration assay, PC-CYS and CHI-TG were found to have 148.42 ± 4.16 μmol and 41.17 ± 2.34 μmol of thiol groups per gram of polymer, respectively.
Mechanical Properties of Gel Formulations
A syringe or applicator is required for successful administration of semi-solid formulations via the vaginal route. Upon this application, dosage forms are rapidly removed by vaginal secretion. Hence, the drug release of vaginally administered dosage forms is subjected to variations and difficult to predict (33). In order to qualify as a mucoadhesive formulation, several criteria such as the ease of removal of the gel from the primary package, the ease of application of the product to the desired region and the retention at the application site without disintegration need to be accomplished (34). The vaginal semi-solid formulations should also have appropriate mechanical properties to boost clinical efficiency. The mechanical properties such as hardness, compressibility, elasticity, cohesiveness and adhesiveness of gel formulations were investigated using texture profile analysis (32, 34–36) (Table II).
Table II.
Mechanical and Mucoadhesive Properties of Gel Formulations (n = 6)
| Formulation | Hardness N ± SD | Compressibility (Nmm) ± SD | Adhesiveness (Nmm) ± SD | Cohesiveness ± SD | Elasticity ± SD | Work of Mucoadhesion (mJ cm−2) ± SD |
|---|---|---|---|---|---|---|
| PC1 | 0.058 ± 0.004 | 0.309 ± 0.028 | 0.182 ± 0.011 | 0.919 ± 0.035 | 0.590 ± 0.034 | 0.063 ± 0.003 |
| PC2 | 0.199 ± 0.007 | 1.235 ± 0.107 | 0.428 ± 0.052 | 0.865 ± 0.030 | 0.609 ± 0.045 | 0.074 ± 0.007 |
| PC3 | 0.399 ± 0.024 | 2.032 ± 0.126 | 0.666 ± 0.048 | 0.856 ± 0.024 | 0.665 ± 0.040 | 0.092 ± 0.007 |
| PC-CYS1 | 0.060 ± 0.002 | 0.312 ± 0.034 | 0.219 ± 0.018 | 0.922 ± 0.039 | 0.602 ± 0.048 | 0.082 ± 0.005 |
| PC-CYS2 | 0.204 ± 0.014 | 1.225 ± 0.132 | 0.472 ± 0.059 | 0.868 ± 0.026 | 0.605 ± 0.053 | 0.098 ± 0.005 |
| PC-CYS3 | 0.387 ± 0.021 | 2.005 ± 0.130 | 0.790 ± 0.054 | 0.859 ± 0.029 | 0.676 ± 0.036 | 0.118 ± 0.009 |
| PC-CYS4 | 0.052 ± 0.007 | 0.285 ± 0.016 | 0.234 ± 0.012 | 1.034 ± 0.022 | 0.575 ± 0.026 | 0.110 ± 0.008 |
| PC-CYS5 | 0.186 ± 0.011 | 1.112 ± 0.059 | 0.519 ± 0.043 | 1.018 ± 0.015 | 0.582 ± 0.021 | 0.136 ± 0.011 |
| PC-CYS6 | 0.348 ± 0.016 | 1.834 ± 0.112 | 0.856 ± 0.051 | 1.002 ± 0.026 | 0.625 ± 0.032 | 0.209 ± 0.010 |
| CHI1 | 0.017 ± 0.001 | 0.014 ± 0.001 | 0.031 ± 0.004 | 1.023 ± 0.027 | 0.732 ± 0.012 | 0.012 ± 0.001 |
| CHI2 | 0.018 ± 0.001 | 0.022 ± 0.006 | 0.041 ± 0.011 | 1.012 ± 0.014 | 0.744 ± 0.016 | 0.017 ± 0.001 |
| CHI3 | 0.072 ± 0.001 | 0.305 ± 0.008 | 0.202 ± 0.014 | 0.998 ± 0.037 | 0.763 ± 0.013 | 0.023 ± 0.001 |
| CHI-TG1 | 0.016 ± 0.001 | 0.025 ± 0.004 | 0.045 ± 0.008 | 1.030 ± 0.023 | 0.744 ± 0.018 | 0.021 ± 0.001 |
| CHI-TG2 | 0.017 ± 0.001 | 0.028 ± 0.012 | 0.064 ± 0.009 | 1.016 ± 0.012 | 0.754 ± 0.021 | 0.032 ± 0.002 |
| CHI-TG3 | 0.074 ± 0.001 | 0.301 ± 0.013 | 0.242 ± 0.017 | 1.003 ± 0.014 | 0.778 ± 0.026 | 0.038 ± 0.002 |
| CHI-TG4 | 0.015 ± 0.003 | 0.021 ± 0.005 | 0.065 ± 0.012 | 1.035 ± 0.032 | 0.730 ± 0.013 | 0.036 ± 0.002 |
| CHI-TG5 | 0.019 ± 0.002 | 0.024 ± 0.007 | 0.085 ± 0.011 | 1.021 ± 0.026 | 0.741 ± 0.033 | 0.054 ± 0.003 |
| CHI-TG6 | 0.069 ± 0.007 | 0.293 ± 0.012 | 0.278 ± 0.023 | 1.013 ± 0.015 | 0.753 ± 0.026 | 0.075 ± 0.006 |
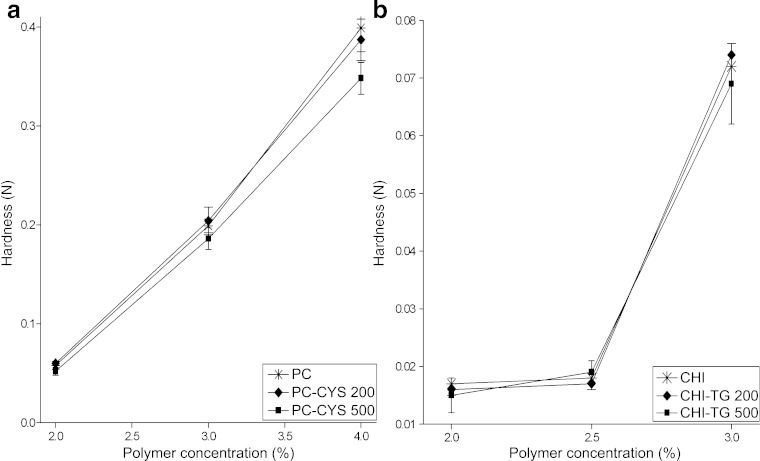
The hardness test was performed to measure the required force to produce deformation of gels. The hardness influences the applicability of the gel to the desired site. Vaginal gels are required to have a low hardness value for ease of administration to the vagina (35). Ferrari et al. (37) described increased hardness of aqueous polymeric gels as a function of polymeric concentration. Jones et al. (38), reported that the product hardness were dependent on the concentration of polymers such as hydroxyethylcellulose (HEC), polyvinylpyrrolidone and PC in hydrogel formulations. It was also reported that the textural properties of gel formulations were significantly affected by the molecular weight, deacetylation degree and concentration of CHI (39).
