Abstract
The aim of this study was to prepare bi-layer tablet of Metoclopramide Hydrochloride (MTH) and Ibuprofen (IB) for the effective treatment of migraine. MTH and IB were formulated as immediate and sustained release layer respectively. MTH was formulated as immediate release layer by using various disintegrants like Ac-Di-Sol, Polyplasdone XL, Explotab, Agar and Gellan Gum. Treated form of gellan gum and agar was prepared and compared for their disintegrant efficiency with other disintegrants. IB was formulated as sustained release layer using hydrophilic matrix (hydroxypropylmethylcellulose [HPMC K4M]). The effect of concentration of hydrophilic matrix (HPMC K4M), binder (polyvinylpyrollidone [PVP K30]) and buffer (sodium bicarbonate) on IB release was studied. The dissolution study of sustained release layer showed that an increasing amount of HPMC or PVP K30 results in reduced IB release. The inclusion of buffer (sodium bicarbonate) enhanced the release of IB from sustained release layer. The rational for formulation of bi-layer tablet of these two drugs in combination was (1) MTH increases the absorption of acidic non-steroidal anti-inflammatory drug (NSAID) by increasing gastric motility. So sequential release of MTH (as immediate release) and IB (as sustained release) was suitable for treatment of migraine. (2) MTH was degraded when prolonged contact with acidic NSAID. Bi-layer tablet was suitable for preventing direct contact of these two drugs and thus to maximize the efficacy of combination of two drugs for migraine.
Key words: bi-layer tablet, gellan gum, ibuprofen, metoclopramide hydrochloride
INTRODUCTION
Bi-layer tablets are prepared with one layer of drug for immediate release while second layer designed to release drug, later, either as second dose or in an extended release manner. Bi-layer tablet is suitable for sequential release of two drugs in combination, separate two incompatible substances, and also for sustained release tablet in which one layer is immediate release as initial dose and second layer is maintenance dose (1).
Metoclopramide Hydrochloride (MTH), 4-amino-5-chloro-N-[2-(diethylamino)ethyl]-2 methoxybenzamide monohydrochloride monohydrate, is one of the potent anti-emetic drug. MTH apparently antagonizes dopamine at the receptor sites. This action can explain its sedative, central anti-emetic (blocks dopamine in the chemo-receptor trigger zone), extrapyrimidal, and prolactin secretion stimulation effects. The gastrointestinal (GI) stimulatory effects of MTH may affect the absorption of many drugs. Drugs that dissolve, disintegrate and/or are absorbed in the stomach (e.g., digoxin) may be absorbed less. MTH may enhance absorption of drugs that are absorbed primarily in the small intestine (e.g., cimetidine, tetracycline, aspirin and diazepam). Immediate release formulation of MTH hydrochloride is suitable for its immediate action and that to enhance absorption of non-steroidal anti-inflammatory drug (NSAID) agents whose absorption is less due to gastric stasis especially in migraine (2).
Ibuprofen (IB), 2-(4-Isobutyl-phenyl)-propionic acid, is one of the nonsteroidal anti-inflammatory agent with analgesic and antipyretic properties. The anti-inflammatory effects of IB, and most of its other pharmacological effects, are generally thought to be related to its inhibition of cyclooxygenase and consequent decrease in prostaglandin concentration. After oral administration of IB, well over 80% of the dose is absorbed from the GI tract (mainly the intestine) (3). IB has shorter plasma half-life (t1/2 = 2 h), thus sustained release formulation is suitable to prevent frequent administration. Combination of MTH and IB is suitable for treatment of migraine.
Gellan gum (GG) is a linear anionic polysaccharide, biodegradable polymer obtained from Pseudomonos elodea consisting of a linear tetrasaccharide repeat structure and used as a food additive. Agar (AG) is the dried gelatinous substance obtained from Gelidium amansii (Gelidanceae) and several other species of red algae like, Gracilaria (Gracilariaceae) and Pterocadia (Gelidaceae). Both were reported earlier as disintegrant (4).
In formulation of immediate release layer of MTH, various disintegrants like Ac-Di-Sol, Polyplasdone XL, Explotab, agar and gellan gum were used. Treated form of agar (TAG) and gellan gum (TGG) were prepared to enhance disintegrants efficiency of agar and gellan gum respectively. Formulation of sustained release layer of IB, hydrophilic matrix (HPMC K4M) was used. Effect of buffer (sodium bicarbonate) and binder (PVP K30) and hydrophilic polymer (HPMC K4M) concentration were studied on in-vitro release of IB from hydrophilic matrix.
The aim of the present study was to design and evaluate bi-layer tablet to avoid chemical incompatibility between MTH and IB for the effective treatment of migraine, in which the immediate release layer was fabricated to release the MTH within 10 min in stomach, then sequential release of sustained release of second layer of IB in small intestinal for sustained action. The study was also extended to investigate the disintegrant efficiency of TAG and TGG.
