Abstract
The purpose of the present study was to develop and characterize the chitosan sponges loading with doxycycline hyclate and their antibacterial activities. The pore density of chitosan sponge prepared with freeze drying technique was increased as the higher concentrated chitosan solution was used. The sponge prepared from 10% w/w of the chitosan solution and crosslinking with glutaraldehyde solution was utilized for loading with doxycycline hyclate. The drug release and sustainable antibacterial activity of fabricated sponge were assessed using dissolution test and agar diffusion test, respectively. Drug release from non-crosslinked sponge into phosphate buffer pH7.4 was slower than that from crosslinked sponge since the former could absorb the medium and form gel to retard the initial drug diffusion. Sustainable antibacterial activity of developed sponge was evident against S. aureus and E. coli. In conclusion, the in vitro release profile and antibacterial efficiency indicated that doxycycline hyclate could be sustained using chitosan sponge.
Key words: antibacterial activity, chitosan sponge, doxycycline hyclate, drug release
INTRODUCTION
The main driving force in the advanced investigations and applications of chitosan comes from its naturally abundant, satisfactorily biocompatible, biodegradable and nontoxic. Many pharmaceutical applications have been focused on chitosan since its structure is cellulose-like, and the free amino groups on this polymeric chain contribute the reactive and polycationic nature that exhibit complexation properties. The potential applications of chitosan as an excipient in oral formulation and other delivery systems are reported (1,2). Commercially, nowadays chitosan is prepared by purification and then N-deacetylation of chitin with an aqueous solution of sodium hydroxide. Many investigators manifested the efficiency of chitosan as biodegradable material for controlling the transport of diffusive molecules (3). Products made from chitosan are variously useful, and especially safety to human beings. In order to increase in structural strength of chitosan products, many scientists attempted to cross-linked chitosan chains with a crosslinking agent or converting its functional group with chemical reaction. The chemical cross-linking notably enhances the flexibility, reduces the swelling index and also affects the release of active compounds. The applications of chitosan and its derivatives in the field of tissue engineering have been progressed and recognized (4). Chitosan sponge was especially claimed to be used in field of tissue engineering and also drug delivery. The high capacity of drug loading and the biodegradability promote the research on chitosan sponge. Chitosan wound dressing consisting of silver sulfadiazine was successful for controlling the wound infections (5). Chitosan sponge reinforced by beta-tricalcium phosphate and calcium phosphate was used for gentamicin sulfate loading (6). Other antibiotics have been loaded in chitosan sponge to control the wound infections with sustainable drug delivery (5,7). Release rate of platelet-derived growth factor could be controlled by chitosan sponge (8).
Doxycycline (α-6-deoxy-5-oxytetracyclin), a well-known broad-spectrum antibiotic, was derived synthetically from oxytetracycline. It is a bacteriostatic, inhibiting the bacterial protein synthesis due to the disruption of transfer RNA and messenger RNA at the ribosomal sites (9,10). It is also used for malaria treatment (11). This study focused on the antibacterial activity and the drug release from chitosan sponge loaded with doxycycline hyclate.
MATERIALS AND METHODS
Materials
Chitosan (type OC) (Aqua premier, Chonburi, Thailand) having degree of deacetylation 99.3% and molecular weight 70kDa was sieved through sieve No. 80 mesh before used. Doxycycline hyclate (Batch No. 20050124, Handan Jiupeng Chemical Engineering Co., Ltd., China) was used as received. Glutaraldehyde and pentasodium triphosphate were purchased from Sigma (St. Louis, USA). Tryptic soy agar (TSA) (lot No. 3056695, Difgo, USA) and tryptic soy broth (TSB) lot 4259, Difgo, USA), were used as received. Acetic acid and other reagents were of analytical grade.
