Abstract
The objective of the present research was to investigate the feasibility of using non-ionic surfactant vesicles (niosomes) as carriers for the ophthalmic controlled delivery of a water soluble local antibiotic; gentamicin sulphate. Niosomal formulations were prepared using various surfactants (Tween 60, Tween 80 or Brij 35), in the presence of cholesterol and a negative charge inducer dicetyl phosphate (DCP) in different molar ratios and by employing a thin film hydration technique. The ability of these vesicles to entrap the studied drug was evaluated by determining the entrapment efficiency %EE after centrifugation and separation of the formed vesicles. Photomicroscopy and transmission electron microscopy as well as particle size analysis were used to study the formation, morphology and size of the drug loaded niosomes. Results showed a substantial change in the release rate and an alteration in the %EE of gentamicin sulphate from niosomal formulations upon varying type of surfactant, cholesterol content and presence or absence of DCP. In-vitro drug release results confirmed that niosomal formulations have exhibited a high retention of gentamicin sulphate inside the vesicles such that their in vitro release was slower compared to the drug solution. A preparation with 1:1:0.1 molar ratio of Tween 60, cholesterol and DCP gave the most advantageous entrapment (92.02% ± 1.43) and release results (Q8h = 66.29% ± 1.33) as compared to other compositions. Ocular irritancy test performed on albino rabbits, showed no sign of irritation for all tested niosomal formulations.
Key words: controlled release, gentamicin sulphate, niosomes, ophthalmic
INTRODUCTION
Gentamicin belongs to a class of antibiotic aminoglycosides that have been extensively used in human therapeutics. Gentamicin has an important potential use against a wide spectrum of Gram-negative and Gram-positive bacteria, however, many of its applications are restricted to low dose administrations, mainly because of the appearance of residues in the kidney and the high cost of the treatment (1). It is administrated in the form of injection, cream, ointment, suspension and it is also used in veterinary medicine (2).
Drug delivery in ocular therapeutics is a challenging problem and is a subject of interest to scientists working in the multi-disciplinary areas pertaining to the eye, including chemical, biochemical, pharmaceutical, medical, clinical, and toxicological sciences (3). The current treatment of ocular infections requires frequent topical antimicrobial drug administration in microbial keratitis and repeated injection of antimicrobial drugs into the site of infection in endophthalmitis (4). The therapeutics requires the association of more than one antimicrobial treatment for several weeks, which often leads to poor patient compliance, contributing to low therapy efficiency (5). In order to overcome the problems of conventional ocular therapy, such as short residence time, loss of drug through nasolacrimal drainage, impermeability of corneal epithelium and frequent instillation; newer ocular delivery systems for gentamicin are being explored by many researchers (6–8). It is now common knowledge that topical controlled delivery of ophthalmic drugs improves their ocular bioavailability with respect to traditional eye drops, by decreasing the rate of drug elimination from the precorneal area (9).
The advantage of vesicular systems does not only reside in providing prolonged and controlled action at the corneal surface but also involves providing controlled ocular delivery by preventing the metabolism of the drug from the enzymes present at the tear/corneal epithelial surface. Moreover, vesicles offer a promising avenue to fulfill the need for an ophthalmic drug delivery system that has the convenience of a drop, but will localize and maintain drug activity at its site of action. The penetration of drug molecules into the eye from a topically applied preparation is a complex phenomenon. In vesicular dosage forms, the drug is encapsulated in lipid vesicles, which can cross cell membrane. Vesicles, therefore, can be viewed as drug carriers which can change the rate and extent of absorption as well as the disposition of the drug. Vesicular drug delivery systems used in ophthalmics broadly include liposomes and niosomes (3).
Niosomes are formed from the self-assembly of non-ionic amphiphiles in aqueous media resulting in closed bilayer structures (10), which can entrap both hydrophilic and lipophilic drugs either in an aqueous layer or in vesicular membrane (11). Niosomes in topical ocular delivery are preferred over other vesicular systems because of the following reasons: (1) chemical stability; (2) low toxicity because of their non-ionic nature; (3) handling surfactants with no special precautions or conditions; (4) the ability to improve the performance of the drug via better availability and controlled delivery at a particular site; (5) being biodegradable, biocompatible and non-immunogenic (12).
Various studies have demonstrated the successful use of niosomes as ocular drug delivery carriers where these vesicles significantly improved the ocular bioavailability of cyclopentolate, with respect to reference buffer solution. No irritation with the niosomal formulation was observed (13). Vyas et al. (14) reported that there was approximately a 2.5 times increase in the ocular bioavailability of timolol maleate (a water soluble drug) encapsulated in niosomes as compared to timolol maleate solution. An increased ocular bioavailability of water-soluble drugs, entrapped in niosomes, may be due to the fact that surfactants act as penetration enhancers by removing the mucus layer and breaking junctional complexes (15–18).
Based on the aforementioned reasons, the purpose of the current study was to prepare gentamicin encapsulated niosomes possessing a high drug loading capacity in order to be used as ophthalmic carriers for topical ocular infections’ treatment. Vesicle dispersions were characterized by light microscopy and transmission electron microscopy for vesicle formation and morphology. Laser diffraction was used to evaluate the particle size of the formed vesicles. Factors affecting the entrapment efficiency, such as surfactant type, cholesterol content and the absence or presence of Dicetyl phosphate (DCP), were evaluated and optimized. The in vitro release studies of gentamicin sulphate from niosomes compared to that of the drug solution in a simulated lacrimal fluid were performed and analyzed. Finally, an ocular irritancy test performed on albino rabbits was done in order to evaluate the optimized formulations upon application to the eye.