MATERIALS AND METHODS
Materials
MTH (260 μm) was obtained as a gift sample from Ipca Laboratory (Mumbai, India). IB (96.44 μm) was obtained as a gift sample from Merck Pharmaceutical (Mumbai, India). Ac-Di-Sol (NMT 2% retained # 200, NMT 10% retained # 325), Polyplasdone XL (<400 μm) and PVP K30 were obtained from Wockhardt Research Centre (Aurangabad, India). Explotab (35–55 μm) and microcrystalline cellulose (50 μm) were obtained as a gift samples from JRS Pharma (USA). Agar (671 μm) was obtained as a gift sample from Marine Chemical (Mumbai, India), Gellan gum (355 μm) was obtained as a gift sample from Burzin and Leons (Mumbai, India). HPMC K4M was obtained as a gift from Colorcon (Mumbai, India). Sodium bicarbonate was obtained from Loba Chemical (Mumbai, India). Magnesium stearate and colloidal silicone-dioxide was obtained from SD Fines (Mumbai, India). All reagents used in these experiments were analytical grades.
Methods
Treatment of Agar and Gellan Gum
TAG and TGG powders were prepared by taking 10 g (AG, GG) powder separately with sufficient distilled water (100 mL) and allowed to swell for 1 day at 37 ± 1 °C. Then spread mechanically in petri-dish and allowed for drying up to 3 days in incubator at 37 ± 1 °C and then pulverized by pulveriser (D.P. industries, Mumbai).
Powder Characterization
Particle Size Distribution
The particle size distribution was measured using sieving method (Remik, Karnavati engineering Ltd., Ahmedabad, India) (5).
Photo-microscope Study
Photo-microscope image of TGG and GG was taken (X 450 magnifications) by photomicroscope (Motic B1 series microscope, Japan) (5).
Angle of Repose
The diameter of the powder cone was measured and the angle of repose was calculated using the following equation (6).
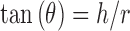 |
1 |
where h and r are the height and radius of the powder cone.
Moisture Sorption Capacity
All disintegrants have capacity to absorb moisture from atmosphere which affects moisture sensitive drugs. Moisture sorption capacity was performed by taking 1 g of disintegrant uniformly distributed in petri-dish and kept in stability chamber (Programmable environmental test chamber, Remi) at 37 ± 1 °C and 100% relative humidity for 2 days and investigated for the amount of moisture uptake by difference between weights (6).
Density
The loose bulk density (LBD) and tapped bulk density (TBD) were determined and calculated using the following formulas (7).
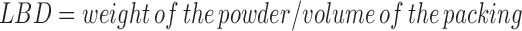 |
2 |
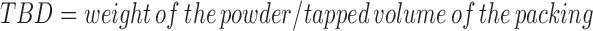 |
3 |
Compressibility
The compressibility index of the disintegrant was determined by Carr’s compressibility index (7).
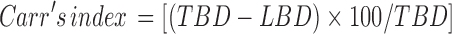 |
4 |
Swelling Capacity
The swelling capacity of disintegrants was measured according to the reported method and swelling capacity was expressed as percentage and calculated according to the equation (8,9).
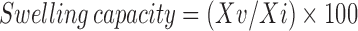 |
5 |
Where, Xv = Final volume occupied by swollen material after 24 h.
Xi = Initial volume of the powder in measuring cylinder.
Hydration Capacity
The hydration capacity was calculated according to following formula (9,10)
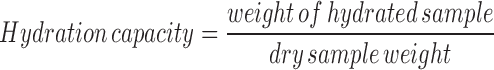 |
6 |
Drug–Excipient Interaction Study (11)
Thin Layer Chromatography
Physical mixture of drug and excipient was filled in the clean ampules and sealed. The sealed ampules were kept at 37 ± 0.5 °C and 75% RH for 28 days in stability chamber. Then Rf values of plain drug, excipient and their mixture were determined. Mobile phases for MTH and IB (methanol: ammonia [1.5: 0.2] and methanol: toluene [1.0: 0.6] respectively) were used. Aluminium backed silica gel 60 F254 HPTLC plates (10 × 20 cm, layer thickness 0.2 mm, E-Merck, Darmstad, Germany) prewashed with methanol was used for the study.
DSC Study
Thermograms of MTH, IB, immediate release layer, and sustained release layer were performed using DSC (Perkin Elmer Cyris-DSC). Indium was used as standard to calibrate the DSC temperature and enthalpy scale. The sample were hermetically sealed in aluminum pans and heated at a constant rate of 10°C/min over a temperature range of 50–200°C.
Preparation of Immediate Release Tablets of MTH
MTH and microcrystalline cellulose were mixed with disintegrant for 15 min in porcelain mortar, passed through 60 # sieve. This blend was mixed with colloidal silicon dioxide and magnesium stearate for 5 min and processed for direct compression by using 7 mm round flat-faced punch of rotary tablet machine (Karnavati, India). Compression force was maintained at constant level and magnesium stearate as lubricant was fixed at 2% w/w for all formulations. Disintegrants were used at 8% in tablets. Composition of all batches was represented in Table I.
Table I.