Preparation of Sponge
Chitosan powder was dissolved in 2% w/w acetic acid solution overnight, thereafter the system was passed through polyester cloth to obtain the clear solution. The 0.3g chitosan solution with different concentrations (4, 7 and 10% w/w) without drug was filled into the 24-well plates. The filled well plates were frozen at −30°C before freeze drying using a freeze dryer (type 77560-01, Labconco, Missouri, USA) to evaporate the utilized solvent from the systems. Fabricated sponges were crosslinked with 1% w/v glutaraldehyde solution or 1% w/v pentasodium triphosphate solution for 30min before carefully washing with distilled water several times before drying with hot air oven at 60°C overnight. Glutaraldehyde was removed with that rinsing medium until no traces of glutaraldehyde could be detected at 235 and 280nm using UV–Vis spectrophotometer (Perkin-Elmer, Germany). The plain and cross-linked chitosan sponges were loaded with 125mg/mL doxycycline hycate solution using methanol as solvent to obtain the drug at amount of 50, 100 and 150mg/sponge. The accurate amount of drug/sponge could be obtained since the micropipette was used to load the drug solution into each chitosan sponges. Drug-loaded sponges were dried with hot air oven at 40°C for 2h and then they were kept in desiccator before tested.
Evaluation of Sponge
Sponge Morphology
Morphology of empty chitosan sponge and sponge containing the drug was characterized under a scanning electron microscope (Maxim2005, Cam scan, UK) operated at an accelerating voltage of 20keV. The samples were stuck on a metal stub using carbon double adhesive and sputter-coated with gold before test.
Drug Release
A test of drug release was undertaken using a dissolution apparatus (type 1) (Prolabo, France) with the basket method at 100rpm (n = 3). A volume of 900-mL phosphate buffer pH7.4 equilibrated at 37°C was utilized as dissolution fluid. Samples were collected at specific time intervals and assayed using a UV–Vis spectrophotometer (Perkin-Elmer, Germany) at a wavelength of 349nm.
Water Sorption
The water sorption capacity was carried out using phosphate buffer pH7.4 as immersion medium (n = 3). Firstly, each sponge was carefully weighed (W1) and then immersed in phosphate buffer pH7.4. The sponge remnants were wiped off excess surface water using filter paper and weighed (W2) at different time intervals. The immersed sponges were dried at 60°C for 24h and kept in a desiccator for 48h prior to reweigh (W3). The water sorption was calculated using the following formula:
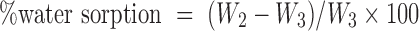 |
Dissolution Profile Fitting
Least square fitting the experimental dissolution data (cumulative drug release >10% and up to 80%) to the mathematical equations (power law, first order, Higuchi’s and zero order) was carried out using a nonlinear computer programme, Scientist for Windows, version 2.1 (MicroMath Scientific Software, Salt Lake City, UT, USA). The coefficient of determination (r2) was used to indicate the degree of curve fitting. Goodness-of-fit was also evaluated using the Model Selection Criterion (MSC) (12), given below.
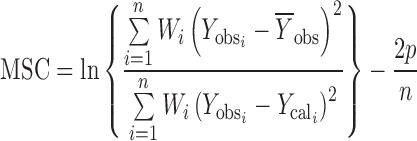 |
Where 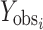 and
and 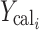 are observed and calculated values of the i-th point, respectively, and Wi is the weight that applies to the i-th point, n is number of points and p is number of parameters.
are observed and calculated values of the i-th point, respectively, and Wi is the weight that applies to the i-th point, n is number of points and p is number of parameters.
Antibacterial Activity Testing
Antibacterial activity of prepared sponges was assessed using agar diffusion method. The standard microbes were used in this study were Staphylococcus aureus (ATCC 6538) and Escherichia coli (ATCC 8740). The isolated colony from TSA was inoculated to TSB and further incubated at 37°C for 18–24h. The bacterial culture was transferred to new TSB for 4h incubation and adjusted the inoculums by the standard 0.5M MacFarland. After the 0.1mL inoculum spreading, the developed sponges were placed on agar. The plates were incubated overnight at 37°C. The diameter of clear zone was measured. The tested sponges were moved to the new agar diffusion plate to evaluate the efficiency of sustainable drug release (n = 3).