MATERIALS AND METHODS
Materials
Gentamicin sulphate USP XXIII was kindly donated by CID Co. Giza, Egypt. Polyoxyethylene 20 sorbitan monostearate (Tween 60), polyoxyethylene 20 sorbitan monooleate (Tween 80), polyoxyethylene 23 laurylether (Brij 35), cholesterol, dicetyl phosphate (DCP), o-phthaldialdehyde, 2-mercaptoethanol and ammonium molybdate were purchased from Sigma-Aldrich, USA. Isopropanol, methanol, chloroform, boric acid, sodium hydroxide, sodium bicarbonate, potassium chloride, calcium chloride dehydrate, magnesium chloride hexahydrate, sodium chloride, potassium dihydrogen phosphate and disodium hydrogen phosphate were purchased from Adwic, El-Nasr Chemical Co., Cairo, Egypt. Spectra/Por dialysis membrane, 12,000–14,000 molecular weight cutoff was purchased from Spectrum Laboratories Inc., USA. Double-distilled water was used throughout the study.
Methods
Preparation of Gentamicin Sulphate Niosomes
The composition of the tested niosomal formulae are reported in Table I. Niosomes containing gentamicin sulphate were prepared by thin film hydration technique (19,20). Briefly, surfactants, cholesterol and DCP, in different molar ratios, were accurately weighed into a long necked quick fit round-bottom flask and dissolved in 10 ml chloroform. The organic solvent was slowly evaporated at 60 °C under reduced pressure, using a rotary evaporator (Buchi R-110 Rotavapor, Switzerland) at 150 rpm such that a thin dry film of the components was formed on the inner wall of the rotating flask. The dried thin lipid film was then hydrated with 10 ml of phosphate buffered saline (PBS, pH 7.4), containing 10 mg gentamicin sulphate, by rotating the flask in a water bath using a rotavapor under normal pressure in order to ensure complete hydration of the film. The niosomal suspension was left to mature overnight at 4 °C. For sterility, all the above mentioned steps were done under aseptic conditions. All glassware was sterilized by autoclaving, phosphate buffered saline was passed through a 0.22 μm membrane filter, and the entire procedure was carried out in a laminar flow hood (Esco, Singapore).
Table I.
Formulae Composition (Molar Ratio)
| Formula | Components | ||||
|---|---|---|---|---|---|
| Tween 60 | Tween 80 | Brij35 | Cholesterol | Dicetyl phosphate | |
| F1 | 1 | – | – | 0.5 | – |
| F2 | 1 | – | – | 1 | – |
| F3 | 1 | – | – | 0.5 | 0.1 |
| F4 | 1 | – | – | 1 | 0.1 |
| F5 | – | 1 | – | 0.5 | – |
| F6 | – | 1 | – | 1 | – |
| F7 | – | 1 | – | 0.5 | 0.1 |
| F8 | – | 1 | – | 1 | 0.1 |
| F9 | – | – | 1 | 0.5 | – |
| F10 | – | – | 1 | 1 | – |
| F11 | – | – | 1 | 0.5 | 0.1 |
| F12 | – | – | 1 | 1 | 0.1 |
Determination of Gentamicin Sulphate Entrapment Efficiency %EE
The proportion of encapsulated gentamicin sulphate was obtained by ultra-centrifugating 1 ml of the niosomal suspension at 15,000 rpm for 1 h using a cooling centrifuge at 4 °C (Beckman, Fullerton, Canada). The niosomes were separated from the supernatant and were washed twice, each time with 1 ml phosphate buffered saline, and recentrifuged again for 1 h. The amount of entrapped gentamicin sulphate was determined by lysis of the separated vesicles with isopropanol. A 100 μl sample of niosomes was mixed with 1 ml of isopropanol, the volume was completed to 10 ml with phosphate buffered saline and covered with parafilm to prevent evaporation. The concentration of the drug was determined spectrophotometrically (Shimadzu, model UV-1601 PC, Kyoto, Japan) after derivatization with o-phthaldialdehyde reagent by Zhang’s method (21). Briefly, the o-phthaldialdehyde reagent was formulated by adding 2.5 g o-phthaldialdehyde, 62.5 ml methanol and 3 ml 2-mercaptoethanol to 560 ml sodium borate solution, pH 8. The reagent was stored in a brown bottle in a dark chamber for at least 24 h before use, as it is light sensitive. This reagent could be used only up to 3 days. Gentamicin sulphate solution, o-phthaldialdehyde reagent, and isopropanol (to avoid precipitation of the products formed) were mixed in similar proportions and stored for 30 min at room temperature. The o-phthaldialdehyde reagent reacted with gentamicin amino groups and chromophoric products were obtained, whose absorbance was measured at 332 nm.
The entrapment efficiency is defined as follows (22):
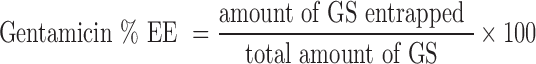 |
Where GS is gentamicin sulphate.