Similar results were obtained in our study. We have found that the hardness values of the PC gels and CHI gels increased significantly by seven and four times, respectively, due to the increase in the polymer concentration from 2% to 4% for PC gels and from 2% to 3% for CHI gels (p < 0.05; Fig. 2). The addition of 0.2 g PC-CYS did not significantly change the hardness of gel formulations but when a higher amount of PC-CYS (0.5 g) was added, the hardness of PC gels was slightly reduced (p > 0.05). However, the addition of CHI-TG conjugate did not significantly change the hardness of CHI gels (p > 0.05).
Fig. 2.
Hardness of the gel formulations (n = 6)
Compressibility is defined as the work that is required to achieve compaction of the product along a definite distance (29,40). A low compressibility value is required to enable ease of removal of the gel from the container and the ease of the spreadibility of the gel on the application site (35,36). It was previously reported by several workers that increasing the concentration of each polymeric component significantly increased formulation compressibility (40–42). Jones et al. (43) also reported that the compressibility was affected by the concentration of HEC, carbopol and/or PC in gel formulations.
In this study, it was apparent that both concentration and type of polymer affected product compressibility. The results indicate that the compressibility of gels increased 6.5 times (PC1: 0.309 ± 0.028 Nmm; PC3: 2.032 ± 0.126 Nmm) and 21.5 times (CHI1 0.014 ± 0.001 Nmm; CHI3: 0.305 ± 0.008 Nmm) for PC and CHI parent polymer, respectively, upon increasing the polymer concentration in gel formulations (p < 0.05; Fig. 3). While the addition of the PC-CYS conjugate was slightly reduced the compressibility of PC gels (PC-CYS3: 2.005 ± 0.130 Nmm; PC-CYS6: 1.834 ± 0.112 Nmm), the compressibility was not changed in formulations containing CHI-TG conjugate (CHI-TG3: 0.301 ± 0.013 Nmm; CHI-TG6: 0.293 ± 0.012 Nmm). It was evident that the gel formulations containing CHI and its conjugate showed less compressibility value than gels containing PC and its conjugate (Fig. 3). When these results were compared with previous studies (36), all formulations had suitable compressibility in the point of view of applicability to the vaginal site.
Fig. 3.
Compressibility of the gel formulations (n = 6)
The elasticity is defined as the direction of re-construction of the gel after its deformation by compression in a defined period of time (44). The increase in the numerical value of elasticity obtained during texture profile analysis shows the decrease in the elasticity of gel (43). The basic physical mechanism of bioadhesion is related to the elasticity of the polymer chains whereby a high elasticity is required for strong bioadhesion between the polymer and the mucosal surface (45). It was noted that the elasticity of gels is decreased due to the increase in the polymer concentration in the gels (43). The results indicated that the elasticity slightly reduced for PC gels (PC1: 0.590 ± 0.034; PC3: 0.665 ± 0.040) and CHI gels (CHI1: 0.732 ± 0.012; CH3: 0.763 ± 0.013) upon increasing the polymer concentration in gel formulations (Fig. 4). The type of polymer also influenced the elasticity of gels. The elasticity of the PC and PC/PC-CYS gels were 1.24–1.35 times higher than that of CHI and CHI/CHI-TG gels. The addition of 0.5 g PC-CYS conjugate slightly increased the elasticity of gels at all concentrations of polymers tested (PC-CYS4: 0.575 ± 0.026; PC-CYS5: 0.582 ± 0.021; PC-CYS6: 0.625 ± 0.032). Interestingly, the addition of CHI-TG conjugates did not change the elasticity of CHI gels (p > 0.05; Fig. 4). It was seen that the elasticity of gel formulations containing PC (PC1-PC3) and its conjugate (PC-CYS1–PC-CYS6) was in more acceptable range (between 0.590 ± 0.034 and 0.676 ± 0.036) compared to that of gels (between 0.732 ± 0.012 and 0.778 ± 0.026) containing CHI (CHI1-CHI3) and its conjugate (CHI-TG1–CHI-TG6).