RESULTS AND DISCUSSION
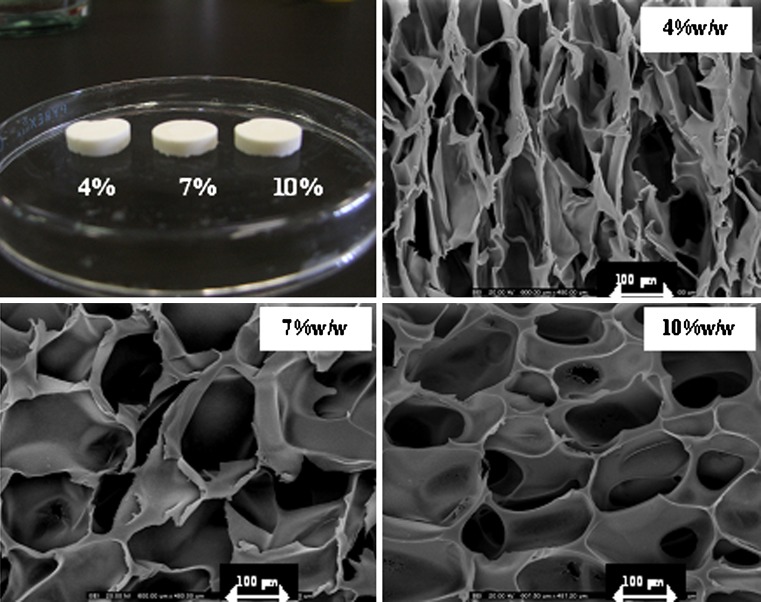
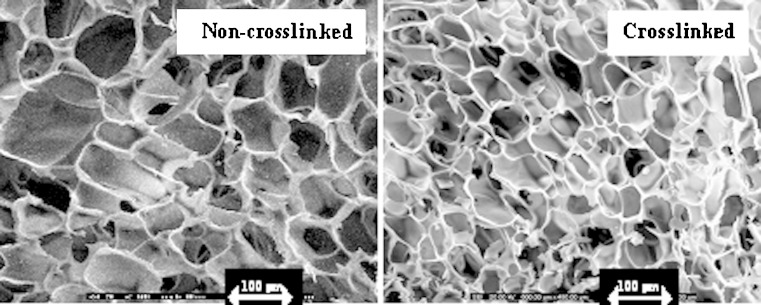
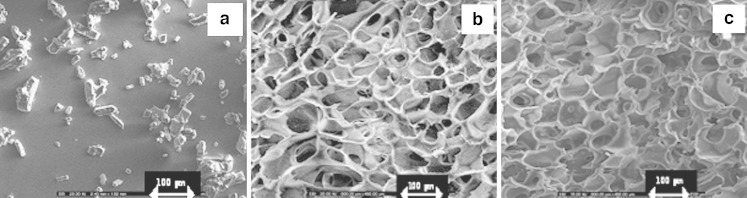
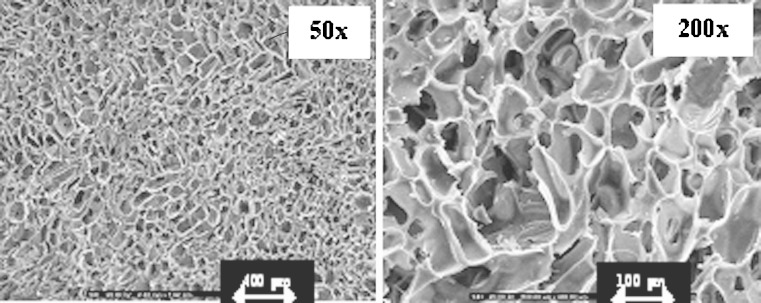
Freeze drying technique could produce the sponge with the light stretchy structure that full of holes. The sponges obtained from 7% and 10% w/w of chitosan solution exhibited the microstructural consistency, whereas that produced from 4% w/w chitosan solution was rather soft. The pore density was increased for the sponge prepared from the high concentration of chitosan solution as presented in Fig. 1. The sponge prepared with 10% w/w chitosan solution contained the pores with consistency in shape and volume than that prepared from 4% and 7% w/w chitosan solutions. The micrograph also showed that the pores were well interconnected throughout the sponge. Diameter of pores was in rank about 80–130μm. However, the higher concentration of chitosan solution greater than 10% w/w could not be prepared since there was the limitation of the polymer solubility and the formation of high viscous system that could not obtain the clearly flowable solution suitable to fill into the well. Apparently, pore volume of chitosan sponge was diminished after crosslinking with glutaraldehyde solution as shown in Fig. 2. Crosslinking reaction occurring between the long chains of the polymer promoted the network structure in the chitosan mass and the shrinkage of sponge structure. Some investigation indicated that the crosslinked high molecular weight chitosan sponges contained the larger pore volume that formed the connections (channels) with the interior of the sponge than crosslinked low molecular weight chitosan (13). By comparison, chitosan