Composition of MTH Immediate Release Tablet
| Ingredients | Batch F1 | Batch F2 | Batch F3 | Batch F4 | Batch F5 | Batch F6 | Batch F7 |
|---|---|---|---|---|---|---|---|
| Metoclopramide HCl | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Ac-Di-Sol | 08 | – | – | – | – | – | – |
| Polyplasdone XL | – | 08 | – | – | – | – | – |
| Explotab | – | – | 08 | – | – | – | – |
| GG | – | – | – | 08 | – | – | – |
| TGG | – | – | – | – | 08 | – | – |
| AG | – | – | – | – | – | 08 | – |
| TAG | – | – | – | – | – | – | 08 |
| MCC | 79 | 79 | 79 | 79 | 79 | 79 | 79 |
| Magnesium stearate | 02 | 02 | 02 | 02 | 02 | 02 | 02 |
| Colloidal silicon-dioxide | 01 | 01 | 01 | 01 | 01 | 01 | 01 |
Each values represented in mg
MTH Metoclopramide hydrochloride, HCl hydrochloride, GG gellan gum, TGG treated gellan gum, AG agar, TAG treated agar
Evaluation of MTH Blend
Prior to the compression, the blends of all batches were evaluated for angle of repose, bulk density, compressibility index and Hausner’s ratio.
Evaluation of MTH Tablet
Tablets were evaluated for weight variation, friability, hardness, thickness was performed according to the IP ’96 (12).
Drug Content
The drug content of the tablets was measured according to the IP ‘96.
It has been reported that metoclopramide HCl can be detected at 273 & 305 nm. Drug content uniformity of MTH was carried out at 305 nm because successive extraction was done using chloroform for which good absorption observed at 305 nm as reported in Pharmacopoeia. During dissolution test study, metoclopramide HCl shown good absorption at 273 nm by using pH 1.2 HCl buffer solution as a dissolution media.
Disintegration Test
Randomly six tablets were selected from each batch for disintegration test. Disintegration test was performed without disc in simulated gastric fluid (37 ± 0.5 °C) using United States Pharmacopcia (USP) disintegration apparatus. The mean ± standard deviation (SD) of six tablets were calculated (13).
Dissolution Test
Dissolution test of MTH tablet was performed using simulated gastric fluid with USP dissolution apparatus II at 50 rpm and 37 ± 0.5 °C temperature. Test sample (5 mL) was withdrawn at particular time interval (1, 2, 5, 10, 15, 20 and 30 min) and replaced with fresh dissolution media maintained at 37 ± 0.5 °C. The test sample was filtered (membrane filter, 0.45 μm) and the concentration of dissolved drug was determined using ultraviolet (UV) spectrophotometer at λmax 273 nm. This test was performed on six tablets and mean ± SD calculated.
Wetting Time
The test was performed according to the reported method (14). Results were reported as mean ± SD calculated.
Maximal Water Uptake Capacity
Modified method and apparatus was used for the water uptake study. The apparatus consists of a sample holder and a liquid holding vessel (petri-dish), set on an electronic digital balance. When tablet was placed into perforated sample holder, then fluid was passively withdrawn in to the tablet. The loss of weight from the liquid holder was read from the digital balance. Test was performed in triplicate. All results were reported as mean ± SD (15).
Preparation of Sustained Release Tablets of IB
IB and HPMC K4M were mixed with other excipient for 15 min in porcelain mortar except magnesium stearate, and the mass was prepared using isopropyl alcohol as a granulating fluid. Then passed the mass through 16 # sieve and granules were allowed to dry in oven at 40 °C for 30 min. Dried granules passed through 12 # sieve. Then 10% fine was added in the granules and mixed with magnesium stearate for 5 min and processed for compression by using 13 mm round flat-faced punches of rotary tablet machine (Karnavati, India). Composition of all batches was represented in Table II. Prior to the compression, granules were evaluated for several tests.
Table II.
Composition of IB Sustained Release Tablet
| Ingredients (mg) | Batch I1 | Batch I2 | Batch I3 | Batch I4 | Batch I5 | Batch I6 | Batch I7 | Batch I8 | Batch I9 | Batch I10 | Batch I11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IB | 600 | 600 | 600 | 600 | 600 | 600 | 600 | 600 | 600 | 600 | 600 |
| HPMC K4M | 125 | 125 | 125 | 125 | 125 | 125 | 115 | 100 | 100 | 100 | 100 |
| Sodium bicarbonate | – | – | – | – | – | – | – | – | 10 | 20 | 30 |
| PVP | 5 | 10 | 20 | 30 | 40 | 50 | 50 | 50 | 50 | 50 | 50 |
| MCC | 62 | 57 | 47 | 37 | 27 | 17 | 27 | 42 | 32 | 22 | 12 |
| Isopropyl alcohol | Q.S. | Q.S. | Q.S. | Q.S. | Q.S. | Q.S. | Q.S. | Q.S. | Q.S. | Q.S. | Q.S. |
| Magnesium stearate | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
IB Ibuprofen, HPMC hydroxypropyl methylcellulose, PVP polyvinylpyrollidone, MCC microcrystalline cellulose, Q.S. quantity sufficient
Precompression Parameter of IB Tablet
Angle of Repose, Bulk Density, Compressibility Index and Hausner’s Ratio
Granules were evaluated by various parameters like angle of repose, bulk density (TBD, LBD), compressibility index and Hausner’s ratio as per the reported methods described in previous section.
