Abstract
The objective of the study was to investigate in vitro transdermal delivery of venlafaxine hydrochloride across the pigskin by passive diffusion and iontophoresis. For passive diffusion, experiments were carried out in Franz diffusion cell whereas for iontophoretic permeation, the diffusion cell was modified to contain both the donor and return electrode on the same side of skin. Anodal iontophoresis was carried out using a current density of 0.5 mA/cm2. Donor concentrations used were 585.5 mg/ml (saturated solution) and 100 mg/ml. Experiments initially performed to determine the transport efficiency of venlafaxine ions showed promising results. Iontophoresis increased the permeation rate at both concentration levels over their passive counterparts (P < 0.01), but surprisingly higher steady-state flux was obtained from lower donor drug load (P < 0.01). The favorable pH of the unsaturated solutions is suggested to be the cause for this effect. Mild synergistic effect was observed when iontophoresis was carried out incorporating peppermint oil in the donor but the same was not found in passive diffusion. Highest steady-state flux obtained in the experiment was 3.279 μmol/cm2/h when peppermint oil (0.1%) was included in the donor. As the maintenance requirement of venlafaxine hydrochloride is approximately 9.956 μmol/h, the results suggested that the drug is a promising candidate for iontophoretic delivery.
Key words: iontophoresis, menthol, peppermint oil, transdermal, venlafaxine hydrochloride
INTRODUCTION
Depression is the most common of all major mental illnesses. The lifetime probability of developing an episode of major depression is around 17% (1). Depression is also associated with a high mortality rate as a large fraction of the depressive patients suffer from suicidal tendency. Approximately 15% of all hospitalized depressed patients commit suicide, which unfortunately does not show any tendency of decline (2). Medication noncompliance is a major factor resulting in failure of antidepressant therapy (3). Efficacy of anti-depressant medicines relies upon their continued presence at the site of action over a prolonged period of time while programmed release capability of the conventional orals and parenterals is limited. The saw-tooth pattern of plasma drug concentrations following oral drug administration often causes adverse events at maxima and loss of therapeutic effect at minima, which leads to intolerability in case of many antidepressants (4).
Oral administration, though the most accepted route of drug delivery, is thought to be non-ideal for most anti-depressant medications and there is a recent focus on alternative drug delivery for optimizing the ideal antidepressant treatment. As transdermal administration avoids the first pass metabolism and offers steady controlled permeation, it has already been investigated for the delivery of certain tricyclic antidepressants (5). Efforts have already been undertaken for the development of transdermal systems of imipramine and amitriptyline (6). Selegiline has recently been marketed and the first reported clinical trial of this transdermally delivered antidepressant has established its superiority over its oral counterparts (7).
Venlafaxine hydrochlorde (VH) is a first line drug in the treatment of depression. It inhibits central serotonin and norepinephrine neuronal reuptake and has proven efficacy in the treatment of depression and anxiety disorders (8). The drug is effective in the treatment resistant depression and thought to be superior to selective serotonin reuptake inhibitors in preventing the recurrence of depression (9,10). Two recent small, placebo-controlled trials on extended release VH had proved that it also has analgesic efficacy against neuropathic pain as well as premenstrual dysphoric disorders (11,14). However, a drug of low half-life (4.9 h), VH, needs frequent administration to maintain a blood level of effective therapeutic concentration (15). After oral administration, it undergoes extensive metabolism in liver and gets converted into the active metabolite, O-desmethyl venlafaxine (16). The oral use of VH is associated with a number of predictable adverse effects like tachycardia, increased blood pressure, fatigue, headache, dizziness, sexual dysfunction, and dry mouth etc. (17). Dose dependant elevation of creatinine kinase has also been reported in the patients suffering from VH toxicity. High prevalence of acute muscle injury or rhabdomyolysis was observed with VH overdose too (8). Statistical data generated from the usage pattern of the drug had revealed a potential risk factor of fatal overdose suggesting the need for cautioning patients with suicidal ideation (18). Hence to control the plasma concentration within an acceptable range, several companies have launched the extended release oral formulations. VH is also mentioned in a number of transdermal patents (US patent no. US2004101551, European Patent no. EP1313457).