used in this study was low molecular weight, therefore the result was also corresponding to that of above reported. The obtained crosslinked chitosan sponge could be effectively loaded with doxycycline hyclate of 50, 100, 150mg. Because of high pore surface area, the high amount drug could be loaded. There was no structural change of sponge before and after loading with 50mg doxycycline hyclate (Fig. 3a and b, respectively). No drug crystal appeared onto the wall indicating the homogeneous dispersion of drug into the sponge. However, the crystals of the incorporated tramadol hydrochloride was detected on the lamellae and within pores in chitosan sponges previously reported (14). Morphology of this drug is presented in Fig. 3a. The shape of drug was rectangular and irregular. The integrity of drug-loaded crosslinked sponge was found during dissolution test. High porous structure of tested sponge was still remained after dissolution (Fig. 4). However, the thinner wall of its structure was evident. The result indicated that the polymeric wall of sponge was gradually eroded with time. However this erosion might also result from the washing step after sponge crosslinking process.
Fig. 1.
Photograph and SEM micrographs of sponges prepared with chitosan solution at different concentrations at vertical side (magnification of ×200)
Fig. 2.
SEM micrographs of non-crosslinked and crosslinked sponges prepared with chitosan solution at concentration of 10% w/w at vertical side (magnification of ×200)
Fig. 3.
SEM micrographs of doxycycline hyclate powder a and crosslinked sponges prepared with 10% w/w chitosan solution before b and after c loading with 50 mg doxycycline at vertical side (magnification of ×200)
Fig. 4.