Fig. 4.
Elasticity of the gel formulations (n = 6)
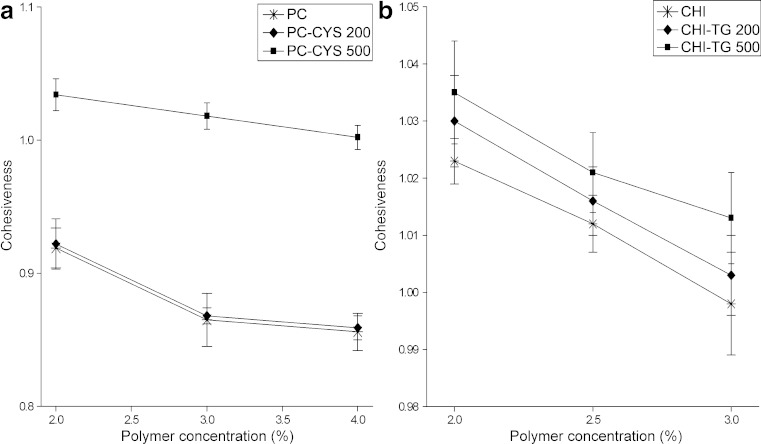
The vagina is an ideal site for the application of systemic and local drug administration due to its large surface area for drug absorption. The main disadvantage of the vaginal dosage forms is their rapid removal from the vaginal system (33). The retention of most formulations in the vaginal site is limited due to the presence of vaginal secretions and weak cohesive forces (46). The cohesiveness of the gel is important to determine the reconstruction ability of the gel after application (40,46). The high cohesiveness value increases the performance of the product at the application site by providing full structural recovery following gel application (36). It was observed the greater reduction in cohesiveness of gel formulations containing the higher concentrations of each polymer such as HEC, carbopol or PC due to the proportionally greater semi-solid character of gels (40). In this study, the cohesiveness of PC gels (PC1: 0.919 ± 0.035; PC3: 0.856 ± 0.024) and CHI gels (CHI1: 1.023 ± 0.027; CHI3: 0.998 ± 0.037) was decreased slightly upon increasing the polymer concentration as previously described (40) (Fig. 5). The addition of 0.5 g PC-CYS conjugate increased the cohesiveness of gels at all concentrations of polymers tested (PC-CYS4: 1.034 ± 0.022; PC-CYS5: 1.018 ± 0.015; PC-CYS6: 1.002 ± 0.026). Similar results were obtained with gels containing 0.5 g CHI-TG conjugate (CHI-TG4: 1.035 ± 0.032; CHI-TG5: 1.021 ± 0.026; CHI-TG6: 1.013 ± 0.015) (Fig. 4). According to results, it was seen that the gel formulations containing highest amounts of PC-CYS conjugate (PC-CYS4 - PC-CYS6) and highest amounts of CHI-TG conjugate (CHI-TG4–CHI-TG6) showed highest and acceptable cohesiveness.
Fig. 5.
Cohesiveness of the gel formulations (n = 6)
A product designed for topical application to mucous membranes should preferably possess adhesive properties, as these will enhance the time of location at the site of application and hence improved clinical efficiency (29). Adhesiveness in texture profile analysis is commonly defined as the work required to overcome the attractive forces between the surface of the sample and the probe surface (32). It was reported that the increase in gel adhesiveness caused by an increase in polyacrylic acid concentration might be attributed to the greater ability of polyacrylic acid to chemically interact with the probe (36).
In our study, the type of polymer used in the preparation of gels influenced the adhesiveness of the resultant gel. PC gels were at least four times more adhesive than CHI gels when compared to gels prepared with same amount of polymer (p < 0.05). The adhesiveness of gels increased upon increasing the polymer concentration from 2% to 4% for PC gels (PC1: 0.182 ± 0.011 Nmm; PC3: 0.666 ± 0.048 Nmm) and from 2% to 3% for CHI gels (CHI1: 0.031 ± 0.004 Nmm; CH3: 0.202 ± 0.014 Nmm). Addition of the PC-CYS conjugates at increasing amounts were found to increase the adhesiveness of PC gels, particularly significant improvement in adhesion was observed when the highest concentration of polymer (4%) were used whereby the adhesion results were 0.666 ± 0.048 Nmm and 0.856 ± 0051 Nmm for PC6 and PC-CYS6 formulations, respectively (p < 0.05; Fig. 6). There was no significant difference in adhesiveness of CHI gels prepared using 2% (CH1: 0.031 ± 0.004 Nmm) and 2.5% (CH2: 0.041 ± 0.011 Nmm) polymer (p > 0.05), but adhesiveness of gels was significantly increased when 3% polymer was used (CH3: 0.202 ± 0.014 Nmm) (p < 0.05). Addition of 0.5 g CHI-TG conjugate increased the adhesiveness of gel formulations at all concentrations of polymers tested (p < 0.05; Fig. 6). While the lowest adhesiveness was seen for CHI1 (0.031 ± 0.004 Nmm), PC-CYS3 (0.790 ± 0.054 Nmm) and PC-CYS6 (0.856 ± 0.051 Nmm) formulations exhibited the highest adhesiveness (Table II).
Fig. 6.
