Abstract
The purpose of this study is to formulate in situ implants containing doxycycline hydrochloride and/or secnidazole that could be used in the treatment of periodontitis by direct periodontal intrapocket administration. Biodegradable polymers [poly (lactide) (PLA) and poly (lactide-co-glycolide) (PLGA)], each polymer in two concentrations 25%w/w, 35%w/w were used to formulate the in situ implants. The rheological behavior, in vitro drug release and the antimicrobial activity of the prepared implants were evaluated. Increasing the concentration of each polymer increases the viscosity and decreases the percent of the drugs released after 24 h. PLA implants showed a slower drugs release rate than PLGA implants in which the implants composed of 25% PLGA showed the fastest drugs release. The in vitro drug release and antimicrobial activity results were compared with results of Atridox®. Results revealed that the pharmaceutical formulation based on 25% PLGA containing secnidazole and doxycycline hydrochloride has promising activity in treating periodontitis in comparison with Atridox®.
Key words: Atridox®, biodegradable polymers, doxycycline, implants, secnidazole
INTRODUCTION
Periodontal diseases are a group of inflammatory conditions affecting the supportive structures of the teeth characterized by destruction of the periodontal ligament, resorption of the alveolar bone and the migration of the junctional epithelium along the tooth surface with the formation of a space (pocket) between the gum and the teeth and can eventually cause tooth loss. The formed pocket provides an ideal environment for the growth and proliferation of microbes (1).
Current periodontal therapy includes the removal of the bacterial deposits from the tooth surface and shifting the pathogenic microorganism to healthy one. Conventional root planning and scaling relies on the mechanical debridement of tooth surfaces as the primary antimicrobial measure (2). However, as specific bacteria are thought to play a major role in disease process, antimicrobial agents have been used as adjuncts to mechanical treatment (3). The potential side-effects in administering systemic antibiotics and the inability of antiseptic mouthwashes to penetrate the periodontal pocket have raised interest in the localized delivery of therapeutic agents within the periodontal pocket, thus ensuring a high effective concentration of antimicrobial agent at the site of infection.
The use of drug delivery systems based on biodegradable polymers that can achieve a sustained release of the drug over days had been used in the treatment of periodontal diseases (4,5), with the advantages of self-elimination, avoiding the need to remove the polymer system from the site of implantation after its use (6). Among the biodegradable polymers used, poly (lactic acid; PLA) and copoly (lactic: glycolic acid; PLGA) drug delivery systems are prepared by dissolving the polymers in biocompatible organic solvents. Drug is added into the polymer solution, where it forms a homogenous solution or a suspension depending on its solubility. The drug suspension or solution is injected subcutaneously, forming an “in situ implant”, which slowly releases the drug over time. Organic solvents are fully or partially water-miscible, and hence the in situ implants were formed by a non-solvent induced phase separation mechanism (7).
Tetracyclines and metronidazole were reported to be effective for the treatment of periodontal diseases (8,9). Doxycycline hydrochloride is chosen for this study due to its activity against putative periodontal pathogens, and is the most potent for collagenase inhibition (10,11). Secnidazole is chosen due to its activity against anaerobic microorganisms, and has a longer terminal elimination half-life than metronidazole (12). The objective of this study is to formulate in situ implants containing both secnidazole and doxycycline hydrochloride to increase the spectrum of the antimicrobial activity against the microorganisms causing periodontal diseases (aerobic and anaerobic) in comparison to Atridox®.
MATERIALS AND METHODS
Materials
Secnidazole (SC) was kindly supplied by EPICO, Cairo, Egypt. Doxycycline hydrochloride (DH) was kindly supplied by El-Nile pharmaceutical Company, Cairo, Egypt. Poly (d,l-lactide) (PLA; Mw = 75,000–120,000) and poly (d,l-lactide-co-glycolide) (PLGA; lactide/glycolide ratio is 50:50, Mw = 40,000–75,000), N-methyl-2-pyrrolidone (NMP) were purchased from Sigma Chemical Co., USA. Paper points were purchased from El Gomhorya, Cairo, Egypt, gas packs were purchased from Basingstoke, England. All other used materials were of analytical grades. Atridox® (doxycycline hyclate, 10% in the Atrigel® Delivery System; Atrix Laboratories) (13).
Methods
Preparation of PLA and PLGA In Situ Implants
The calculated amount of either PLA or PLGA to prepare 25% w/w and 35% w/w of each polymer were dissolved in N-methyl-2-pyrrolidone (NMP) by horizontal shaking of filled glass vials (20 ml) at 37°C, then 5% w/w of secnidazole and/or doxycycline hydrochloride was added to the formed solution and mixed. Solutions were stored overnight in refrigerator prior to experimentations (14). All preparations were evaluated freshly prepared to avoid the epimerization of DH and loss of activity (15).