SEM micrographs of crosslinked sponges prepared with 10% w/w chitosan solution loading with 50 mg doxycycline after dissolution test at vertical side (magnification of ×50 and ×200)
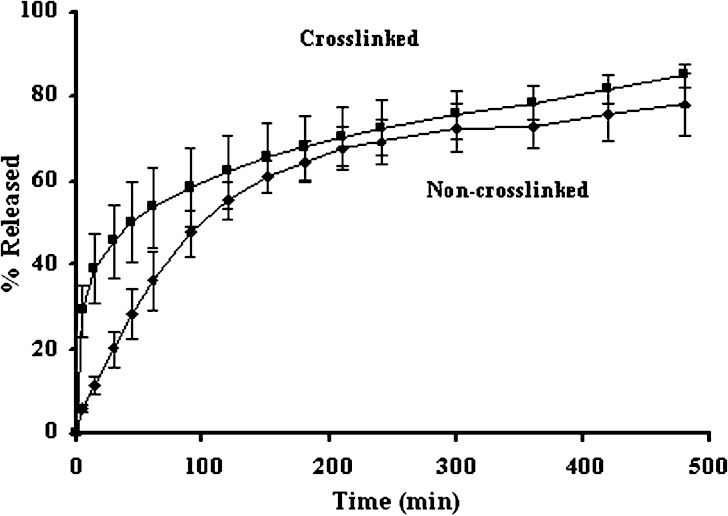
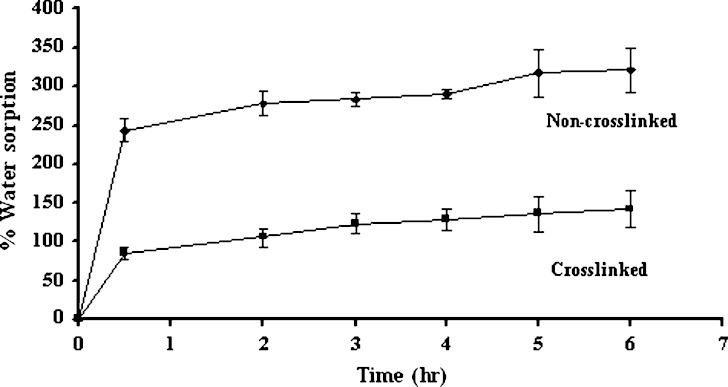
The drug release from non-crosslinked chitosan sponge was slower than that from crosslinked chitosan sponge as shown in Fig. 5. The initial rapid drug release from the crosslinked sponge was due to the release of deposited drug from insoluble sponge surface. The more retardation of initial drug release of non cross-linked sponge resulted from the gel formation of the system after exposure to the dissolution medium and the gel could retard the drug diffusion from the sponge. However, both systems could prolong the drug release. Similar result was reported for the release of silver sulfadiazine from the bilayer chitosan dressing displayed a burst release on the first day and then tapered off to a much slower release (6). The sustainable release of triamcinolone acetonide from crosslinked chitosan sponge has been reported (14). There was the entrapment of drug molecule with incomplete erosion of chitosan structure therefore the total loaded drug was not completely released. The data from water sorption study was corresponding to that of the drug dissolution. Since the apparently high water sorption was appeared for non-crosslinked sponge owing to its hydration and gel formation after contacting with the medium (Fig. 6), the drug release was slower than that of crosslinked sponge. Both crosslinked with glutaraldehyde and non-crosslinked sponges absorbed water more than 100% of sponge weight, which the water absorption capacity of non-crosslinked sponges was about 2.5 times higher than crosslinked sponges. However the non-crosslinked sponge structure was damaged after overnight incubation in phosphate buffer. Decrease in hydrophilic groups from crosslinking reaction diminished the water hydration and water sorption of chitosan sponge.
Fig. 5.
Dissolution profiles of doxycycline hyclate from non-crosslinked and crosslinked chitosan sponge (n = 3)
Fig. 6.
The percentage of water sorption of non-crosslinked and crosslinked chitosan sponges prepared with 10% w/w chitosan solution (n = 3)
Power law equation gained popularity for analysis of release data (15). The n value from power law is the diffusional exponent which characterizes the transport mechanism of the drug. The transport mechanisms are classified based on the value that n assumes. For a cylinder, the drug transport mechanism is by Fickian diffusion when n = 0.45, if 0.45 < n < 0.89, it indicates anomalous (non-Fickian) transport and for values of n = 0.89, Case II or zero order release kinetics is indicated (16). Case II relates to polymer relaxation, while non-Fickian release is described by two mechanisms; the coupling of drug diffusion and polymer relaxation (15,17). The large value of coefficient of determination (r2) or model selection criteria (MSC) indicated a superiority of the dissolution profile fitting to mathematical equations. Fitting experimental drug dissolution profiles from crosslinked sponge to power law equation provided high r2 and MSC indicating a superiority of this model whereas that from non crosslinked was rather poor fitting as presented in Table I. By comparison, both profiles could be fitting with first order equation better than Higuchi’s equation and zero order equation, respectively. Estimate parameters from curve fitting to power law and first order equations are shown in Tables II and III, respectively. The n values from the fitting of both drug dissolution profiles were less than 0.45, indicating the release was corresponded to other release kinetic. To compare the release rate, curve fitting to first order kinetic which could provide the large r2 and MSC values (Table I) was used. Drug release rate of the crosslinked sponge into medium was higher than that from the non-crosslinked sponge as exhibited in Table III.