Adhesiveness of the gel formulations (n = 6)
Mucoadhesion Studies
The mucoadhesion test was performed to measure the adhesive strength of gel formulations to the vaginal mucosa. Importantly, factors such as the type, molecular weight and architecture of polymer and the polymer concentration in the gel can influence the mucoadhesive performance of the formulation (5,21). PC was reported to possess good bioadhesive properties (40). Chang et al. (47) developed the mucoadhesive thermosensitive gel formulations composed of poloxamers 407, 188 and PC for the effective treatment of vaginal candidiasis and reported that the formulations with higher contents of PC had shown stronger mucoadhesiveness. CHI was also reported to be a linear polycation that readily adheres to negatively charged surfaces at acidic pH simulating vaginal conditions (48).
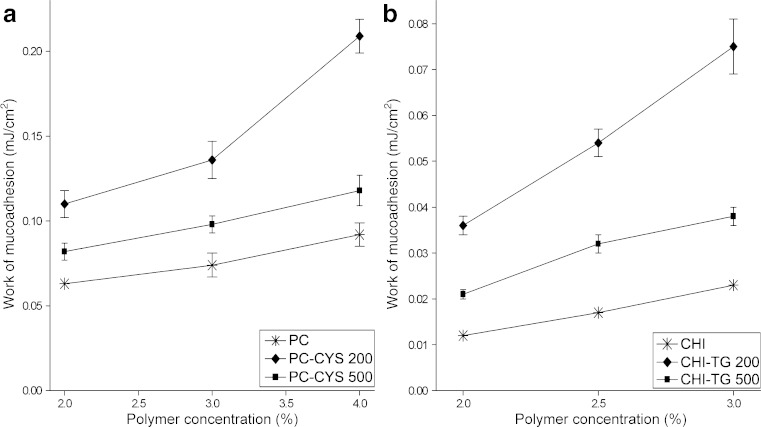
In our study, when the polymer concentration was increased from 2% to 4% for PC gels and from 2% to 3% for CHI gels, the mucoadhesion ability was also increased (Table II, Fig. 6). The type of polymer used in the preparation of gels influenced the mucoadhesion ability of gels and PC gels were found more mucoadhesive than CHI gels.
These findings were in accordance with literature (32,49). It was reported that due to the existence of more than 90% of the acid groups of polyacrylic acids in non-ionized form at pH 5 and below, polymer cannot be charged electrostatically and exhibits lower swelling ability. Hence, the polymer binds directly to polysaccharides or proteins with hydrogen bonds and increases the mucoadhesion (32). On the contrary, Bonferoni et al. found that the higher lactic acid content reduce ionisation of the acidic moieties of sialic acids of the mucin chains which are usually known to contribute to strengthening the mucoadhesive bonds with cationic polymers such as CHI and correspond to poor mucoadhesiveness (49). In this study, gels were prepared in lactate buffer and this could be the reason of limited interaction between CHI and vaginal mucosa.
The addition of increasing amount of thiol conjugates significantly enhanced the mucoadhesion ability of parent gels. For example, the work of mucoadhesion for the 4% polymer gels containing PC alone, PC plus 0.2 g conjugate (PG-CYS3) and PC plus 0.5 g conjugate (PG-CYS6) were 0.092 ± 0.007 mJ cm−2, 0.118 ± 0.009 mJ cm−2 and 0.209 ± 0.010 mJ cm−2, respectively (p < 0.05). Similar results were obtained with gels containing CHI. Upon addition of CHI-TG conjugates at increasing amounts, the work of mucoadhesion of gel formulations containing CHI were also increased from 0.038 ± .0.002 mJ cm−2 (CHI-TG3) to 0.075 ± 0.006 mJ cm−2 (CHI-TG6; p < 0.05), albeit higher than that of CHI alone (CH3: 0.023 ± 0.001 mJ cm−2; p < 0.05). The addition of thiol conjugate was found to have a higher effect on mucoadhesion ability in PC gels than in CHI gels. Since the presence of thiol groups were reported to enhance the mucoadhesive ability of the parent polymer (25,27,28), it was believed that the higher amounts of thiol groups per gram of polymer in the PC conjugates (148.42 ± 4.16 μmol) compared to that of CHI conjugates (41.17 ± 2.34 μmol) may have enhanced the mucoadhesive ability of the former. PC-CYS6 formulation has exhibited the highest mucoadhesive property (0.209 ± 0.010 mJ cm−2) in all formulations analysed (Fig. 7).
Fig. 7.