Rheological Measurements and Data Analysis
Steady shear measurement was conducted where the rheograms of the prepared solutions was performed at 25 ± 0.1°C using cone and plate programmable viscometer (Brookfield Engineering Laboratories Inc., Model HADV-II, USA) connected to a digital thermostatically controlled circulating water bath (Polyscience, Model 9101, USA) with spindle 52, the shear rates range from 50 to 400 s−1 corresponding to 25 to 200 rpm with 10 s between each two successive speeds and then in a descending order. Equilibration of the sample for 5 min was made following loading of the viscometer. Ramp time for each viscosity stage was reading after 20 s. All studies were performed in triplicates and the average was taken.
Rheological data was fitted to different models (Bingham, Power law, Casson) to examine the pattern of flow and the presence of yield value (16).• Bingham:
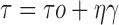 |
1 |
• Power law:
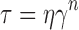 |
2 |
• Casson:
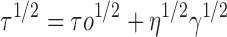 |
3 |
Where τ is the shear stress, τ0 the yield value, η a constant called the apparent viscosity or the consistency index, γ the shear rate and n is the flow index. In case of Newtonian behavior n = 1 and τo = 0, whereas in case of pseudoplastic (shear thinning) behavior 0 < n < 1 and τo = 0; for plastic behavior it is the same as pseudoplastic but with τo > 0, while in case of dilatant flow (shear thickening) n > 1. The software employed was Graph Pad Prism® version 4 and the level of significance was set at 5%.
In Vitro Drug Release from PLA and PLGA In Situ Implants
The in vitro release was performed using shaking water bath (Köttermann, Germany), in which 0.5 g of the prepared solutions were placed in 20-ml vials, then 10 ml phosphate buffer pH 6.8 (to simulate the gingival crevicular fluid) (15) was added thereafter. The sample vials were shaken in sealing condition to avoid partial evaporation of the fluids at 37°C ± 0.1°C with a constant agitation (100 oscillation/min) (14).
One-milliliter samples were withdrawn and replaced with equal volumes of fresh buffer.
UV Measurements of SC and DH
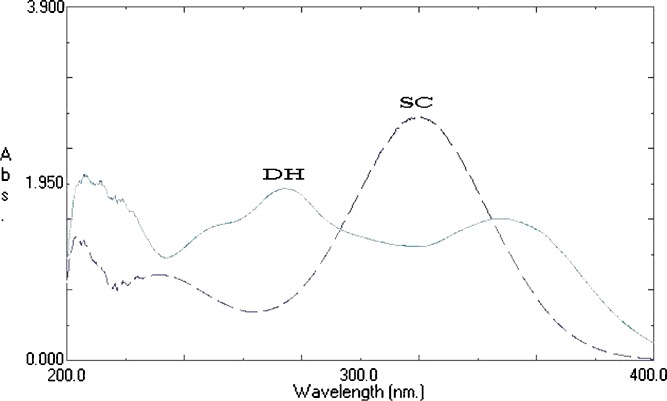
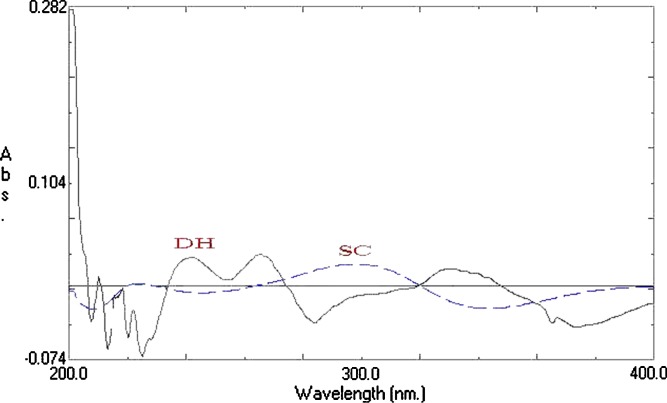
Samples were diluted and measured spectrophotometrically (Shimadzu UV-1601 PC, double beam spectrophotometer, Kyoto, Japan) at λmax = 320 nm for SC and 274 nm for DH (when each drug used alone), Fig. 1. For implants containing the two drugs together, first derivative spectra was constructed, Fig. 2, in which SC can be measured at 350 nm and DH can be measured at 264 nm.
Fig. 1.
Ultraviolet spectra of SC and DH in phosphate buffer (pH 6.8)
Fig. 2.
First derivative spectra of SC and DH in phosphate buffer (pH 6.8)
Drugs Release Data Analysis
Drug release data generated from the release study were fitted to the following general release equation (17) using logarithmic transformations and least squares regression analysis:
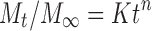 |
4 |
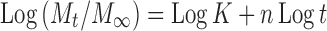 |
5 |
Where Mt/M∞ is the fraction of released drug at time t; k the release constant incorporating structural and geometrical characteristics of the delivery system; n is the release exponent, a measure of the primary mechanism of drug release.
Microbiological Evaluation of the Selected Formulations
Patient’s specimen
This study included 20 patients suffering from periodontal diseases were randomly chosen from the flow of the Outpatient Diagnosis Clinic, Department of Oral Medicine, Diagnosis and Periodontology, Faculty of Dentistry, Ain Shams University. After removing of supragingival plaque, two paper points were inserted into a periodontal pocket for 10 s. The first paper point was inoculated on peptone water as transport medium for aerobes and the second paper point was inoculated on Robertson cooked meat broth, as transport medium for anaerobes (18).