Recently iontophoresis, a physical enhancement technique is getting investigated for the transdermal delivery of drug molecules. Iontophoresis refers to the facilitated movement of ions of soluble salts through application of an electrical field. It has an advantage over passive diffusion of being able to increase the delivery of agents across the dermal barrier by 3 to 4 orders of magnitude (3).
Iontophoresis achieves enhanced transport of solutes by three additional mechanisms besides the pure diffusion or the ‘passive’ transdermal delivery. First, the ion-electric field interaction provides an additional force which drives ions through the skin; secondly the electrical flow increases permeability through the skin; thirdly the electroosmosis produces bulk motion of the solvent that carries ionic or neutral solutes with the solvent stream (19). The objective of the present study was to investigate the potential of VH for iontophoretic delivery.
MATERIALS AND METHODS
Materials
Iontophoretic direct current source (model no. PSU 2510/Lab, digital display, current 0–10 mA, voltage 0–25 V) was purchased from C-Tech Mumbai, India. For both passive and iontophoretic permeation, modified Franz diffusion cell was purchased from Neutron Scientific Ltd., Calcutta, India. Magnetic water bath for stirring was obtained from Spectralabs, Mumbai, India. Silver/silver chloride electrodes were prepared in the laboratory as per the standard procedure (20). Silver rods (99.99% pure, 1.0 mm thickness) obtained from local jewelers were used as connecting wire. Menthol (99% pure) and peppermint oil (containing 44% menthol) were purchased from S.D. Fine Chemicals, Mumbai, India. All other reagents/chemicals used were of analytical grade. Experiments were conducted with ultra pure water (resistivity 18.2 MΩ cm) obtained from Milli-Q academic system (Millipore Pvt. Ltd., Bangalore, India).
Methods
Solubility Determination
Excess amount of VH was taken into glass vials and dissolved in vehicles (normal saline and water) to get saturated solutions. The solutions were kept at rest for 24 h to assist the attainment of equilibrium with the un-dissolved drug particles. The supernatants were decanted and filtered through filter paper (Whatman no. 42) (21). The filtrates were suitably diluted to measure the concentrations by high performance liquid chromatography (HPLC).
Partition Coefficient
Octanol and water were mutually saturated by shaking in a separating funnel, allowed to stand for 24 h and separated. Standard solution of the drug was prepared in this pre-saturated water. Octanol (10 ml) was added to equal volume of this standard drug solution in a separating funnel and was kept for 24 h at 37 °C with intermittent shaking. Finally, the water layer was separated, clarified by centrifugation and assayed for drug content by HPLC (22).
pH and pKa determination
pH was determined by using Digisun DI-707 pH meter. A glass electrode combined with a silver–silver chloride reference system was used. The sensor electrode was dipped into the drug solutions and pH was directly recorded from the digital display. pKa was calculated as suggested by Silcocks (23). Solutions were made at different concentration levels and pH was noted. The pKa was determined on the basis of the equation:
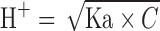 |
Where ‘Ka’ is ionization constant and C is molar concentration of the drug in solution.
Preparation of Skin Membrane
From a local abattoir, ears were obtained from freshly slaughtered pigs. Pig-ear skin is usually favored for assessment of skin permeability as it is very similar to human skin in terms of follicular density. Both the skin types have approximately 11 follicles/cm2 and statistically significant correlation has been reported between the values obtained by in vitro permeation through pigskin and that of human skin in vivo (24). The skin was separated carefully from the underlying cartilage with a scalpel. Fat adhering to the dermal side was removed using isopropyl alcohol and average thickness of the skin was found to be 0.95 mm. Finally the skin was washed with tap water, packed in aluminum foil, stored at −20 °C in refrigerator and used within 2 days (25,26).