Evaluation of IB Tablets
Weight Variation, Drug Content, Friability, Hardness and Thickness
Tablet weight variation, thickness and friability were measured using the USP methods. Drug content was determined by following procedure. Twenty tablets were triturated. An accurately weighed amount of triturated powder from IB tablet equivalent to (10 mg) was dissolved in phosphate buffer (pH 7.2) and the solution was filtered through 0.45 μm membrane filter then adjust volume up to 100 mL with phosphate buffer (pH 7.2). The absorbance was measured at 221 nm after suitable dilution. All results were represented as a mean ± SD.
Dissolution Test
The in-vitro dissolution studies were carried out using USP apparatus type II at 50 rpm. The dissolution medium (900 mL) consisted of simulated gastric fluid (pH 1.2 HCl buffer) was used for the first 2 h and then replaced with phosphate buffer (pH 7.2) for 3 to 10 h (900 mL), maintained at 37 ± 0.5 °C. The drug release at different time interval was measured by UV–visible spectrophotometer at 221 nm. The release studies were conducted on (six tablets in each batch), and the mean values were plotted versus time with SD.
Drug Release Kinetics
To study the release kinetics, data obtained from in vitro drug release studies were plotted in various kinetic models: zero order (Eq. 8) as cumulative amount of drug released vs time, first order (Eq. 9) as log cumulative percentage of drug remaining vs time, and Higuchi’s model (Eq. 10) as cumulative percentage of drug released vs square root of time (16).
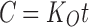 |
8 |
Where K0 is the zero-order rate constant expressed in units of concentration/time and t is the time in hours. A graph of concentration vs time would yield a straight line with a slope equal to K0 and intercept the origin of the axes.
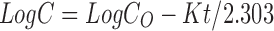 |
9 |
Where C0 is the initial concentration of drug, K is the first order constant, and t is the time.
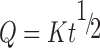 |
10 |
Where K is the constant reflecting the design variables of the system and t is the time in hours.
Mechanism of Drug Release (16)
To evaluate the mechanism of drug release from IB sustained release tablet, data for the drug release were plotted in Korsmeyer et al.’s equation (Eq. 11) as log cumulative percentage of drug released vs log time, and the exponent n was calculated through the slope of the straight line (16).
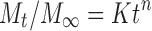 |
11 |
Where Mt/M∞ is the fractional solute release, t is the release time, K is a kinetic constant characteristic of the drug/polymer system, and n is an exponent that characterizes the mechanism of release of drug. For cylindrical matrix tablets, if the exponent n < 0.5, then the drug release mechanism is quasi-Fickian diffusion, if n = 0.5 then Fickian diffusion, 0.5 < n < 1, then it is anomalous diffusion. An exponent value of 1 is indicative of Case-II Transport or typical zero-order and n > 1 non-Fickian supercase II. The diffusion exponent is based on Korsmeyer–Peppas equation.
Preparation of Bi-layer Tablets of MTH and IB
Optimized batch of MTH (batch F1) and IB (batch I11) was selected for formulation of bi-layer tablet. As previously reported procedure granules of IB layer and powder blend of MTH layer were prepared separately. One by one both layer were filled in rotary tablet machine and compressed
Evaluation of Bi-layer Tablet
Weight Variation, Friability, Hardness, Thickness and Content Uniformity
Bi-layer tablets were evaluated for weight variation, friability, hardness and thickness as per the procedure previously mentioned. Content uniformity of both drugs in bi-layer tablet was measured by separating both layer of bi-layer tablet and measured individually.
Dissolution Test
The in-vitro dissolution studies were carried out using USP apparatus type II at 50 rpm. The dissolution medium (900 mL) consisted of simulated gastric fluid was used for the first 2 h and then replaced with phosphate buffer (pH 7.2) for 3 to 10 h (900 mL), maintained at 37 ± 0.5 °C. The drug release at different time intervals was measured by UV–visible spectrophotometer at 221 and 273 nm for IB and MTH respectively. The release studies were conducted on (six tablets), and the mean values were plotted versus time with SD.
RESULTS AND DISCUSSION
Powder Characterization
Particle Size Distribution
Particle size distribution of GG and TGG was found different (data not shown). Particles retained over 100 # sieve was 21% and 64% for GG and TGG respectively. Water treatment produces coarse particles in TGG than GG. Particle size of disintegrant is one of the factor that affects disintegration activity (17). A larger particle size and hence, increased porosity leads to a faster wicking and swelling of disintegrants (10). Larger particle size probably yielded greater pore size and altered the shape of the pore (17).
Photo-microscope Study
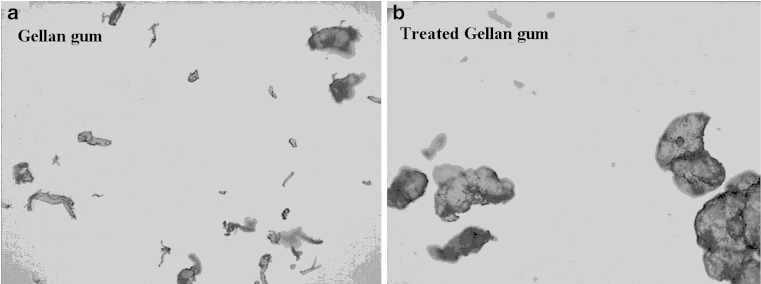
Coarse particles of TGG compared to GG also confirmed by photomicroscope image (Fig. 1a and b). Larger particles of disintegrants swelled more rapidly and to a greater extent than did the smaller particle (17). TGG had taken less time for disintegration of tablet than GG containing tablet. Similar results were found in case of AG and TAG (5).