Work of mucoadhesion of the gel formulations (n = 6)
Drug Release Studies
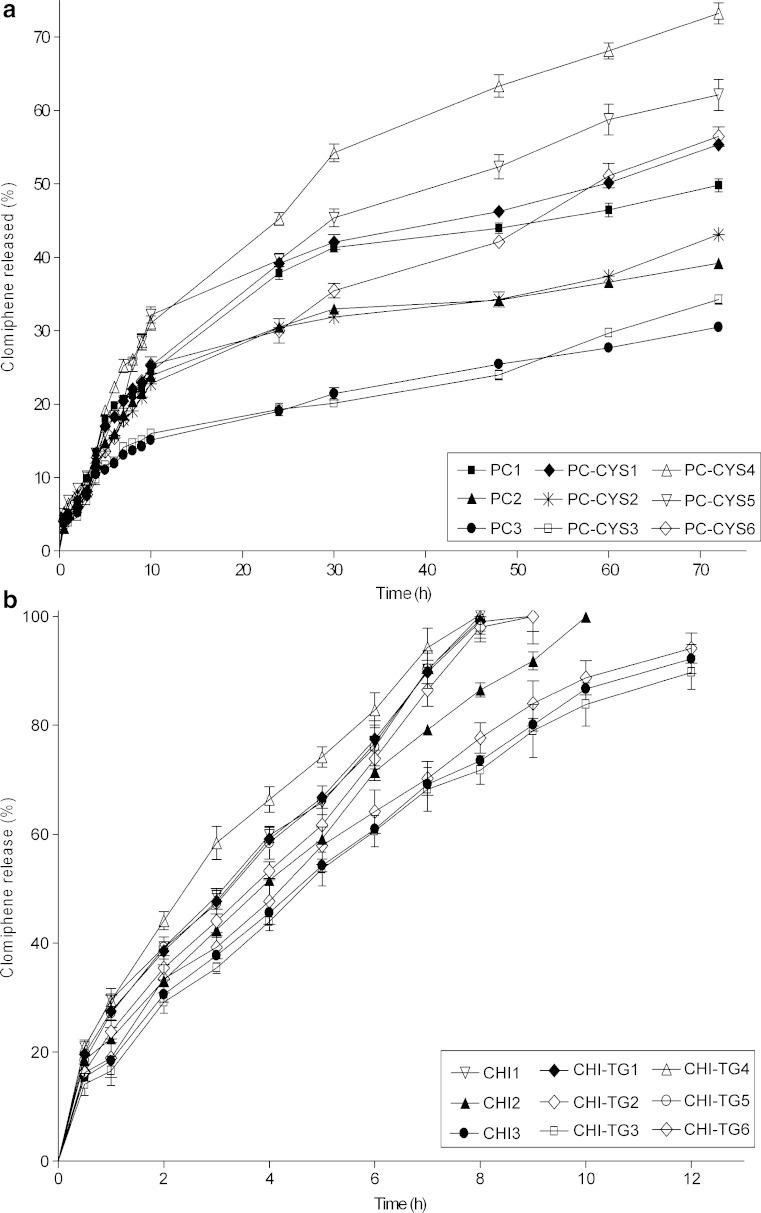
According to the in vitro drug release study results, the release of CLM from gels was found to decrease with increasing concentrations of polymer in PC and CHI gel formulations.
PC and its thiol conjugate were found to prolong the release of CLM longer than 72 h unlike gel formulations prepared using CHI and CHI-TG which were found to release CLM in only 9–12 h. 49.8%, 39.2% and 30.5% CLM release were achieved in 72 h for the formulations composed of 2%, 3% and 4% PC (PC1-PC3), respectively (Fig. 8a). The amount of CLM released from formulations including CHI was 100% at 8th h for CHI1, 100% at 10th h for CHI2 and 92.1% at 12th hour for CHI3 (Fig. 8b). In comparison of the in vitro drug release profiles, a significant difference was determined between PC and CHI gel formulations (p < 0.05). Upon addition of conjugate at increasing amounts, the drug release rate from PC gels (PC-CYS1–PC-CYS6; Fig. 8a) and CHI gels (CHI-TG1–CHI-TG6; Fig. 8b) was increased accordingly (p < 0.05).
Fig. 8.
In vitro drug release profiles of CLM from the gel formulations including a polycarbophil and polycarbophil–cystein conjugate and b chitosan and chitosan–thioglycolic acid conjugate (n = 3)
The drug release from gels formulated using 0.2 g PC-CYS conjugate (PC-CYS1–PC-CYS3) and from gels formulated using 0.5 g conjugate (PC-CYS4–PC-CYS6) was, respectively, 10.0–12.5% (p < 0.05) and 47.0–85.5% (p < 0.05) higher than formulations including the same amount of the polymer without conjugate (PC1–PC3). However, when the formulations including CHI-TG conjugate (CHI-TG1–CHI-TG6) were compared with formulations including the same amount of the parent polymer (CHI1–CHI3), no significant difference was seen between the drug release profiles (p > 0.05).
Stability Test
The stability of CLM was observed by keeping all gel formulations in stability cabinet at 25 ± 2°C, 60 ± 5% RH; 30 ± 2°C, 65 ± 5% RH; 40 ± 2°C, 75 ± 5% RH for 3 months. According to the stability test, no significant difference in the CLM concentrations of all formulations (p > 0.05) were observed and the amount of CLM was determined between 98.76 ± 2.15% and 103.07 ± 2.48% at the end of the stability study. Stability data of CLM in PG-CYS6 and CHI-TGA6 formulations was given in Fig. 9 (Data belong to other gel formulations are not shown).
Fig. 9.
Stability graph of CLM in a PG-CYS6 and b CHI-TGA6 formulations
CONCLUSION
HPV is a sexually transmitted disease and the infected patients may develop venereal warts in the genital region. CLM is a non-steroidal ovulation-promoting substance and acts by competitively inhibiting the binding of HPV to the estrogenic receptors in cervix glandular cells, metaplastic cells and the basal cells of the squamous epithelium. Due to this effect, CLM is highly effective in the treatment of HPV infections. The clinical efficiency of CLM in HPV treatment could be increased by delivering CLM via mucoadhesive vaginal delivery systems.