Isolation and Identification of The Microorganisms
The samples were cultured aerobically from peptone water on Colombia blood agar plate and cultured anaerobically on Colombia blood agar plate with added kanamycin at concentration of 75 μg/ml (Kanamycin is selective for obligate anaerobes). The first plate was incubated aerobically at 37°C for 24 h while the second plate was incubated anaerobically in anaerobic jar at 37°C for 2–4 days. Identification of isolated colonies was done using Gram stain, morphology, UV lamp and biochemical reactions (19).
Antimicrobial Activity of the Selected Formulations of SC and DH on the Isolated Micro-organisms
Inoculums of 1.5 ml of a suspension containing each identified isolated organism was transferred to sterile Petri dish, then 30 ml of Mueller Hinton agar was added to the inoculums and mixed well and left to solidify (20). Wells were done by punching stainless steel cylinders (internal diameter 11 mm) onto the plate and removing the agar to form a well. Each well was aseptically filled with accurate weight of the selected tested formulation. For aerobic organisms; the Mueller Hinton plates were incubated aerobically at 37°C for 24 h. For anaerobic organisms the plates were incubated anaerobically in anaerobic jar at 37°C for 24 h. After incubation, the inhibition zone diameter was measured using a ruler. The extent of release (zone of inhibition) was measured by taking the average of three readings and compared with the inhibition zone formed by the global product Atridox®. Results were analyzed using one-way ANOVA followed by post hoc statistical analysis performed by Tukey–Kramer test for multiple comparisons. The software employed was Graph Pad Instat® V2.04 and the level of significance was set at 5%.
RESULTS AND DISCUSSION
Rheological Measurements and Data Analysis
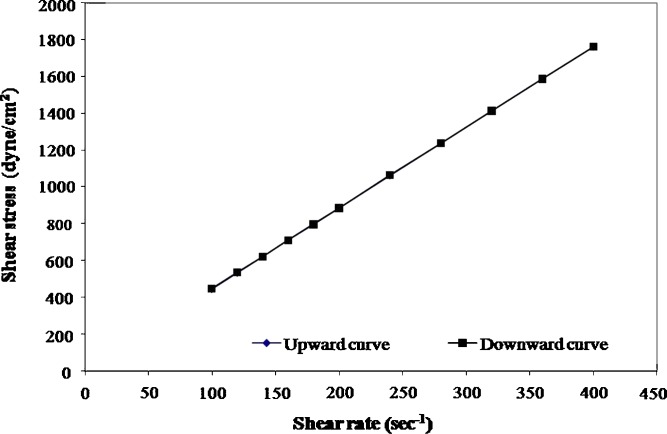
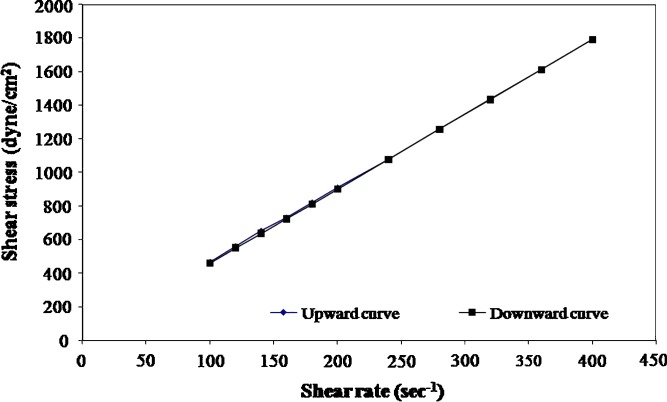
The rheological parameters of the prepared formulations, namely, the flow index (n) the consistency index (η) and the apparent viscosity measured at shear rate 50 s−1 at 25°C are shown in Table I. The rheological data of the different formulations are best fitted to power law model. Based on the values of n, it is obvious that all the formulations exhibit nearly Newtonian behavior except 35% PLA solutions which have n values in the range of 0.8, indicating that 35% PLA solutions have a shear thinning (pseudoplastic) behavior. All formulations show a non-thixotropic behavior where the up and down curves coincide [examples of rheograms are shown in Figs. 3 and 4].
Table I.