Fig. 1.
Photo-microscope image of disintegrant powder of a GG and b TGG
Angle of Repose, Moisture Sorption Capacity, Density and Compressibility
Compressibility index for TGG was found to be excellent, while for GG was found to be poor. Tapped bulk density of TGG was found to be less than GG (TGG = 0.45 g/mL, GG = 0.62 g/mL) that indicates more porous structure of TGG than GG. Therefore, tablets prepared from TGG had faster wicking and swelling then GG and hence faster disintegration of tablets containing TGG as compared to GG.
Swelling and Hydration Capacity
Swelling and hydration capacity of disintegrants are the important parameters for comparing disintegration efficiency (Data not shown). Higher swelling and hydration capacity (Capability of absorbing water) of Ac-Di-Sol leads to faster disintegration of batch F1 (32.33 ± 5.53 s), hence optimized for immediate release layer. Higher swelling and hydration capacity of TGG and TAG leads to faster disintegration than GG and AG. Less swelling capacity of polyplasdone XL than AG, GG, TAG and TGG but disintegration was found to be faster than AG, GG, TAG and TGG because the capillary activity of polyplasdone XL for water is responsible for its tablet fast disintegration (18) Explotab was found to be less swelling capacity than Ac-Di-Sol but it found to be more effective then polyplasdone XL.
Drug–Excipient Interaction Study
Thin Layer Chromatography
The results of TLC study indicate no change in the Rf value of drugs and no interaction between drug and excipient.
DSC Study
DSC study demonstrate, there is no change in the melting point of drug (MTH = 183 °C and IB = 75–78 °C) which shows that no drug–excipient interaction.
Evaluation of MTH Blend
Angle of Repose, Bulk Density, Compressibility Index and Hausner’s Ratio
Precompression parameter like angle of repose, lose bulk density, tapped bulk density, compressibility index and Hausner’s ratio of all batches of MTH was represented in Table III. Hausner’s ratio <1.25 for all batches indicates good flow properties.
Table III.
Precompression Parameter of MTH Blend
| Batch | Angle of repose | LBD (g/mL) | TBD (g/mL) | Compressibility index (%) | Hausner’s ratio |
|---|---|---|---|---|---|
| F1 | 28.61 | 0.27 | 0.31 | 14.81 | 1.15 |
| F2 | 29.98 | 0.27 | 0.29 | 6.80 | 1.07 |
| F3 | 29.05 | 0.27 | 0.30 | 10.00 | 1.11 |
| F4 | 32.00 | 0.28 | 0.31 | 9.67 | 1.10 |
| F5 | 29.98 | 0.27 | 0.29 | 6.89 | 1.07 |
| F6 | 30.96 | 0.27 | 0.29 | 6.80 | 1.07 |
| F7 | 29.05 | 0.26 | 0.28 | 7.14 | 1.08 |
MTH Metoclopramide hydrochloride
Evaluation of MTH Tablet
Weight Variation, Friability, Hardness, Thickness, Drug Content and Disintegration Time
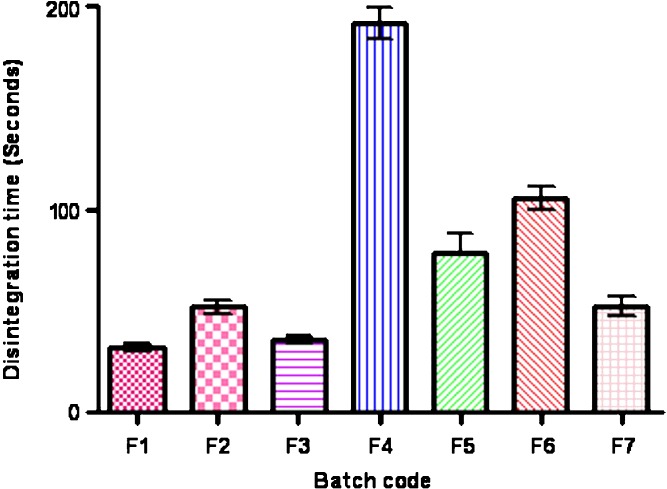
Tablet properties like weight variation, thickness, hardness, friability, disintegration time and drug content was represented in Table IV. Results of hardness and thickness measurement indicates that the TAG and TGG were harder to make compact than the AG and GG. Tablets prepared from GG and AG was found to thinner and harder than tablets prepared from TGG and TAG. All batches pass weight variation (between 90–110%). Thickness of tablet was found variation less than 5%. Friability of all batches were found <1%. Tablet of batch F1 was disintegrate faster than all other batches. Drug content of all batches was found within limit (90–110%). Graphical presentation of disintegration time represented in Fig. 2.