Mucoadhesive systems could increase the retention time of the CLM at the application site and reduce clinically beneficial dose. These sustained drug delivery systems may also improve patient compliance by reducing the frequency of administration. In this study, the mucoadhesive gel formulations of CLM using PC and CHI and their conjugates including thiol groups were prepared and their mechanical, mucoadhesive properties, drug release characteristics were evaluated. By increasing the polymer concentrations, gels with higher hardness, compressibility, adhesiveness and mucoadhesion ability can be achieved. Furthermore, a slower rate of CLM release from gels was achieved when the polymer concentrations were increased in the gel formulations. PC and its thiol conjugates were found to prolong the release of CLM longer than 72 h unlike gel formulations prepared using CHI and its thiol conjugates which were able to release CLM up to 12 h. The increasing in the amount of the thiol conjugates was found to enhance the elasticity, cohesiveness, adhesiveness and mucoadhesion of the gel formulations but not its hardness, compressibility when compared to gels prepared using their respective parent formulations. The type of polymer used to prepare gels had a profound effect on the mechanical properties of gels. PC gels are harder, more elastic and adhesive than CHI gels, although PC gels have roughly similar cohesiveness as CHI gels. Stability of CLM was preserved in all the formulations tested after incubation in a stability cabinet at 25 ± 2°C, 60 ± 5% RH; 30 ± 2°C, 65 ± 5% RH; 40 ± 2°C, 75 ± 5% RH for 3 months. On the basis of the obtained data PC-CYS6 formulation was found as the most appropriate formulation in the vaginal treatment of HPV infection due to its suitable mechanical properties, exhibiting high cohesion and mucoadhesion and releasing the drug in vitro longer than 72 h.
Acknowledgement
This work was supported by the Istanbul University. Project number: T-138/11112002.
References
- 1.Ferrera A., Velema J. P., Figueroa M., Bulnes R., Toro L. A., Claros J. M., De Barahona O., Melchers W. J. G. Human papillomavirus infection, cervical dysplasia and invasive cervical cancer in Honduras: A case-control study. Int. J. Cancer. 1999;82:799–803. doi: 10.1002/(SICI)1097-0215(19990909)82:6<799::AID-IJC5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Newton D. C., McCabe M. P. The impact of stigma on couples managing a sexually transmitted infection. Sex Relat. Ther. 2005;20:51–63. doi: 10.1080/14681990500058341. [DOI] [Google Scholar]
- 3.Czerwenka K. F., Schön H. J., Manavi M., Zeillinger R., Kubista E., Gitsch E. Reliability of in-situ hybridization of smears and biopsies for papilloma virus genotyping of the uterina cervix. Eur. J. Clin. Chem. Clin. Biochem. 1991;29:139–145. [PubMed] [Google Scholar]
- 4.Schön H. J., Czerwenka K. F., Schurz B., Kramar R., Kubista E. Papanicolaou test and enzyme-linked in-situ hybridization a combined diagnostic system for papilloma virus infections with high prognostic value. Eur. J. Clin. Chem. Clin. Biochem. 1991;29:131–138. [PubMed] [Google Scholar]
- 5.Park J. S., Oh Y. K., Kang M. J., Kim C. K. Enhanced mucosal and systemic immune responses following intravaginal immunization with human papilloma virus 16L1 virus-like particle vaccine in thermosensitive mucoadhesive delivery systems. J. Medical Virology. 2003;70:633–641. doi: 10.1002/jmv.10442. [DOI] [PubMed] [Google Scholar]
- 6.McLeish M. J. Clomiphene citrate. In: Florey K., editor. Analytical Profiles of Drug Substances and Excipients. New York: Academic; 1998. pp. 85–120. [Google Scholar]
- 7.Jimenez-Castellanos R., Zia H., Rhodes C. T. Mucoadhesive drug delivery systems. Drug Dev. Ind. Pharm. 1993;19:143–194. doi: 10.3109/03639049309038765. [DOI] [Google Scholar]
- 8.Choi H. G., Oh Y. K., Kim C. K. In situ gelling and mucoadhesive liquid suppository containing acetaminophen: enhanced bioavailability. Int. J. Pharm. 1998;165:23–32. doi: 10.1016/S0378-5173(97)00385-2. [DOI] [Google Scholar]
- 9.Ryu J. M., Chung S. J., Lee M. H., Kim C. K., Shim C. K. Increased bioavailability of propranolol in rats by retaining thermally gelling liquid suppositories in the rectum. J. Control Release. 1999;59:163–172. doi: 10.1016/S0168-3659(98)00189-8. [DOI] [PubMed] [Google Scholar]
- 10.Valenta C., Kast C. E., Harich I., Bernkop-Schnürch A. Development and in vitro evaluation of mucoadhesive vaginal delivery system for progesterone. J. Control. Release. 2001;77:323–332. doi: 10.1016/S0168-3659(01)00520-X. [DOI] [PubMed] [Google Scholar]
- 11.Cevher E., Sahin O., Araman A. Investigations on topical formulations of clomiphene citrate for treatment of HPV lesion. Pharmazie. 2002;57:72–73. [PubMed] [Google Scholar]
- 12.Y. W. Chien, C. H. Lee. Drug Delivery-Vaginal Route. In J. Swarbrick, J. C. Boylan (eds.), Encyclopedia of Pharmaceutical Technology, second edition, PharmaceuTech, Pinehurst, North Carolina, 2002, pp. 961–985.