Table I.
Comparison of Degree of Goodness-of-fit from Curve Fitting of Drug Dissolution in Phosphate Buffer pH 7.4 to Different Release Models
| Chitosan sponge | Power law | First order | Higuchi’s | Zero order | ||||
|---|---|---|---|---|---|---|---|---|
| r 2 | MSC | r 2 | MSC | r 2 | MSC | r 2 | MSC | |
| Non crosslinked | 0.9586 | 2.76 | 0.9138 | 2.17 | 0.8826 | 1.86 | 0.7565 | 1.13 |
| Crosslinked | 0.9999 | 8.48 | 0.9358 | 2.46 | 0.9029 | 2.05 | 0.8431 | 1.57 |
Table II.
Estimate Parameters from Curve Fitting of Drug Dissolution in Phosphate Buffer pH 7.4 to Power Law Expression
| Chitosan sponge | k ± SD | n ± SD | Tl ± SD |
|---|---|---|---|
| Non crosslinked | 0.1130 ± 0.0195 | 0.3251 ± 0.0316 | 14.24 ± 0.02 |
| Crosslinked | 0.2254 ± 0.0015 | 0.2129 ± 0.0013 | 1.76 ± 0.15 |
Table III.
Estimate Parameters from Curve Fitting of Drug Dissolution in Phosphate Buffer pH 7.4 to First Order Equation
| Chitosan sponge | k ± SD × 10−3 | Tl ± SD |
|---|---|---|
| Non crosslinked | 4.0639 ± 0.4364 | −41.12 ± 16.28 |
| Crosslinked | 4.5019 ± 0.7583 | −73.39 ± 24.92 |
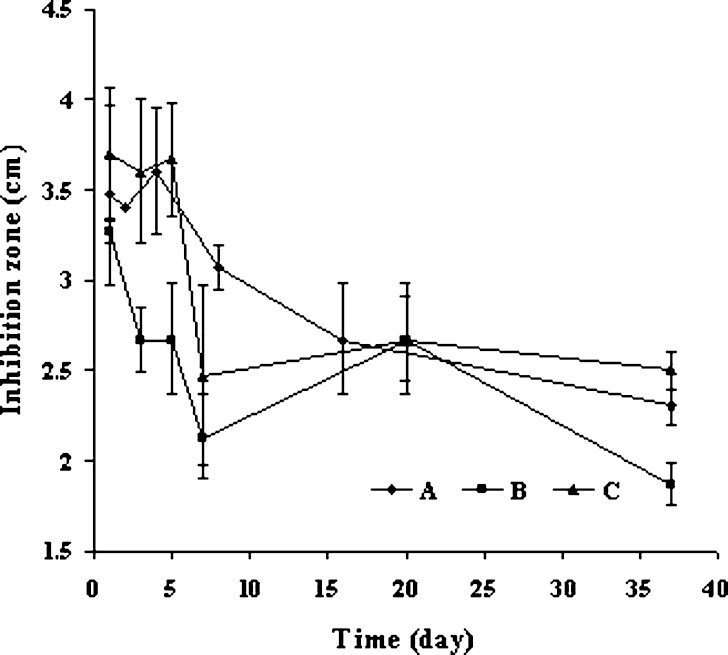
For preliminary study the antimicrobial activity of prepared sponges, the structural stability of drug-loaded sponge was also assessed by agar diffusion technique. After 1day incubation, sponges containing a high amount of drug (100 and 150mg) collapsed (data not shown), but the structural integrity of 50mg-doxycycline sponge was unchanged. Due to acidic property of drug, high drug loading produced the acidic environment and promoted the solubility of chitosan and induced the disintegration of sponge. In order to improve the structural strength, glutaradehyde and pentasodium triphosphate were separately used for chitosan crosslinking. Efficiency of pentasodium triphosphate was less than that of glutaraldehyde since the sponge crosslinked with pentasodium tripolyphosphate was apparently collapsed during water sorption test (data not shown). Doxycycline hyclate loading into the crosslinked and the non-crosslinked chitosan was successful since loaded sponges were stable after incubation onto TSA several days. However, the integrity of crosslinked sponge was rather more stable than that of non-crosslinked sponge, especially at the late stage of incubation. From antibacterial assay, the inhibition zones were found in both crosslinked and non-crosslinked sponge (Fig. 7 A). The diameter of clear zone of the crosslinked sponge was declined more rapid than the non-crosslinked after transfer to new incoculated plate, which was corresponding to the rapid release of crosslinked sponge from dissolution test comparing to the non-crosslinked sponge. After transfer to new culture agar for four times, the diameter of inhibition zone was rather stable both crosslinked and non-crosslinked systems (Fig. 7 A). The antibacterial activity of doxycycline against E. coli was still evident from chitosan sponge after six times repeating transfer during 37 days. The similar result was found for the antibacterial activity test of non-crosslinked sponge against S. aureus (Fig. 7 B). It has been reported by another group that the silver sulfadiazine wound dressing made from chitosan could affect against Pseudomonas aeruginosa and Staphylococcus aureus for 1 week incubation (6). The result from this study manifested that the sponge fabricated with freeze–drying could be used for antibacterial delivery. Doxycycline hyclate chitosan sponge might be developed to many products such as wound dressing or implant for the local applications that could reduce side effect and promote the therapeutic effectiveness.
Fig. 7.
Antibacterial activity of non-crosslinked chitosan sponges loaded with 50 mg doxycycline against E. coli (A) and crosslinked chitosan sponges loaded with 50 mg doxycycline against E. coli (B) and S. aureus (C) in six times of transfer (in 37 days period) (n = 3)
CONCLUSION
Sustainable antimicrobial system of doxycycline hyclate could be fabricated using chitosan sponge as carrier device. The release of drug from non-crosslinked sponge was slower than that from crosslinked owing to the hydration and gel formation retarding the drug diffusion of the first system. It could be suggested that the utilization of chitosan could give rise to sponge exhibiting sustained drug release and antibacterial activity.
Acknowledgments
The authors wish to thank Thailand Research Fund (Grant No. DBG5080014) for research grant support and T. Man Pharma Ltd., PART. This work was under kindly provision by Faculty of Pharmacy, Silpakorn University. The authors would like to express my appreciation to Mrs. Kamnuam Kongsupalak, Dr. Wanchai Chongcharoen, Miss Kullap Jirangkul, Mr. Suthep Sutthipongkiat and Miss Suchada Wattananawinrat for their help and comment.