The Rheological Parameters of Different Prepared Formulations
| Formula | Drug | Flow index (n) | Consistency index (K) | Regression coefficient (r 2) | Viscosity (P)a | |
|---|---|---|---|---|---|---|
| PLA | 25% | SC | 0.997 | 7.4958 | 0.9999 | 743 ± 34.80 |
| DH | 0.992 | 7.5038 | 1 | 721 ± 31.30 | ||
| SC + DH | 0.995 | 7.4994 | 1 | 730 ± 23.45 | ||
| 35% | SC | 0.811 | 55.116 | 0.9842 | 1940 ± 70.50 | |
| DH | 0.818 | 55.717 | 0.9855 | 2044 ± 55.20 | ||
| SC + DH | 0.815 | 55.408 | 0.989 | 1850 ± 40.54 | ||
| PLGA | 25% | SC | 0.992 | 4.6207 | 1 | 442 ± 16.80 |
| DH | 0.965 | 5.4837 | 0.9998 | 455 ± 21.70 | ||
| SC + DH | 0.978 | 5.0401 | 1 | 445 ± 18.76 | ||
| 35% | SC | 0.985 | 5.8748 | 1 | 542 ± 34.50 | |
| DH | 0.990 | 5.3376 | 1 | 507 ± 23.60 | ||
| SC + DH | 0.987 | 5.6055 | 1 | 530 ± 21.78 |
aAverage of three determination ± standard deviation, measured at shear rate 50 s−1 at 25°C
Fig. 3.
Flow curves of 25% PLGA solution containing SC at 25°C
Fig. 4.
Flow curves of 25% PLGA solution containing DH at 25°C
Increasing the concentration of PLA or PLGA polymer increases the viscosity of the solutions. However, solutions based on 35% PLA show the highest viscosity followed by 25% PLA, while 35% PLGA and 25% PLGA solutions have lower viscosity values. It is revealed that the incorporation of each drug or both together causes no change in the rheological properties of the prepared solutions.
In Vitro Release of SC or DH from PLA and PLGA In Situ Implants
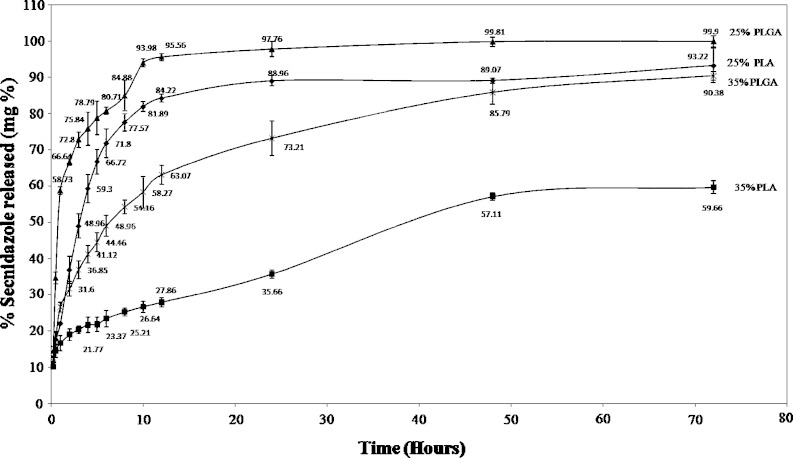
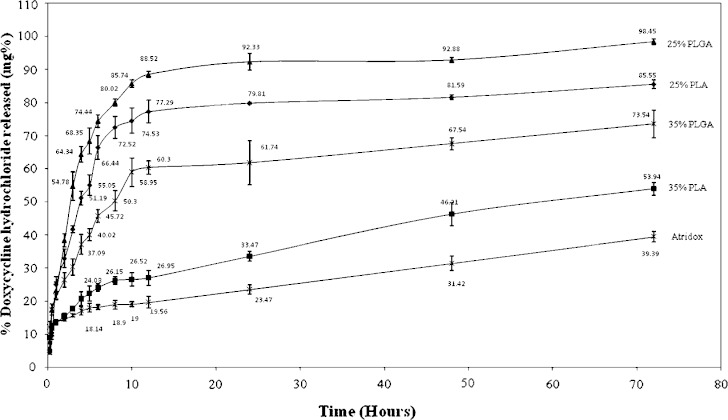
The release of SC or DH from different formulations and Atridox® (25% PLA, 35% PLA, 25% PLGA and 35% PLGA) is illustrated in Figs. 5 and 6 respectively. All preparations show an initial burst effect after 15 min, which ranges from 10–15% of the drug for SC when used alone and from 4–12% for DH when used alone, while Atridox® shows a burst effect of 8% of DH. Same initial burst effect are observed upon the combination of the two drugs in which all in situ implants show an initial burst effect which ranges from 8–18% of the total percent drug released (figures not shown). In situ implants based on 25% PLGA containing SC or/and DH show a continuous fast rate of release, followed by 25% PLA implants with a slower release rate and same release profile. In situ implants based on 35% PLGA show slower rate of release, finally, in situ implants based on 35% PLA show the slowest rate of release followed by Atridox®.
Fig. 5.
In vitro release of SC from different concentrations of PLA and PLGA in situ implants
Fig. 6.
In vitro release of DH from different concentrations of PLA and PLGA in situ implants
An initial burst effect was observed by other workers as Schmidt et al. (21) and Yoo et al. (22). High release rates at the beginning of an antibiotic therapy are very important because the efficiency of antibiotics often depends on high drug concentrations at the beginning of a therapy at the site of infection (21,22). The initial drug release from conventional biodegradable systems is normally attributed to the surface-associated drug (23–25), which was in a good agreement of Lin et al. (26).