Table IV.
Evaluation Parameter of MTH Tablet
| Batch | Weight (mg)a | Thickness (mm)a | Hardness (Kg/cm2)a | Friability (%)b | Disintegration time (s)a | Drug content (%)a |
|---|---|---|---|---|---|---|
| F1 | 101.0 (1.4) | 2.24 (0.05) | 4.50 (0.00) | 0.33 | 32.33 (5.53) | 99.9 (1.38) |
| F2 | 101.4 (1.2) | 2.30 (0.00) | 5.00 (0.49) | 0.19 | 52.16 (12.98) | 101.4 (0.87) |
| F3 | 99.2 (1.68) | 2.23 (0.04) | 4.00 (0.00) | 0.64 | 35.66 (4.58) | 101.2 (0.84) |
| F4 | 100.8 (1.2) | 2.17 (0.04) | 4.75 (0.35) | 0.29 | 191.50 (31.23) | 99.7 (2.08) |
| F5 | 99.9 (1.21) | 2.24 (0.05) | 4.10 (0.00) | 0.37 | 78.66 (22.34) | 101.3 (1.28) |
| F6 | 100.2 (1.0) | 2.19 (0.03) | 4.90 (0.14) | 0.58 | 105.80 (14.16) | 101.2 (0.84) |
| F7 | 100.1 (1.3) | 2.27 (0.04) | 3.90 (0.14) | 0.74 | 52.50 (12.27) | 102.2 (0.80) |
MTH Metoclopramide hydrochloride
aEach value represents as mean ± SD of three determinations
bEach value represents as singly
Fig. 2.
Disintegration time of MTH tablet (batch F1–F7)
Disintegration and dissolution test of all batches were performed in simulated gastric fluid, represent that Ac-Di-Sol was found to be best among all disintegrants which supports the earlier report (18) TGG and TAG containing tablets were found to disintegrate faster that GG and AG containing tablets.
Dissolution Test, Wetting Time, and Maximal Water Uptake Capacity
Cumulative % of drug released at 1, 3 and 5 min represents as Q1 min, Q3 min, and Q5 min to investigate positive correlation between the maximal water uptake and the cumulative % of drug dissolved at 1, 3 and 5 min. Higher water uptake leads to faster disintegration and dissolution of tablets. TGG and TAG containing tablets showed faster disintegration. Cumulative % of drug released vs time plot of each batch shown in Fig. 3. Faster wetting occur of batch F2 containing polyplasdone XL because it shows disintegration of tablet by capillary mechanism.
Fig. 3.
Dissolution test of MTH tablet (batch F1–F7)
Wetting time of tablet was in following decreasing order polyplasdone XL, Ac-Di-Sol, Explotab, TAG, TGG, AG and GG. The comparative wetting time study of GG, TGG, AG and TAG containing tablets was also performed. Above all studied parameter indicates batch F1 was suitable as an immediate release layer for bi-layer tablet.
Precompression Parameter of IB Tablet
Angle of Repose, Bulk Density, Compressibility Index and Hausner’s Ratio
Precompression parameters like angle of repose, loose bulk density, tapped bulk density, compressibility index and Hausner’s ratio of all batches of IB are represented in Table V. The precompression parameters of tablet granules study demonstrate all batches have good compressibility. Flow properties also found to be good for all batches (Angle of repose between 23–35 indicates good flow). Hausner’s ratio (<1.25) for all batches indicates good flow properties.
Table V.
Precompression Parameter of IB Sustained Release Tablet
| Batch | Angle of repose (θ) | LBD (g/mL) | TBD (g/mL) | Compressibility index (%) | Hausner’s ratio |
|---|---|---|---|---|---|
| I1 | 28.26 | 0.167 | 0.182 | 8.24 | 1.08 |
| I2 | 28.36 | 0.160 | 0.182 | 12.09 | 1.14 |
| I3 | 27.86 | 0.167 | 0.200 | 16.50 | 1.19 |
| I4 | 28.62 | 0.167 | 0.182 | 8.24 | 1.09 |
| I5 | 28.78 | 0.154 | 0.166 | 7.23 | 1.08 |
| I6 | 28.89 | 0.143 | 0.154 | 7.04 | 1.07 |
| I7 | 29.88 | 0.180 | 0.200 | 10.00 | 1.11 |
| I8 | 29.32 | 0.200 | 0.220 | 10.10 | 1.10 |
| I9 | 30.76 | 0.200 | 0.220 | 10.10 | 1.05 |
| I10 | 30.40 | 0.240 | 0.260 | 7.69 | 1.04 |
| I11 | 30.05 | 0.250 | 0.270 | 6.25 | 1.08 |
IB Ibuprofen
Evaluation of IB Tablet
Weight Variation, Thickness, Hardness, Friability and Drug Content
Tablet properties like weight variation, thickness, hardness, friability and drug content of each batch was represented in Table VI. All batches pass the weight variation test and found to be within range (100 ± 5%). Friability of all batches except batch I1 and I2 were found less than 1% and indicates that tablet surfaces are strong enough to withstand mechanical shock or attrition during storage and transportation and until they are consumed. Hardness of tablet increase as PVP K30 amount increase (batch I1–I6) and friability also decreases as PVP K30 amount increases. Drug content of all batches was found within limit (90–110%). Thickness variation of tablet was also found within limit (5%).