Photomicroscopy and Transmission Electron Microscopy
The formation of niosomal vesicles as well as their morphological aspects were evaluated by using photo and transmission electron microscopy.
Various niosomal formulations were examined under optical microscope (Lecia Image, Germany) and photographed at a magnification of ×40, by means of a fitted camera (JVC, Japan).
Gentamicin sulphate niosomal samples were also examined by transmission electron microscope (model JEM-1230, Jeol, Tokyo, Japan) at 70 kV, after being negatively stained. A saturated ammonium molybdate aqueous solution was used as the staining agent (23).
Determination of Vesicle Size
Niosomal vesicle size of the prepared formulae were determined by light scattering based on laser diffraction using the Malvern Mastersizer (Malvern Instruments Ltd., Worcestershire, UK) (24). The measurements were performed using a 45 mm focus objective and a beam length of 2.4 mm.
In Vitro Release of Gentamicin Sulphate from Niosomes
The release of gentamicin sulphate from niosomes was determined using the membrane diffusion technique (25–27). An accurately measured amount of gentamicin sulphate niosomal formulations, equivalent to 1 mg drug, was suspended in 0.3 ml phosphate buffered saline (pH 7.4) and transferred to a glass cylinder having the length of 10 cm and diameter of 2.5 cm fitted at its lower end with presoaked cellulose membrane (Spectra/Por dialysis membrane 12,000–14,000 Mwt cutoff). Then, the glass tube was suspended in the dissolution flask of a USP dissolution apparatus (Hanson research, Chatsworth, USA) containing 50 ml simulated lacrimal fluid (pH 7.4) (28,29). The glass tube was allowed to rotate at a constant speed (50 rpm).
The lacrimal fluid was maintained at a temperature of 37 ± 0.5 °C (30). At predetermined time intervals for a total period of 8 h, aliquots were withdrawn and the drug content was determined spectrophotometrically at 332 nm after derivatization with o-phthaldialdehyde reagent, as previously mentioned. The results were the mean values of three runs.
Ocular Irritancy Test of Niosome-Encapsulated Gentamicin
The potential ocular irritancy and/or damaging effects of niosomal formulations F4, F6 and F12 were evaluated by observing them for any redness, inflammation, or increased tear production, upon application to the eyes of albino rabbits. Each formulation was tested on three albino rabbits; the experiment was performed by a single instillation (50 μl) of the niosomal preparation under test into the conjunctival sac of one eye, whilst the contralateral eye served as control. Both eyes of the rabbits under test were examined for any irritation signs, such as conjunctival: corneal edema and/or hyperemia on the basis of direct visual observation using a slit lamp, before treatment, and 1, 24 and 48 h after instillation (31,32). The animal experiments were conducted in full compliance with local, national, ethical and regulatory principles for animal care and was approved by the Cairo University Animal Care Committee
RESULTS AND DISCUSSION
Entrapment Efficiency %EE
The entrapment efficiencies of all niosomal formulations are reported in Table II. In order to attain high gentamicin sulphate encapsulation efficiency, several factors, including the type of surfactant, presence of DCP and the ratio of cholesterol added were evaluated and optimized.
Table II.
Entrapment Efficiency %EE, Vesicle Size and Q8h of Prepared Gentamicin Sulphate Niosomes
| Niosomal formulations | %EEa (% ± SD) | Vesicle size (μm; average ± SD) | Q8h b (% ± SD) |
|---|---|---|---|
| F1 | 65.91 ± 1.70 | 1.25 ± 0.0173 | 70.76 ± 0.86 |
| F2 | 46.12 ± 2.02 | 1.2 ± 0.0173 | 72.58 ± 1.41 |
| F3 | 74.02 ± 2.19 | 1.34 ± 0.036 | 67.70 ± 1.03 |
| F4 | 92.02 ± 1.43 | 1.37 ± 0.0659 | 66.29 ± 1.33 |
| F5 | 27.82 ± 0.99 | 1.09 ± 0.0295 | 78.20 ± 1.94 |
| F6 | 40.35 ± 1.49 | 1.1 ± 0.0324 | 76.59 ± 1.57 |
| F7 | 25.64 ± 1.23 | 1.09 ± 0.0271 | 79.03 ± 1.01 |
| F8 | 23.43 ± 0.99 | 1.04 ± 0.0276 | 84.90 ± 1.58 |
| F9 | 6.55 ± 0.27 | 0.79 ± 0.0116 | 91.65 ± 1.46 |
| F10 | 5.74 ± 0.08 | 0.76 ± 0.089 | 97.05 ± 2.62 |
| F11 | 11.48 ± 0.31 | 0.96 ± 0.0163 | 88.12 ± 2.74 |
| F12 | 13.19 ± 0.21 | 0.98 ± 0.0106 | 86.99 ± 2.86 |
aEach value is an average of three determinations
bQ8h: % gentamicin sulphate released after 8 h.