Increasing the concentration of the polymer decreases the rate of release of SC or DH, Figs. 5 and 6. This may be attributed to the nature of system, which is a solution of water insoluble polymer (PLA, PLGA) in biocompatible solvent (NMP). Such system precipitates upon contact with water as NMP diffuses into the dissolution media, which lead to the polymer precipitation forming in situ implant and entrapping the drug, which is released as the polymer degrades. Therefore, it is believed that increasing the concentration of the polymer will increase the time required for degradation of the implant formed, as higher polymer concentration lead to the formation of a dense polymer matrix structure (27).
The release profile shown in Figs. 5 and 6 illustrates that implants formed of PLA have a slower rate of release when compared with others made from PLGA of the same concentration. Results are in a good agreement with previous study (28). PLGA polymers containing 50:50 ratio of lactic and glycolic acids are hydrolyzed much faster than those containing higher proportion of either of the two monomers. Polyglycolide acid is highly crystalline because it lacks the methyl side groups of the PLA, however, when it was prepared with D, L PLA it became amorphous so both PLA and PLGA are amorphous but PGA is more hydrophilic so it absorbs more water and degrade more quickly (29). Similar finding was obtained from Mundargi et al. (30), where the authors revealed that doxycycline release rate decreased as the ratio of PLGA decreased.
Previous studies have shown that the physico-chemical properties of the incorporated drug in the polymer matrix are important factors in polymer degradation and drug release (31,32). SC is a water soluble drug having the properties of weak base, while DH is an acidic drug. However no significant difference has been observed in the percent of drug released from the formulations containing each drug, as revealed in Table II, which shows the cumulative percent drug released after 24 h. Also there are no significant differences in the percent released after 24 h when each drug was used separately or combined with the other drug upon statistical analyses at p < 0.05.
Table II.
The Cumulative Drug Percent Released After 24 h and the Values of the Kinetic Release Parameters for Different In Situ Implants
| Formula | Drug | Cumulative % drug released after 24 h | Release exponent (n) | Kinetic constant | Correlation coefficient |
|---|---|---|---|---|---|
| 25% PLA + SC | SC | 88.96 ± 1.41 | 0.5929 | 25.14 | 0.9907 |
| 25% PLA + DH | DH | 79.81 ± 0.26 | 0.8087 | 22.52 | 0.9638 |
| 35% PLA + SC | SC | 35.66 ± 1.10 | 0.2889 | 15.15 | 0.9503 |
| 35% PLA + DH | DH | 33.47 ± 1.51 | 0.3065 | 13.46 | 0.9887 |
| 25% PLGA+ SC | SC | 97.76 ± 2.10 | 0.7373 | 48.31 | 0.9039 |
| 25% PLGA + DH | DH | 92.33 ± 2.51 | 0.8066 | 22.52 | 0.9638 |
| 35% PLGA+ SC | SC | 73.21 ± 4.75 | 0.4021 | 23.62 | 0.9918 |
| 35% PLGA+ DH | DH | 61.74 ± 6.63 | 0.4091 | 21.35 | 0.9890 |
| 25% PLA + SC + DH | SC | 86.34 ± 2.99 | 0.6864 | 23.60 | 0.9830 |
| DH | 85.59 ± 2.34 | 0.6901 | 24.55 | 0.9787 | |
| 35% PLA + SC + DH | SC | 43.60 ± 3.61 | 0.3411 | 15.97 | 0.9748 |
| DH | 45.32 ± 3.12 | 0.3459 | 15.59 | 0.9797 | |
| 25% PLGA + SC + DH | SC | 98.76 ± 3.45 | 0.6870 | 39.21 | 0.9290 |
| DH | 95.65 ± 0.76 | 0.5249 | 42.82 | 0.8817 | |
| 35% PLGA + SC + DH | SC | 67.98 ± 0.86 | 0.3347 | 25.17 | 0.9926 |
| DH | 61.84 ± 0.68 | 0.3186 | 24.96 | 0.9685 | |
| Atridox® | DH | 23.47 ± 1.43 | 0.2350 | 12.19 | 0.9496 |
SC secnidazole, DH doxycycline hydrochloride
Table II shows the release kinetics of SC or DH from different PLA and PLGA in situ implants when each drug used separately or combined together. Implants based on 25% PLA and 25% PLGA containing SC or/and DH show (n) values > 0.5 indicating that the mechanism of release is anomalous diffusion (non-Fickian), while implants based on 35% PLA and 35% PLGA show (n) values of <0.5 which indicate that the mechanism of release also is non-Fickian diffusion, nevertheless these low values of (n) indicate a situation where drug release occur by means of diffusion, partially through a swollen matrix and partially through water filled spaces (17).
Microbiological Evaluation of the Selected Formulations
Identification of the Microorganisms
Table III shows the number and the percent of isolated microorganisms from the mouth of 20 patients suffering from periodontal diseases. It is revealed that anaerobic microorganisms represent 78.57% of the total isolated organisms from different periodontal infections in which Bacteroid species represent 50% of these anaerobes. This is in agreement with Tomazinho and Avila-Campos (33), who reported that Bacteroid species as anaerobic bacteria are predominant components of the bacterial florae of mucous membranes and, therefore Bacteroid species are a common cause of endogenous infections.