Table VI.
Evaluation Parameter of IB Sustained Release Tablet
| Batch | Weight variation (mg)a | Thickness (mm)a | Hardness (Kg/cm2)a | Friability (%) | Drug content (%)a |
|---|---|---|---|---|---|
| I1 | 810.37 (8.87) | 6.21 (0.07) | 3.66 (0.28) | 2.560 | 101.40 (0.66) |
| I2 | 820.35 (5.81) | 6.30 (0.04) | 4.00 (0.00) | 2.060 | 102.80 (0.66) |
| I3 | 817.70 (8.96) | 6.35 (0.05) | 4.17 (0.28) | 0.890 | 101.86 (0.33) |
| I4 | 811.90 (5.86) | 6.26 (0.05) | 4.66 (0.28) | 0.240 | 101.75 (0.83) |
| I5 | 809.20 (6.47) | 6.25 (0.07) | 4.93 (0.12) | 0.020 | 100.69 (1.32) |
| I6 | 813.89 (3.79) | 6.37 (0.05) | 5.83 (0.29) | 0.010 | 101.28 (0.49) |
| I7 | 807.98 (5.16) | 6.28 (0.04) | 6.06 (0.12) | 0.006 | 100.58 (0.49) |
| I8 | 812.44 (5.15) | 6.38 (0.06) | 5.60 (0.17) | 0.014 | 100.93 (0.33) |
| I9 | 810.33 (5.32) | 6.39 (0.03) | 5.50 (0.50) | 0.038 | 100.58 (0.49) |
| I10 | 808.08 (4.84) | 6.38 (0.04) | 5.47 (0.50) | 0.032 | 100.93 (0.99) |
| I11 | 811.19 (3.82) | 6.34 (0.05) | 5.60 (0.17) | 0.030 | 101.75 (0.83) |
IB Ibuprofen
aEach value represents as mean ± SD of three determinations.
Dissolution Test
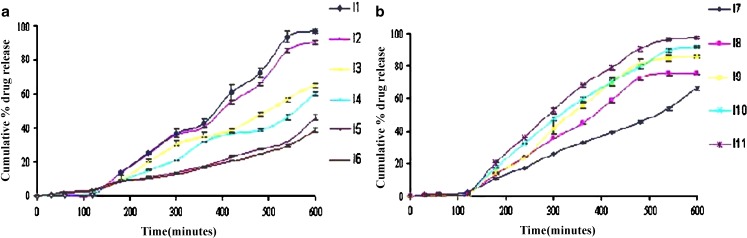
Dissolution study data demonstrated that as PVP K30 amount increases that lead to decrease the release of drug (Fig. 4a). As HPMC K4M amount decrease that leads to increase the release of drug (Fig. 4b). IB is a weak acid (PKa = 4.41), making it more soluble in basic conditions. Buffering agents have been included in tablet formulations to increase the dissolution and to decrease the gastric irritation of weak acidic drugs, such as IB. Buffers can compete for the water of hydration and reduce the hydrophilic matrix integrity and IB solubility increased in presence of buffering agent. Above all studied parameters indicate batch I11 was suitable as a sustained release layer for bi-layer tablet.
Fig. 4.
Dissolution test of IB sustained release tablet [a batch I1–I6, and b batch I7–I11]
Drug Release Study
The zero-order rate (Eq. 8) describes the system where the drug release rate is independent on its concentration. The first order (Eq. 9), which describes the release from systems where the release rate is concentration dependent. Higuchi’s model (Eq. 10) describes the release of drugs from an insoluble matrix as a square root of a time-dependent process based on Fickian diffusion. The release constant was calculated from the slope of the appropriate plots, and the regression coefficient (R2) was determined.
Mechanism of Drug Release
The corresponding plot (log cumulative percent drug release vs log time) for the Korsmeyer–Peppas equation indicated a good linearity (R2 = 0.9208). The release exponent n was 0.4831, which appears to indicate drug release mechanism is Quasi-Fickian diffusion.
Evaluation of Bi-layer Tablet of MTH and IB
Weight Variation, Thickness, Hardness, Friability and Content Uniformity
Properties of bi-layer tablets such as weight variation, thickness, hardness and friability was checked. Weight variation of bi-layer tablet was found (915.57 ± 3.72 mg) within limit (100 ± 5%). Friability of bi-layer tablet was found (0.40%) less than 1%. Hardness was found 5.88 Kg/cm2 and thickness variation was found (7.30 ± 0.14) less than 5%. Content uniformity of MTH and IB in bi-layer tablet was found 101.2 ± 0.80 and 101.70 ± 0.58 respectively.
Dissolution Test
Cumulative % drug release vs time plot for MTH and IB represented in Fig. 5a and b respectively. The dissolution study suggested that MTH was released within 10 min in simulated gastric fluid. While IB released in very less amount (1.71%) within 2 h. After replacing media with phosphate buffer (pH 7.2), there was found that IB dissolution was found to be increased. The 10 h dissolution study, 97.31% IB was released.