The entrapment efficiencies for niosomes prepared using Tween 60 were superior to those prepared using Tween 80 which in turn were superior to those prepared with Brij 35. This may be explained by the fact that the vesicles obtained from stearyl (C18) chain surfactants (Tween 60) produce higher entrapment efficiencies than surfactants with lauryl (C12) chain (Brij 35), as the length of alkyl chain is a crucial factor of permeability. Thus, long chain surfactants produce high entrapment (33). Additionally, the alkyl chain length influences the HLB value of the surfactant which in turn directly influences the drug entrapment efficiency (34). The lower the HLB of the surfactant the higher will be the drug entrapment efficiency and stability as in the case of niosomes prepared using Tween 60 (HLB = 14.9), whilst Brij 35 with a high HLB of 16.9, showed very low entrapment efficiency. Only Tween 80 has an unsaturated alkyl chain. The introduction of double bonds made the chains bend, which led to the formation of a niosomal membrane that is not sufficiently tight. Thus, the membrane formed was more permeable, which possibly explains the lower entrapment efficiency of the Tween 80 formulations compared to Tween 60 formulations (33).
Cholesterol is one of the common additives included in the formulation in order to prepare stable niosomes. Cholesterol stabilizes bilayers, prevents leakiness, and retards permeation of solutes enclosed in the aqueous core of these vesicles. Cholesterol is known to abolish the gel to lipid phase transition of niosome systems (35), which could be able to effectively prevent leakage of drug from niosomes (36). Cholesterol is thus usually included in a 1:1 molar ratio (non-ionic surfactant: cholesterol) in most formulations. However even after the addition of cholesterol, the intrinsic phase transition behaviour of vesicle forming surfactants still influences the properties of the dispersion: notably the membrane permeability, encapsulation efficiency and bilayer rigidity (10). DCP, a charged molecule, is often used to prevent niosome aggregation (35) and increase the stability of niosome dispersions (37).
Data in Table II reveals that DCP had a great effect on the niosomal formulations. In case of niosomes composed of Tween 60 (F1–F4) or Brij 35 (F9–F12), the incorporation of DCP was found to increase the encapsulation efficiency of gentamicin sulphate. In presence of DCP, equal molarity of these non-ionic surfactants and cholesterol showed higher entrapment efficiency than a 1:0.5 molar ratio. This may be due to the fact that cholesterol in the presence of DCP was more efficiently able to stabilize the structure of the niosomal membrane in a molar ratio of 1:1 (non-ionic surfactant: cholesterol) (10). On the other hand, in the absence of DCP, the formulations exhibited higher entrapment efficiency within a 1:0.5 molar ratio than 1:1 molar ratio (non-ionic surfactant: cholesterol). This is possibly attributed to that an increase in cholesterol ratio may in certain cases disrupt the regular linear structure of the formed niosomal membrane (38,39).
It is obvious from Table II that the inclusion of DCP into niosomes composed of Tween 80 (F5–F8) led to a decrease in the percentage entrapment efficiency of gentamicin. In presence of DCP, equal molarity of this surfactant and cholesterol illustrated lower entrapment efficiency than a 1:0.5 molar ratio. These results could be attributed to the structure formed with Tween 80 which was not sufficiently stabilized by DCP (33).
It is clear that formula F4 composed of Tween 60, cholesterol and DCP in a 1:1:0.1 molar ratio is most beneficial for the efficient encapsulation of gentamicin sulphate as it exhibited the highest entrapment efficiency (92.02 ± 1.43) compared to the other formulae.
Photomicroscopy and Transmission Electron Microscopy
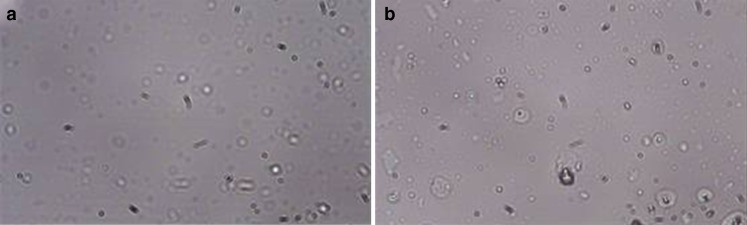
The photomicrographs (×40) of gentamicin sulphate niosomes F4 composed of Tween 60, cholesterol and DCP in a 1:1:0.1 molar ratio and F6 composed of Tween 80 and cholesterol in a 1:1 molar ratio are shown in Fig. 1a, b, respectively. We can observe the spherical morphology of the niosomal preparation F4 and F6. It is also clear that the F4 vesicles are larger in size than those of F6, which is coherent to the entrapment efficiency results.
Fig. 1.
Photomicrographs of gentamicin-loaded niosomes a F4; b F6 (×40)
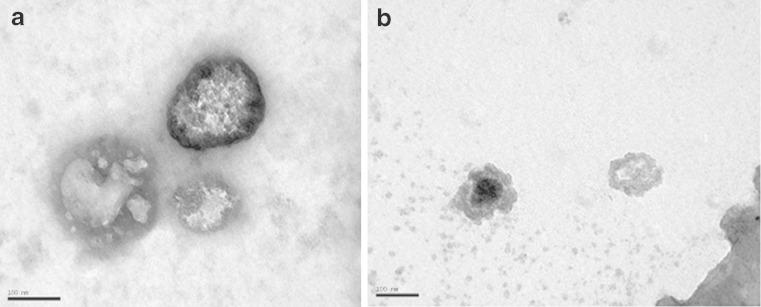
Negative stain transmission electron micrographs of gentamicin F4 and F6 are shown in Fig. 2a, b, respectively. It is demonstrated that the vesicles are well identified and present in a nearly perfect sphere-like shape having a large internal aqueous space.