- 13.Takeuchi H., Matsui Y., Yamamoto H., Kawashima Y. Mucoadhesive properties of carbopol or chitosan-coated liposomes and their effectiveness in the oral administration of calcitonin to rats. J. Control. Release. 2003;86:235–242. doi: 10.1016/S0168-3659(02)00411-X. [DOI] [PubMed] [Google Scholar]
- 14.Han R. Y., Fang J. Y., Sung K. C., Hu O. Y. P. Mucoadhesive buccal disks for novel nalbuphine prodrug controlled delivery: Effect of formulation variables on drug release and mucoadhesive performance. Int. J. Pharm. 1999;177:201–209. doi: 10.1016/S0378-5173(98)00343-3. [DOI] [PubMed] [Google Scholar]
- 15.Ceschel G. C., Maffei P., Lombardi B. S. Design and evaluation of a new mucoadhesive bi-layered tablet containing nimesulide for buccal administration. STP Pharm. Sci. 2001;11:151–156. [Google Scholar]
- 16.Perioli L., Ambrogi V., Rubini D., Giovagnoli S., Ricci M., Blasi P., Rossi C. Novel mucoadhesive buccal formulation containing metronidazole for the treatment of periodontal disease. J. Control. Release. 2004;95:521–533. doi: 10.1016/j.jconrel.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Yahagi R., Machida Y., Onishi H., Machida Y. Mucoadhesive suppositories of ramosetron hydrochloride utilizing Carbopol®. Int. J. Pharm. 2000;193:205–212. doi: 10.1016/S0378-5173(99)00338-5. [DOI] [PubMed] [Google Scholar]
- 18.Sales D., Sae-Lee D., Matsuya S., Ana I. D. Short-term fluoride and cations release from polyacid-modified composites in a distilled water, and an acidic lactate buffer. Biomaterials. 2003;24:1687–1696. doi: 10.1016/S0142-9612(02)00545-8. [DOI] [PubMed] [Google Scholar]
- 19.Yun M., Choi H., Jung J., Kim C. Development of a thermo-reversible insulin liquid suppository with bioavailability enhancement. Int. J. Pharm. 1999;189:137–145. doi: 10.1016/S0378-5173(99)00227-6. [DOI] [PubMed] [Google Scholar]
- 20.Ceulemans J., Vermeire A., Adriaens E., Remon J. P., Ludwig A. Evaluation of a mucoadhesive tablet for ocular use. J. Control. Release. 2001;77:333–344. doi: 10.1016/S0168-3659(01)00522-3. [DOI] [PubMed] [Google Scholar]
- 21.Smart J. D., Kellaway I. W., Worthington H. E. C. An in vitro investigation of mucosa adhesive materials for use in controlled drug delivery. J. Pharm. Pharmacol. 1984;36:295–299. doi: 10.1111/j.2042-7158.1984.tb04377.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee C. H., Chien Y. W. Development and evaluation of a mucoadhesive drug delivery system for dual-controlled delivery Nonoxynol-9. J. Control. Release. 1996;39:93–103. doi: 10.1016/0168-3659(95)00142-5. [DOI] [Google Scholar]
- 23.Park H., Robinson J. R. Physico-chemical properties of water insoluble polymers important to mucin/epithelial adhesion. J. Control. Release. 1985;2:47–57. doi: 10.1016/0168-3659(85)90032-X. [DOI] [Google Scholar]
- 24.Shojaei A. H., Zhou S. L., Li X. Transbuccal delivery of acyclovir (II): feasibility, system design, and in vitro permeation studies. J. Pharm. Pharmaceut. Sci. 1998;1:66–73. [PubMed] [Google Scholar]
- 25.Bernkop-Schnürch A., Zarti H., Walker G. F. Thiolation of polycarbophil enhances its inhibition of intestinal brush border membrane bound aminopeptidase. J. Pharm. Sci. 2001;90:1907–1914. doi: 10.1002/jps.1140. [DOI] [PubMed] [Google Scholar]
- 26.Kast C. E., Valenta C., Leopold M., Bernkop-Schnürch A. Design and in vitro evaluation of a novel bioadhesive vaginal drug delivery system for clotrimazole. J. Control. Release. 2002;81:347–354. doi: 10.1016/S0168-3659(02)00077-9. [DOI] [PubMed] [Google Scholar]
- 27.Langoth N., Kalbe J., Bernkop-Schnürch A. Development of buccal drug delivery systems based on a thiolated polymer. Int. J. Pharm. 2003;252:141–148. doi: 10.1016/S0378-5173(02)00638-5. [DOI] [PubMed] [Google Scholar]
- 28.Kast C. E., Bernkop-Schnürch A. Thiolated polymers-thiomers: development and in vitro evaluation of chitosan–thioglycolic acid conjugates. Biomaterials. 2001;22:2345–2352. doi: 10.1016/S0142-9612(00)00421-X. [DOI] [PubMed] [Google Scholar]
- 29.Jones D. S., Woolfson A. D., Brown A. F. Textural, viscoelastic and mucoadhesive properties of pharmaceutical gels composed of cellulose polymers. Int. J. Pharm. 1997;151:223–233. doi: 10.1016/S0378-5173(97)04904-1. [DOI] [Google Scholar]
- 30.Tamburic S., Craig D. Q. M. An investigation into the rheological, dielectric and mucoadhesive properties of poly(acrylic acid) gel systems. J. Control. Release. 1995;37:59–68. doi: 10.1016/0168-3659(95)00064-F. [DOI] [PubMed] [Google Scholar]
- 31.Tobyn M. J., Johnson J. R., Dettmar P. W. Factors affecting in vitro gastric mucoadhesion I. Test Conditions and instrumental parameters. Eur. J. Pharm. Biopharm. 1995;41:235–241. [Google Scholar]