Fig. 5.
Dissolution test of bi-layer tablet, a MTH release, b IB release
CONCLUSIONS
Optimized immediate release layer of MTH and sustained release layer of IB show satisfactory pre and post compression parameters. Bi-layer tablet of MTH and IB might be suitable for treatment of migraine by sequential release of the drug.
Acknowledgements
Authors specially acknowledge to Ipca Laboratory, Merck Pharma, Colorcon, JRS Pharma, Marine Chemical and Burzin and Leons for providing gift sample of MTH, IB, HPMC K4M, Explotab, Agar and Gellan gum respectively. Authors thanks to Mrs. Agarkar for her manuscript assistance.
References
- 1.Gunsel W. C. Compression-coated and layer tablet. In: Lieberman A.H. , editor. Pharmaceutical dosage forms: tablets. New York: Decker; 1989. pp. 274–284. [Google Scholar]
- 2.Wells B. G., DiPiro J. T., Schwinghammer T. L., Hamilton C. W. Pharmacotherapy Handbook. New York: McGraw-Hill; 2006. pp. 535–547. [Google Scholar]
- 3.Higgins J. D., Gilmor T. P., Martellucci S. A., Bruce R. D. IB. In: Brittain H. G., editor. Analytical profiles of drug substances and excipients. New Delhi, India: Elsevier; 2001. p. 294. [Google Scholar]
- 4.U. S. Bagul, N. S. Bagul, M. S. Kulkarni, S. D. Swant, N. K. Gujar, and A. A. Bidkar. Current status of tablet disintegrants: A review. Available at: http://www.pharmainfo.net. Accessed July 8, 2006.
- 5.Akihiko I., Masayasu S. Development of oral dosage form for elderly patients: use of agar as base of rapidly disintegrating tablets. Chem. Pharm. Bull. 1996;45:2132–2136. doi: 10.1248/cpb.44.2132. [DOI] [PubMed] [Google Scholar]
- 6.Ohwoavworhua F., Adelakun T. Phosphoric acid-mediated depolymerization and decrystallization of α-Cellulose obtained from corn cob: preparation of low crystallinity cellulose and some physicochemical properties. Trop. J. Pharm. Res. 2004;4(2):509–516. [Google Scholar]
- 7.Reddy K. R., Mutalik S., Reddy S. One-daily sustained-release matrix tablets of nicorandil: formulation and in vitro evaluation. AAPS PharmSciTech. 2003;4(4):E61. doi: 10.1208/pt040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magnus A. I., Anthony O. Preliminary investigation into the use of pleurotus tuber-regium powder as a tablet disintegrant. Trop. J. Pharm. Res. 2002;1(1):29–37. [Google Scholar]
- 9.G. Jain, and J. Goswami. Studies on formulation and evaluation of new superdisintegrants for dispersible tablets. Int. J. Pharmaceut Excipient. 37–43 (2005).
- 10.Bussemera T., Peppasb N., Bodmeiera R. Evaluation of the swelling, hydration and rupturing pulsatile drug delivery system. Eur. J. Pharm. Biopharm. 2006;56:261–270. doi: 10.1016/S0939-6411(03)00070-5. [DOI] [PubMed] [Google Scholar]
- 11.Heng P. W., Chah L. W. Drug substance and excipient characterization. In: Parikh D. M., editor. Handbook of Pharmaceutical Granulation Technology. New York: Decker; 1997. pp. 52–55. [Google Scholar]
- 12.Indian Pharmacopoeia. Controller of publications New Delhi, 1996.
- 13.USP/NF. Physical Tests: Disintegration (701). 22/17 ed. Rockville, MD: United StatesPharmacopeial Convention Inc; 1990.
- 14.Mutasem M., Rawas Q. Fast-disintegrating sublingual tablets: effect of epinephrine load on tablet characteristics. AAPS PharmSciTech. 2006;7(2):E1–E7. doi: 10.1208/pt070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao N., Augsburger L. L. Functional comparison of 3 classes of disintegrants in promoting aspirin tablet disintegration and dissolution. AAPS PharmSciTech. 2005;6(4):E634–E640. doi: 10.1208/pt060479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamid A. M., Harris M. S., Jaweria T., Rabia I. Y. Once-daily tablet formulation and in vitro release evaluation of cefpodoxime using hydroxypropyl methylcellulose: a technical note. AAPS PharmSciTech. 2006;7(3):E1–E6. doi: 10.1208/pt070356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Augsburger L. L., Brzecko A. W., Shah U., Hahm H. A. Characterization and functionality of super disintegrants. In: Swarbrick J., Boylan J. C., editors. Encyclopedia of Pharmaceutical Technology. New York, NY: Dekker; 2000. pp. 269–291. [Google Scholar]
- 18.Zhao N., Augsburger L. L. The influence of swelling capacity of superdisintegrants in different pH media on the dissolution of hydrochlorothiazide from directly compressed tablets. AAPS PharmSciTech. 2005;6(1):E120–E126. doi: 10.1208/pt060119. [DOI] [PMC free article] [PubMed] [Google Scholar]