Fig. 2.
Negative stain transmission-electron micrographs of gentamicin-loaded niosomes a F4; b F6
Determination of Vesicle Size
The mean particle diameter of the prepared niosomes is shown in Table II. We note that niosomal formulations composed of Tween 60 (F1–F4) are larger in size than niosomes prepared using Tween 80 (F5–F8) which are in turn larger than the particle size of Brij 35 niosomes (F9–F12). Tween 60 and Tween 80 have longer alkyl chain compared to Brij 35 as mentioned previously and it was reported that surfactants with longer alkyl chains generally give larger vesicles (39). This would account for the higher entrapment efficiencies obtained with Tween 60 niosomes.
In Vitro Release of Gentamicin Sulphate from Niosomes
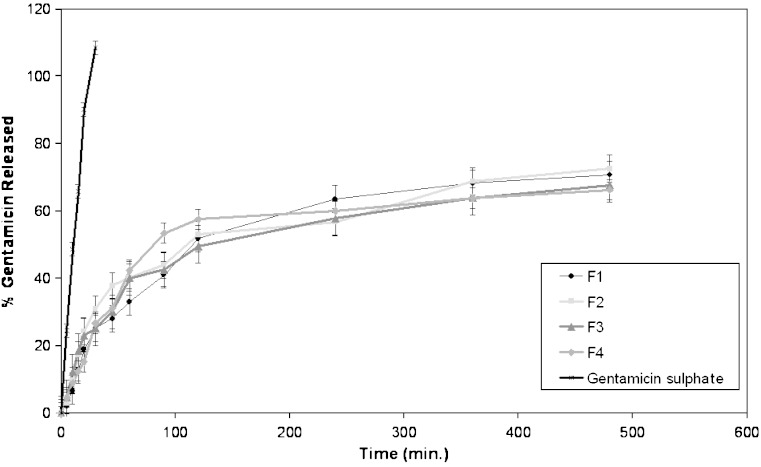
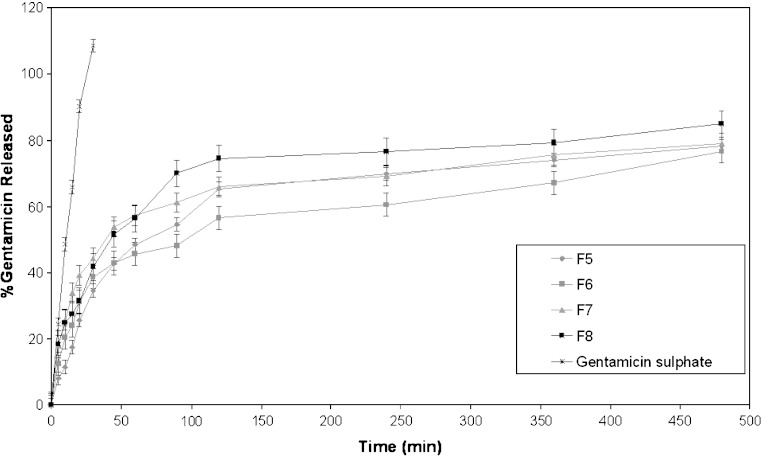
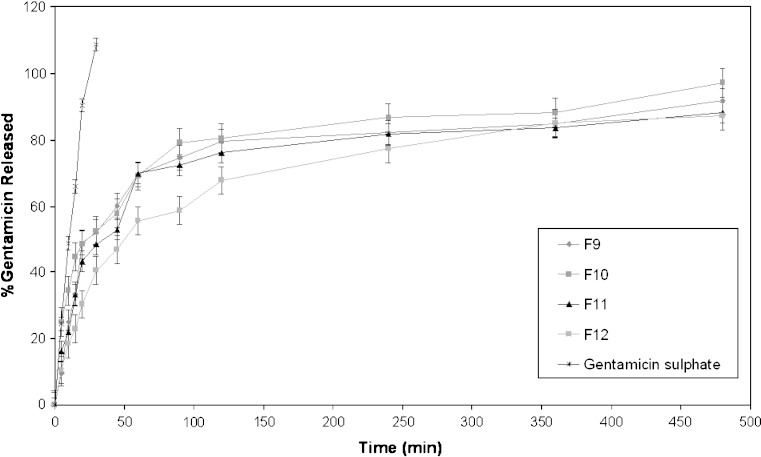
Results of an in vitro study on the release of gentamicin sulphate niosomal vesicles prepared using Tween 60, Tween 80 or Brij 35 are shown in Figs. 3, 4 and 5, respectively. The percentage of drug released after 8 h (Q8h) from the prepared niosomal vesicles are shown in Table II. Niosomal formulations showed slower release rate than gentamicin solution. Significant changes in release were observed upon changing the type of surfactant used in the bilayer of gentamicin niosomes.
Fig. 3.
In-vitro release profiles of gentamicin sulphate in simulated lacrimal fluid from niosomal formulations prepared using Tween 60 as surfactant (F1–F4), compared to drug solution
Fig. 4.