Table III.
The Microorganisms Isolated from 20 Patients Suffering from Periodontal Diseases and the Antimicrobial Susceptibility of Different Microorganisms to SC and DH
| Category | Numbera | Percenta | Organism | Numberb | Percentb | % Antimicrobial susceptibility | |
|---|---|---|---|---|---|---|---|
| SC | DH | ||||||
| Anaerobic Gram negative bacteria | 7 | 50 | Porphyromonas | 4 | 28.57 | 95 | 70 |
| Prevotella | 2 | 14.28 | 100 | 75 | |||
| Fusobacteria | 1 | 7.14 | 100 | Zero | |||
| Anaerobic Gram positive bacteria | 4 | 28.57 | Peptostreptococci | 4 | 28.57 | 70–100 | 25 |
| Total anaerobes | 11 | 78.57 | – | – | – | 70–100 | 25–75 |
| Aerobic bacteria | 3 | 21.43 | Streptococci | 3 | 21.43 | Zero | 100 |
| Total bacteria | 14 | 100 | – | 14 | 100 | – | – |
SC secnidazole, DH doxycycline hydrochloride
aNumber and percent of each category
bNumber and percent of each organism
From Table III, it is revealed that black-pigmented anaerobic rods such as Prevotella and Porphyromonas are involved in the etiology and perpetuation of endodontic infections. Of a total of 14 isolates from periodontal infections 28.57% have Porphyromonas, 14.28% have Prevotella, 7.14% have Fusobacteria, 28.57% have Peptostreptococci and 21.43% have Streptococci. Van Winkelhoff et al. (34), revealed that of 261 isolates from patients with periodontal infections 35% of cases had Prevotella, 37.5% had Fusobacteria and 65% had Streptococci. This difference in the percentage of isolates may be due to small number of the studied cases.
Antimicrobial Activity of the Selected Formulations of SC and DH on the Isolated Micro-organisms
The selected formulations were then tested for the antimicrobial activity against the isolated microbes using cup diffusion method, Table III. Results reveal that anaerobic Gram negative bacteria are highly sensitive to SC in which 95–100% of the inoculums are inhibited by SC, while Gram positive cocci (Peptostreptococci) show only 70% sensitivity to SC. These results agree with Behra-Miellet et al. (35). revealing that all strains of Gram negative anaerobes were inhibited by metronidazole while strains of peptostreptococci acquired resistance to metronidazole, while as expected SC shows no activity against aerobic bacteria.
Studying the susceptibility of different organisms to DH reveals that aerobic Gram positive cocci are highly sensitive to DH (100%), while anaerobic bacteria show some resistance, in which 25%, 70%, and 75% of Peptostreptococci, Porphyromonas and Prevotella respectively show sensitivity to DH, while Fusobacteria are resistant to it, Table III.
Table IV shows the antimicrobial activity of SC and DH (expressed as inhibition zone diameter) from the tested 25% PLGA in situ implants in comparison to the release of DH from Atridox®. Statistical analyses of the results at p < 0.05 showed significant differences among the tested implants and Atridox®. As shown, implants based on 25% PLGA show a greater zone of inhibition than Atridox® and this appears in agreement with the release profile, also addition of SC to DH did not result in change in the extent of inhibition zones of when DH used alone. The pharmaceutical formulation based on 25% PLGA containing secnidazole and doxycycline hydrochloride has promising activity in treating periodontitis in comparison with Atridox®.
Table IV.
Inhibition Zones Formed by the Antibacterial Activity of SC and/or DH in 25% PLGA In Situ Implants Using Isolated Anaerobes and Streptococci as Test Organisms
| Formula | Zone of inhibition (cm) mean ± SD | |
|---|---|---|
| Anaerobes | Streptococci | |
| 25% PLGA + SC | 2.03 ± 0.057 | – |
| 25% PLGA + DH | 2.11 ± 0.05 | 2.09 ± 0.015 |
| 25% PLGA + SC +DH | 2.07 ± 0.015 | 2.10 ± 0.05 |
| Atridox® | 1.93 ± 0.057 | 1.97 ± 0.057 |
CONCLUSION
In conclusion, this study has shown that the combination of secnidazole and doxycycline hydrochloride results in no change in the flow pattern or viscosities of the in situ implants, also no change in the percent of the two drugs released from different poly (lactide) and poly (lactide-co-glycolide) implants after 24 h. Both drugs can be formulated in one drug delivery system to increase the spectrum of the antimicrobial activity against the microorganisms causing periodontal diseases and to decrease the therapy cost. Among formulations tested, 25% PLGA in situ implants containing secnidazole and/or doxycycline hydrochloride show the fastest rate of release with a promising antimicrobial activity against aerobic and anaerobic bacteria.
Acknowledgement
The authors thank Dr. Kawthar El-Gindy, Department of Bacteriology, Faculty of Medicine, Ain Shams University, for her great help in the microbiological part.