- 32.Cevher E., Taha M. A. M., Orlu M., Araman A. Evaluation of mechanical and mucoadhesive properties of clomiphene citrate gel formulations containing carbomers and their thiolated derivatives. Drug Deliv. 2007;15:57–67. doi: 10.1080/10717540701829234. [DOI] [PubMed] [Google Scholar]
- 33.Deshpande A. A., Rhodes C. T., Danish M. Intravaginal drug delivery. Drug Dev. Ind. Pharm. 1992;18:1225–1279. doi: 10.3109/03639049209046329. [DOI] [Google Scholar]
- 34.Schwartz N. O. Adaptation of sensory textile profile method to skin care products. J. Text. Studies. 1975;42:33–42. doi: 10.1111/j.1745-4603.1975.tb01116.x. [DOI] [Google Scholar]
- 35.Jones D. S., Irwin C. R., Woolfson A. D., Djokic J., Adams V. Physochemical characterization and preliminary in vivo efficiacy of bioadhesive, semisolid formulations containing flurbiprofen for the treatment of gingivitis. J. Pharm. Sci. 1999;88:592–598. doi: 10.1021/js9803095. [DOI] [PubMed] [Google Scholar]
- 36.Tan Y. T. F., Peh K. K., Al-Hanbali O. Effect of carpobol and polyvinylpyrrolidone on the mechanical, rheological and release properties of bioadhesive polyethylene glycol gels. AAPS PharmSciTech. 2000;1:E24. doi: 10.1208/pt010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrari F., Bertoni M., Caramella C., La Manna A. Description and validation of an apparatus for gel strength measurements. Int. J. Pharm. 1994;109:115–124. doi: 10.1016/0378-5173(94)90139-2. [DOI] [Google Scholar]
- 38.Jones D. S., Woolfson A. D., Brown A. F. Textural analysis and flow rheometry of novel, bioadhesive antimicrobial oral gels. Pharm. Res. 1997;114:450–457. doi: 10.1023/A:1012091231023. [DOI] [PubMed] [Google Scholar]
- 39.Kachanechaia T., Jantawata P., Pichyangkurab R. The influence of chitosan on physico-chemical properties of chicken salt-soluble protein gel. Food Hydrocoll. 2008;22:74–83. doi: 10.1016/j.foodhyd.2007.04.010. [DOI] [Google Scholar]
- 40.Jones D. S., Woolfson D. A., Brown A. F., O’Neill M. J. Mucoadhesive, syringeable drug delivery systems for controlled application of metronidazole to the periodontal pocket: In vitro release kinetics, syringeability, mechanical and mucoadhesive properties. J. Control Release. 1997;49:71–79. doi: 10.1016/S0168-3659(97)00060-6. [DOI] [Google Scholar]
- 41.Tamburic S., Craig D. Q. M. A comparison of different in vitro methods for measuring mucoadhesive performance. Eur. J. Pharm. Biopharm. 1997;44:159–167. doi: 10.1016/S0939-6411(97)00073-8. [DOI] [Google Scholar]
- 42.Jones D. S., Woolfson A. D., Djokic J., Coulter W. A. Development and mechanical characterization of bioadhesive semi-solid, polymeric systems containing tetracycline for the treatment of periodontal diseases. Pharm. Res. 1996;13:1734–1738. doi: 10.1023/A:1016413428473. [DOI] [PubMed] [Google Scholar]
- 43.D. S. Jones, A. D. Woolfson, A. F. Brown, W. A. Coulter, C. McClelland, C. R. Irwin. Design, characterisation and preliminary clinical evaluation of a novel mucoadhesive topical formulation containing tetracycline for the treatment of periodontal disease. J. Control. Release.67:357–368 (2000). [DOI] [PubMed]
- 44.Jones D. S., Woolfson A. D., Djokic J. Texture profiles analysis of bioadhesive polymeric semisolids: mechanical characterisation and investigation of interactions between formulation components. J. App. Polymer. Sci. 1996;16:2229–2234. doi: 10.1002/(SICI)1097-4628(19960919)61:12<2229::AID-APP24>3.0.CO;2-0. [DOI] [Google Scholar]
- 45.Peppas N. A., Buri P. A. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J. Control. Release. 1985;2:257–275. doi: 10.1016/0168-3659(85)90050-1. [DOI] [Google Scholar]
- 46.Richardson J., Illum L. Routes of delivery: Case studies, the vaginal route of peptide and protein drug delivery. Adv. Drug Del. Rev. 1992;8:341–366. doi: 10.1016/0169-409X(92)90008-E. [DOI] [Google Scholar]
- 47.Chang J. Y., Oh Y. K., Kong H. S., Kim E. J., Jang D. D., Nam K. T., Kim C. K. Prolonged antifungal effects of clotrimazole-containing mucoadhesive thermosensitive gels on vaginitis. J. Control. Release. 2002;82:39–50. doi: 10.1016/S0168-3659(02)00086-X. [DOI] [PubMed] [Google Scholar]
- 48.A. El-Kamel, M. Sokar, V. Naggar, and S. A. Gamal. Chitosan and sodium alginate-based bioadhesive vaginal tablets. AAPS PharmSci. 4:article 44 (2002). [DOI] [PMC free article] [PubMed]
- 49.M. C. Bonferoni, P. Giunchedi, S. Rossi, G. Sandri, and C. Caramella. Chitosan gels for the vaginal delivery of lactic acid: Relevance of formulation parameters to mucoadhesion and release mechanisms. AAPS PharmSciTech. 7:article 104 (2006). [DOI] [PMC free article] [PubMed]