In-vitro release profiles of gentamicin sulphate in simulated lacrimal fluid from niosomal formulation prepared using Tween 80 as surfactant (F5–F8), compared to drug solution
Fig. 5.
In-vitro release profiles of gentamicin sulphate in simulated lacrimal fluid from niosomal formulation prepared using Brij 35 as surfactant (F9–F12) compared to drug solution
By inspection of the data, it could be concluded that niosomal formulations prepared using Tween 60 yielded a lower rate of release compared to Tween 80 niosomes which in turn showed lower release than niosomes formulated with Brij 35. This can be explained by the fact that niosomes exhibit an alkyl chain length-dependent release and the higher the chain length, the lower the release rate (26). By reviewing the data in Table II, Q8h for the niosomal formulations can be arranged in the following decreasing order: F10 > F9 > F11 > F12 > F8 > F7 > F5 > F6 > F2 > F1 > F3 > F4 niosomal vesicles.
By comparing the release data of gentamicin niosomes containing DCP with that of DCP free-niosomes, it is clear that the release is retarded in presence of DCP with Tween 60 and Brij 35. This confirms that DCP stabilizes the structure of niosomal membrane and renders it less permeable. Whilst, for niosomes prepared using Tween 80, the release is slower from DCP free-niosomes. This may be attributed to the structure of Tween 80 niosomal vesicles (33).
From the results, it is obvious that the increase of cholesterol molar ratio from 1:0.5:0.1 to 1:1:0.1 (non-ionic surfactant: cholesterol: DCP) markedly reduced the efflux of the drug from niosomal preparations (F3, F4, F11 and F12), which is in accordance with its membrane stabilizing ability (40). Cholesterol is known to abolish the gel to liquid phase transition of niosome systems (35), resulting in niosomes that are less leaky (36). Therefore, the diffusion of gentamicin entrapped in the hydrophobic regions of the vesicles would be expected to occur over a prolonged period of time. On the contrary to previous results, the increase of cholesterol molar ratio from 0.5 to 1 slightly increased the efflux of the drug from DCP free niosomes (F1, F2, F9 and F10).
The release profiles of gentamicin from niosomes prepared with Tween 80 reveal that the presence of cholesterol in the niosomes stabilizes the bilayers and decreases their permeability. However, contrary to previous results, increase in cholesterol molar ratio from 1:0.5 to 1:1 in absence of DCP gradually reduces the permeability (F5 and F6). Upon incorporation of DCP, increase in cholesterol molar ratio from 0.5 to 1 (F7 and F8) showed increase in vesicle permeability
It is to be noted that the in vitro release results are consistent with those of the entrapment efficiency, as the niosomes composed of Tween 60, cholesterol and DCP (1:1:0.1) molar ratio with the highest entrapment efficiency (92.02%) showed the lowest drug release percent after 8 h (Q8h = 66.29%). The comparative release data indicate that, by encapsulation of drug into niosomes, it is possible to sustain and control the release of the drug for a longer duration (22).
Ocular Irritancy Test of Niosomes
It was observed that over the study period (48 h) none of the tested formulae showed any signs of redness, inflammation or increased tear production. Thus it could concluded that the non ionic surfactants namely Tween 60 [F4], Tween 80 [F6], and Brij 35 [F12], used in the niosomal formulations as well as the other excipients were non-irritant to the eye.
CONCLUSIONS
The results of this study show that cholesterol content, type of surfactant and the presence of charge inducer dicetyl phosphate (DCP), altered the entrapment efficiency %EE and release rate from gentamicin sulphate niosomes. Higher %EE was obtained with niosomes prepared from Tween 60, cholesterol and DCP in a 1:1:0.1 molar ratio. The in-vitro evaluation of gentamicin niosomes in comparison to gentamicin solution showed that gentamicin niosomes composed of Tween 60, cholesterol and DCP were the most effective in the prolongation of drug release from the ocular delivery system. No signs of irritation was observed upon the application of the niosome-encapsulated gentamicin to the eyes of albino rabbits. Niosomes may be considered as promising ophthalmic carriers for the topical application of gentamicin sulphate.