References
- 1.Kinane D. F., Lindhe J. Pathogenesis of periodontitis. In: Lindhe J., Karring T., Lang N. P., editors. Clinical Periodontology and Implant Dentistry. Copenhagen: Munksgaard; 1998. pp. 189–225. [Google Scholar]
- 2.Schwach-Abdellaoui K., Vivien-Castioni N., Gurny R. Local delivery of antimicrobial agents for the treatment of periodontal diseases. Eur. J. Pharm. Biopharm. 2000;50:83–99. doi: 10.1016/S0939-6411(00)00086-2. [DOI] [PubMed] [Google Scholar]
- 3.Goodson J. M. Antimicrobial strategies for treatment of the periodontal diseases. Periodontology 2000. 1994;5:142–168. doi: 10.1111/j.1600-0757.1994.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal K. R., Robison H. D., Maze I. G., Reinhardt A. R. Development and characterization of tetracycline-poly (lactide/glycolide) films for the treatment of periodontitis. J. Control. Release. 1993;23:137–146. doi: 10.1016/0168-3659(93)90039-8. [DOI] [Google Scholar]
- 5.Esposito P., Cortesi R., Cervellati F., Menegatti E., Nastruzzi C. Biodegradable microparticles for sustained delivery of tetracycline to the periodontal pocket: formulatory and drug release studies. J. Microencapsul. 1997;14:175–187. doi: 10.3109/02652049709015331. [DOI] [PubMed] [Google Scholar]
- 6.Jain J. P., Modi S., Domb A. J., Kumar N. Role of polyanhydrides as localized drug carriers. J. Control. Release. 2005;103:541–563. doi: 10.1016/j.jconrel.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 7.Wang L., Venkatraman S., Kleiner L. Drug release from injectable depots: two different in vitro mechanisms. J. Control Rel. 2004;99:207–216. doi: 10.1016/j.jconrel.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Pähkla E. R., Koppel T., Saag M., Pa hkla R. Metronidazole concentrations in plasma, saliva and periodontal pockets in patients with periodontitis. J. Clin. Peridontol. 2005;32:163–166. doi: 10.1111/j.1600-051X.2005.00653.x. [DOI] [PubMed] [Google Scholar]
- 9.Friesen L. R., Williams K. B., Krause L. S., Killoy W. J. Controlled local delivery of tetracyclines with polymer strips in the treatment of periodontitis. J. Periodontol. 2002;73:13–19. doi: 10.1902/jop.2002.73.1.13. [DOI] [PubMed] [Google Scholar]
- 10.Walker C. B., Godowski K. C., Borden L., et al. The effects of sustained release doxycycline on the anaerobic flora and antibiotic-resistant patterns in subgingival plaque and saliva. J. Periodontol. 2000;71:768–774. doi: 10.1902/jop.2000.71.5.768. [DOI] [PubMed] [Google Scholar]
- 11.Sorsa T., Ding Y. L., Ongmam T., et al. Cellular source, activation and inhibition of dental plaque collagenase. J. Clin. Periodontol. 1995;22:709–711. doi: 10.1111/j.1600-051X.1995.tb00831.x. [DOI] [PubMed] [Google Scholar]
- 12.Gillis J. C., Wiseman L. R. Secnidazole: a review of its antimicrobial activity, pharmacokinetic properties and therapeutic use in the management of protozoal infections and bacterial vaginosis. Drugs. 1996;51:621–638. doi: 10.2165/00003495-199651060-00003. [DOI] [PubMed] [Google Scholar]
- 13.Atridox® (Doxycycline Hyclate) 10%. Collagenex Pharmaceuticals Products Website. Available at http://www.collagenex.com/pr_atridox.asp. Accessed December 4, 2007.
- 14.Luan X., Bodmeier R. Influence of the poly(lactide-co-glycolide) type on the leuprolide release from in situ forming microparticle systems. J. Control. Release. 2006;110:266–272. doi: 10.1016/j.jconrel.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Esposito P., Carotta V., Scabbia A., et al. Comparative analysis of tetracycline containing dental gels: poloxamer- and monoglyceride-based formulations. Int. J. Pharm. 1996;142:9–23. doi: 10.1016/0378-5173(96)04649-2. [DOI] [Google Scholar]
- 16.Abdel-Hamid S. M., Abdel-Hady S. E., El-Shamy A. A., El-Dessouky H. F. Formulation of an antispasmodic drug as a topical local anesthetic. Int. J. Pharm. 2006;326:107–118. doi: 10.1016/j.ijpharm.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 17.Peppas N. A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985;60:110–111. [PubMed] [Google Scholar]
- 18.Eick S., Pfister W., Straube E. Antimicrobial susceptibility of anaerobic and capnophilic bacteria isolated from odontogenic abscesses and rapidly progressive periodontitis. Int. J. Antimicrob. Agents. 1999;12:41–46. doi: 10.1016/S0924-8579(99)00056-4. [DOI] [PubMed] [Google Scholar]
- 19.Kinci I., Senel S., Akıncıbay H., Kas S., Ercis S., Wilson C. G., Hıncal A. A. Effect of chitosan on a periodontal pathogen Porphyromonas gingivalis G. Int. J. Pharm. 2002;235:121–127. doi: 10.1016/S0378-5173(01)00974-7. [DOI] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. Approved Standard, 7th edition (M02-A7). Villanova, PA; NCCLS, 2002.