References
- 1.Felt O., Buri P., Gurny R. Chitosan: a unique polysaccharide for drug delivery. Drug Dev. Ind. Pharm. 1998;24(11):979–993. doi: 10.3109/03639049809089942. [DOI] [PubMed] [Google Scholar]
- 2.Berger J., Reist M., Mayer J. M., Felt O., Peppas N. A., Gurny R. Structure and interactions in covalently and ionically cross-linked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004;57:19–34. doi: 10.1016/S0939-6411(03)00161-9. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda H., Takayama K., Nagai T. Drug permeation behavior in chitosan film prepared on the metal plate laded with enteric charge. Chem. Pharm. Bull. 1997;45(1):221–223. [Google Scholar]
- 4.Kato Y., Onishi H., Machida Y. Application of chitin and chitosan derivatives in the pharmaceutical field. Curr. Pharm. Biotechnol. 2003;4(5):303–309. doi: 10.2174/1389201033489748. [DOI] [PubMed] [Google Scholar]
- 5.Mi F. L., Wu Y. B., Shyu S. S., Schoung J. Y., Huang Y. B., et al. Control of wound infections using a bilayer chitosan wound dressing with sustainable antibiotic delivery. J. Biomed. Mater. Res. 2002;59(3):438–449. doi: 10.1002/jbm.1260. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y., Zhang M. Calcium phosphate/chitosan composite scaffolds for controlled in vitro antibiotic drug release. J. Biomed. Mater. Res. 2002;62(3):378–386. doi: 10.1002/jbm.10312. [DOI] [PubMed] [Google Scholar]
- 7.Mi F. L., Shyu S. S., Wu Y. B., Lee S. T., Shyong J. Y., Huang R. N. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials. 2001;22(2):165–173. doi: 10.1016/S0142-9612(00)00167-8. [DOI] [PubMed] [Google Scholar]
- 8.Park Y. J., Lee Y. M., Lee J. Y., Seol Y. J., Chung C. P., Lee S. J. Controlled release of platelet-derived growth factor-BB from chondroitin sulfate–chitosan sponge for guided bone regeneration. J. Control. Release. 2000;67(2–3):385–394. doi: 10.1016/S0168-3659(00)00232-7. [DOI] [PubMed] [Google Scholar]
- 9.Stratton C. W., Lorian V. Mechanisms of action for antimicrobial agents: general principals and mechanisms for selected classes of antibiotics, antibiotics in laboratory medicine. 4. Baltimore: Williams & Wilkins; 1996. [Google Scholar]
- 10.Reynolds J. J., Meikle M. C. Mechanisms of connective tissue matrix destruction in periodontitis. Periodontology. 1997;2000:144–157. doi: 10.1111/j.1600-0757.1997.tb00195.x. [DOI] [PubMed] [Google Scholar]
- 11.Ohrt C., Richie T. L., Widjaja H., Shanks G. D., Fitriadi J., et al. Mefloquine compared with doxycycline for the prophylaxis of malaria in Indonesian soldiers. A randomized, double-blind, placebo-controlled trial. Ann. Int. Med. 1997;126(12):963–972. doi: 10.7326/0003-4819-126-12-199706150-00006. [DOI] [PubMed] [Google Scholar]
- 12.MicroMath Scientist Handbook Rev. 7EEF, MicroMath: Salt Lake City, 1995, p. 467.
- 13.Foda N. H., El-laithy H. M., Tadros M. I. Optimization of biodegradable sponges as controlled release drug matrices. I. Effect of moisture level on chitosan sponge mechanical properties. Drug Ind. Dev. Pharm. 2004;30(4):369–379. doi: 10.1081/DDC-120030931. [DOI] [PubMed] [Google Scholar]
- 14.Oungbho K., Muller B. W. Chitosan sponges as sustained release drug carriers. Inter. J. Pharm. 1997;156:229–237. doi: 10.1016/S0378-5173(97)00201-9. [DOI] [Google Scholar]
- 15.Ritger P. L., Peppas N. A. A simple equation for description of solute release. II. Fickian and anomalous release from swellable devices. J. Control. Release. 1987;5:37–342. doi: 10.1016/0168-3659(87)90035-6. [DOI] [PubMed] [Google Scholar]
- 16.Peppas N. A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta. Helv. 1985;60(4):110–111. [PubMed] [Google Scholar]
- 17.Nerurkar J., Jun H. W., Price J. C., Park M. O. Controlled-release matrix tablets of ibuprofen using cellulose ethers and carrageenans: effect of formulation factors on dissolution rates. Eur. J. Pharm. Biopharm. 2005;61:56–68. doi: 10.1016/j.ejpb.2005.03.003. [DOI] [PubMed] [Google Scholar]