References
- 1.Cabanes A., Reig F., Garcia-Anton J.M. Evaluation of free and liposome-encapsulated gentamicin for intramuscular sustained release in rabbits. Res. Vet. Sci. 1998;64:213–217. doi: 10.1016/S0034-5288(98)90128-X. [DOI] [PubMed] [Google Scholar]
- 2.Megoulas N.C., Koupparis M.A. Development and validation of a novel LC/ELSD method for the quantitation of gentamicin sulphate components in pharmaceuticals. J. Pharm. Biomed. Anal. 2004;36:73–79. doi: 10.1016/j.jpba.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Kaur I.P., Garg A., Singla A.K., Aggarwal D. Vesicular systems in ocular delivery: an overview. Int. J. Pharm. 2004;269:1–14. doi: 10.1016/j.ijpharm.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Frucht-Pery J., Mechoulam H., Siganos C.S., Ever-Hadani P., Shapiro M., Domb A. Iontophoresis–gentamicin delivery into the rabbit cornea, using a hydrogel delivery probe. Exp. Eye Res. 2004;78:745–749. doi: 10.1016/S0014-4835(03)00215-X. [DOI] [PubMed] [Google Scholar]
- 5.Blanco-Prıeto M.J., Lecaroz C., Renedo M.J., Kunkova J., Gamazo C. In vitro evaluation of gentamicin released from microparticles. Int. J. Pharm. 2002;242:203–206. doi: 10.1016/S0378-5173(02)00158-8. [DOI] [PubMed] [Google Scholar]
- 6.Records R.E. Gentamicin in ophthalmology. Surv. Ophthalmol. 1976;21:49–58. doi: 10.1016/0039-6257(76)90048-5. [DOI] [PubMed] [Google Scholar]
- 7.Baeyens V., Kaltsatos V., Boisramé B., Varesio E., Veuthey J.L., Fathi M., Balant L.P., Gex-Fabry M., Gurny R. Optimized release of dexamethasone and gentamicin from a soluble ocular insert for the treatment of external ophthalmic infections. J. Control Release. 1998;52:215–220. doi: 10.1016/S0168-3659(97)00212-5. [DOI] [PubMed] [Google Scholar]
- 8.Baeyens V., Felt-Baeyens O., Rougier S., Pheulpin S., Boisramé B., Gurny R. Clinical evaluation of bioadhesive ophthalmic drug inserts (BODI®) for the treatment of external ocular infections in dogs. J. Control Release. 2002;85:163–168. doi: 10.1016/S0168-3659(02)00284-5. [DOI] [PubMed] [Google Scholar]
- 9.Colo D.G., Zambito Y. A study of release mechanisms of different ophthalmic drugs from erodible ocular inserts based on poly (ethylene oxide) Eur. J. Pharm. Biopharm. 2002;54:193–199. doi: 10.1016/S0939-6411(02)00086-3. [DOI] [PubMed] [Google Scholar]
- 10.Uchegbu I.F., Vyas S.P. Non ionic surfactant based vesicles (niosomes) in drug delivery. Int. J. Pharm. 1998;172:33–70. doi: 10.1016/S0378-5173(98)00169-0. [DOI] [Google Scholar]
- 11.Carafa M., Santucci E., Alhaique F., Coviello T., Murtas E., Riccieri F.M., Lucania G., Torrisi M.R. Preparation and properties of new unilamellar non-ionic surfactant vesicles. Int. J. Pharm. 1998;160:51–59. doi: 10.1016/S0378-5173(97)00294-9. [DOI] [Google Scholar]
- 12.Carafa M., Santucci E., Lucania G. Lidocaine-loaded non ionic surfactant vesicles: characterization and in vitro permeation studies. Int. J. Pharm. 2002;231:21–32. doi: 10.1016/S0378-5173(01)00828-6. [DOI] [PubMed] [Google Scholar]
- 13.Saettone M.F., Perini G., Carafa M., Santucci E., Alhaique F. Non-ionic surfactant vesicles as ophthalmic carriers for cyclopentolate. A preliminary evaluation. S. T. P. Pharma. Sci. 1996;6:94–98. [Google Scholar]
- 14.Vyas S.P., Mysore N., Jaittley V., Venkatesan N. Discoidal niosome based controlled ocular delivery of timolol maleate. Pharmazie. 1998;53:466–469. [PubMed] [Google Scholar]
- 15.Green K., Downs S. Ocular penetration of pilocarpine in rabbits. Arch. Ophthalmol. 1975;93:1165–1168. doi: 10.1001/archopht.1975.01010020871009. [DOI] [PubMed] [Google Scholar]
- 16.Keller N., Moore D., Carper D., Longiwell A. Increased corneal permeability by the dual effect of the transient tear film acidification and exposure to benzalkonium chloride. Exp. Eye Res. 1980;30:203–210. doi: 10.1016/0014-4835(80)90114-1. [DOI] [PubMed] [Google Scholar]
- 17.Burstein N.L. Preservative alteration of corneal permeability in human and rabbits. Invest. Ophthalmol. Vis. Sci. 1984;25:1453. [PubMed] [Google Scholar]
- 18.Kaur I.P., Smitha R. Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev. Ind. Pharm. 2002;28:353–369. doi: 10.1081/DDC-120003445. [DOI] [PubMed] [Google Scholar]
- 19.Baillie A.J., Florence A.T., Hume L.R., Muirhead G.T., Rogerson A. The preparation and properties of niosomes non-ionic surfactant vesicles. J. Pharm. Pharmacol. 1985;37:863–868. doi: 10.1111/j.2042-7158.1985.tb04990.x. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal R., Katare O.P., Vyas S.P. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. Int. J. Pharm. 2001;228:43–52. doi: 10.1016/S0378-5173(01)00810-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X., Wyss U.P., Pichora D., Goosen M.F.A. Biodegradable controlled antibiotic release devices for osteomyelitis: optimization of release properties. J. Pharm. Pharmacol. 1994;46:718–24. doi: 10.1111/j.2042-7158.1994.tb03890.x. [DOI] [PubMed] [Google Scholar]