- 21.Schmidt C., Wenz R., Nies B., Moll F. Antibiotic in vivo/in vitro release, histocompatibility and biodegradation of gentamicin implants based on lactic acid polymers and copolymers. J. Control. Release. 1995;37:83–94. doi: 10.1016/0168-3659(95)00067-I. [DOI] [Google Scholar]
- 22.Yoo J. Y., Kim J. M., Khanga G., Kim M. S., Cho S. H., Lee H. B., Kim Y. S. Effect of lactide/glycolide monomers on release behaviors of gentamicin sulfate-loaded PLGA discs. Int. J. Pharm. 2004;276:1–9. doi: 10.1016/j.ijpharm.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 23.Wang J., Wang B. M., Schwendeman S. P. Characterization of the initial burst release of a model peptide from poly (d,l-lactide-co-glycolide) microspheres. J. Control. Release. 2002;82:289–307. doi: 10.1016/S0168-3659(02)00137-2. [DOI] [PubMed] [Google Scholar]
- 24.K. J. Brodbeck, A. T. Gaynor-Duarte, and T. T. Shen. Gel compositions and methods, US patent 6 130 200, October 10, 2000.
- 25.Graham P. D., Brodbeck K. J., Mchugh A. J. Phase inversion dynamics of PLGA solutions related to drug delivery. J. Control. Release. 1999;58:233–245. doi: 10.1016/S0168-3659(98)00158-8. [DOI] [PubMed] [Google Scholar]
- 26.Lin D. M., Kalachandra S., Valiyaparambil J., Offenbacher S. A polymeric device for delivery of anti-microbial and anti-fungal drugs in the oral environment: effect of temperature and medium on the rate of drug release. Dent. Mater. 2003;19:589–596. doi: 10.1016/S0109-5641(02)00109-4. [DOI] [PubMed] [Google Scholar]
- 27.Cui F., Cun D., Tao A., Yang M., Shi K., Zhao M., Guan Y. Preparation and characterization of melittin-loaded poly (dl-lactic acid) or poly (dl-lactic-co-glycolic acid) microspheres made by the double emulsion method. J. Control. Release. 2005;107:310–319. doi: 10.1016/j.jconrel.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Piñón-Segundo E., Ganem-Quintanar A., Alonso-Pérez V., Quintanar-Guerrero D. Preparation and characterization of triclosan nanoparticles for periodontal treatment. Int. J. Pharm. 2005;294:217–232. doi: 10.1016/j.ijpharm.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Miyajima M., Koshika A., Okada J., Kusai A., Ikeda M. The effects of drug physico-chemical properties on release from copoly (lactic:glycolic acid) matrix. Int. J. Pharm. 1998;169:255–263. doi: 10.1016/S0378-5173(98)00133-1. [DOI] [Google Scholar]
- 30.Mundargi R. C., Srirangarajan S., Agnihotri S. A., Patil S. A., Ravindra S., Setty S. B., Aminabhavi T. M. Development and evaluation of novel biodegradable microspheres based on poly(D,L-lactide-co-glycolide) and poly(ε-caprolactone) for controlled delivery of doxycycline in the treatment of human periodontal pocket: in vitro and in vivo studies. J. Control. Release. 2007;119:59–68. doi: 10.1016/j.jconrel.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Bodmeier R., Chen H. Evaluation of biodegradable poly (lactide) pellets prepared by direct compression. J. Pharm. Sci. 1991;78:819–822. doi: 10.1002/jps.2600781008. [DOI] [PubMed] [Google Scholar]
- 32.Takada S., Kurokawa T., Miyazaki K., Iwasa S., Ogawa Y. Sustained release of a water-soluble GP IIb:IIIa antagonist from copoly (dl-lactic:glycolic) acid microspheres. Int. J. Pharm. 1997;146:147–157. doi: 10.1016/S0378-5173(96)04780-1. [DOI] [Google Scholar]
- 33.Tomazinho L. F., Avila-Campos M. J. Detection of Porphyromonas gingivalis, Porphyromonas endodontalis, Prevotella intermedia, and Prevotella nigrescens in chronic endodontic infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(2):285–288. doi: 10.1016/j.tripleo.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Van Winkelhoff A. J., Herrera D., Oteo A., Sanz M. Antimicrobial profiles of periodontal pathogens isolated from periodontitis patients in The Netherlands and Spain. J. Clin. Periodontol. 2005;32(8):891–892. doi: 10.1111/j.1600-051X.2005.00782.x. [DOI] [PubMed] [Google Scholar]
- 35.Behra-Miellet J., Calvet L., Dubreuil L. Activity of linezolid against anaerobic bacteria. Int. J. Antimicrob. Agents. 2003;22:28–34. doi: 10.1016/S0924-8579(03)00087-6. [DOI] [PubMed] [Google Scholar]