- 22.Ruckmani K., Jayakar B., Ghosal S.K. Nonionic surfactant vesicles (niosomes) of cytarabine hydrochloride for effective treatment of leukemia: encapsulation, storage and in vitro release. Drug Dev. Ind. Pharm. 2000;26:217–222. doi: 10.1081/DDC-100100348. [DOI] [PubMed] [Google Scholar]
- 23.Benchimol M., Piva B., Campanati L., De Souza W. Visualization of the funis of Giardia lamblia by high-resolution field emission scanning electron microscopy-new insights. J. Struc. Bio. 2004;147:102–115. doi: 10.1016/j.jsb.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Arunothayanun P., Bernard M.S., Craig D.Q.M., Uchegbu I.F., Florence A.T. The effect of processing variables on the physical characteristics of non-ionic surfactant vesicles (niosomes) formed from a hexadecyl diglycerol ether. Int. J. Pharm. 2000;201:7–14. doi: 10.1016/S0378-5173(00)00362-8. [DOI] [PubMed] [Google Scholar]
- 25.El-Gazayerly O.N., Hikal A.H. Preparation and evaluation of acetazolamide liposomes as an ocular delivery system. Int. J. Pharm. 1997;158:121–127. doi: 10.1016/S0378-5173(97)00186-5. [DOI] [Google Scholar]
- 26.Devaraj G.N., Parakh S.R., Devraj R., Apte S.S., Rao B.R., Rambhau D. Release studies on niosomes containing fatty alcohols as bilayer stabilizers instead of cholesterol. J. Colloid Interface Sci. 2002;251:360–365. doi: 10.1006/jcis.2002.8399. [DOI] [PubMed] [Google Scholar]
- 27.Glavas-Dodov M., Goracinova K., Mladenovska K., Fredro-Kumbaradzi E. Release profile of lidocaine HCl from topical liposomal gel formulation. Int. J. Pharm. 2002;242:381–384. doi: 10.1016/S0378-5173(02)00221-1. [DOI] [PubMed] [Google Scholar]
- 28.Van Haeringen N.J. Clinical biochemistry of tears. Surv. Ophthalmol. 1981;5:84–96. doi: 10.1016/0039-6257(81)90145-4. [DOI] [PubMed] [Google Scholar]
- 29.Ceulemans J., Vermeire A., Adriaens E., Remon J.P., Ludwig A. Evaluation of a mucoadhesive tablet for ocular use. J. Control Release. 2001;77:33–344. doi: 10.1016/S0168-3659(01)00522-3. [DOI] [PubMed] [Google Scholar]
- 30.Gursoy A., Kut E., Ozkirmli S. Co-encapsulation of isoniazid and rifampicin in liposomes and characterization of liposomes by derivative spectroscopy. Int J Pharm. 2004;271:115–123. doi: 10.1016/j.ijpharm.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 31.Tamilvanana S., Benitab S. The potential of lipid emulsion for ocular delivery of lipophilic drugs. Eur. J. Pharm. Biopharm. 2004;58:357–368. doi: 10.1016/j.ejpb.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 32.Colo D.G., Burgalassi S., Chetoni P., Fiaschi M.P., Zambito Y., Saettone M.F. Gel-forming erodible inserts for ocular controlled delivery of ofloxacin. Int. J. Pharm. 2001;215:101–111. doi: 10.1016/S0378-5173(00)00671-2. [DOI] [PubMed] [Google Scholar]
- 33.Hao Y., Zhao F., Li N., Yang Y., Li K. Studies on a high encapsulation of colchicine by a niosome system. Int. J. Pharm. 2002;224:73–80. doi: 10.1016/S0378-5173(02)00301-0. [DOI] [PubMed] [Google Scholar]
- 34.RajaNaresh R.A., Pillai G.K., Udupa N., Chandrashekar G. Anti-inflammatory activity of niosome encapsulated diclofenac sodium in arthritic rats. Indian. J. Pharmacol. 1994;26:46–48. [Google Scholar]
- 35.C. Cable. An examination of the effects of surface modifications on the physicochemical and biological properties of non-ionic surfactant vesicles. PhD Thesis. University of Strathclyde, Glasgow, UK (1989).
- 36.Rogerson A., Cummings J., Florence A.T. Adriamycin- loaded niosomes-drug entrapment, stability and release. J. Microencap. 1987;4:321–328. doi: 10.3109/02652048709021824. [DOI] [PubMed] [Google Scholar]
- 37.Gianasi E., Cociancich F., Uchegbu I.F., Florence A.T., Duncan R. Pharmaceutical and biological characterization of a doxorubicin polymer conjugate (PK1) entrapped in sorbitan monostearate Span 60 niosomes. Int. J. Pharm. 1997;148:139–148. doi: 10.1016/S0378-5173(96)04840-5. [DOI] [Google Scholar]
- 38.New RRC . Liposomes: a practical approach. Oxford: Oxford University Press; 1990. [Google Scholar]
- 39.Manosroi A., Wongtrakul P., Manosroi J., Sakai H., Sugawara F., Yuasa M., Abe M. Characterization of vesicles prepared with various non ionic surfactants mixed with cholesterol. Colloids Surf. B Biointerfaces. 2003;30:129–138. doi: 10.1016/S0927-7765(03)00080-8. [DOI] [Google Scholar]
- 40.Betageri G.V., Parsons D.L. Drug encapsulation and release from multilamellar and unilamellar liposomes. Int. J. Pharm. 1992;81:235–241. doi: 10.1016/0378-5173(92)90015-T. [DOI] [Google Scholar]