Procedure of Passive Permeation
The in vitro passive permeation studies were conducted using vertical type Franz diffusion cell having a receptor compartment capacity of 25 ml (22). The excised skin was mounted between the half-cells with the dermis in contact with receptor fluid (0.9% NaCl) and equilibrated for 1 h. The area available for diffusion was about 1.94 cm2. The donor cell was covered with an aluminum foil to prevent the evaporation of vehicle. The fluid in the receptor compartment was maintained at 37 ± 0.5 °C. Permeation experiments were carried out at two concentration levels, 585.5 mg/ml and 100 mg/ml. For high concentration experiments (585.5 mg/ml), saturated solution of VH (maintained with the traces of un-dissolved drugs in vehicle) was used in the donor whereas low concentration experiments had standard solution of VH in the donor. For all the experiments, donor vehicle was water. However in experiments with peppermint oil and menthol, 1% alcohol was used as co-solvent to facilitate the dissolution of these hydrophobic moieties. The entire assembly was kept on a magnetic stirrer and then 1 ml of the solution was withdrawn from receptor compartment at hourly intervals and replaced with equal volume of receptor fluid. The concentration of VH was determined by HPLC. The cumulative amount of drug permeated was calculated by multiplying the concentration (VH in receiver fluid) with volume of the receptor fluid and added with the correction factor for quantities lost during sampling.
Procedure of Iontophoretic Diffusion Through Pigskin Membrane
The iontophoretic diffusion cell was designed as suggested by Glikfield et al. (25,27). The apparatus consisted of a set of three glass pieces which when attached together took the shape of a vertical type diffusion cell. The receiving chamber was comparatively large (25 ml) having two parallel ports on the top and a sampling port on the side. Two upper ports were actually cylindrical extension of receiving chamber, which acted as support for the donor and return electrode compartments. Once skin attached to the bottom of two small cylindrical glass tubes were slipped into these ports, two separate compartments were created (donor and return electrode chamber) with skin at the base of each. The inner and outer tubes stayed attached by glass joints. Skin tied to both the chambers touched the receptor fluid at the same depth and each chamber housed one electrode (anode and cathode). VH was delivered in the anodal chamber whereas equal volume of water was placed in the cathodal chamber. The receiver fluid was connected to the donor and return electrode chambers through the skin. The assembly was put in a magnetic water bath to maintain the temperature at 37 ± 0.5 °C. Once the power supply was switched on, current flowed from anode to cathode and forces of electromigration and electroosmosis were established. Direct current (0.5 mA cm−2) was used throughout the experiment. The receptor fluids (5 ml) were withdrawn at hourly intervals and replaced with fresh buffer to maintain sink condition. The study was carried out for a period of 8 h. The samples were assayed by HPLC.
Quantification of VH
Hitachi high performance chromatograph with a reversed phase Kromasil 300-4 C-18, 10 μm column (250 × 4 mm internal diameter) equipped with Hitachi L-7110 pump, L-7400 UV detector and Winchrome-99 software was used. For quantification of VH in the receptor fluid, the procedure of Matoga et al. was modified (28). For making standard graph, working standards were prepared in aqueous solutions (5–20 μg/ml) and injected into the column (20 μl). The column was eluted with the mobile phase consisting of phosphate buffer (PB) 50 mM KH2PO4 and acetonitrile (65:35, adjusted to pH 3.5 by orthophosphoric acid) and the detection wavelength was 220 nm. Delivered at a flow rate of 1 ml/min, the retention time recorded for VH was 4.90 min. The plots of peak area versus respective concentration of VH were found to be linear with the correlation coefficient (r2) of 0.9999. Experiments were done in triplicate and the variation in data was less than 1%. Below 1 μg/ml, the drug could be detected but not quantified. During the measurement of standard solutions, peaks were obtained at 1.84 and 4.90 min. The peak at 1.84 min represented solvent as it appeared at the dead volume mark of the column. As the peak at 4.90 min showed proportional increase with drug concentration, it was confirmed to be representative of VH. However in the skin permeation experiments, an extra peak was noted at 2.12 min. To identify the source of this addition, blank experiments were carried out using vehicles of VH in the donor (1% alcohol, 0.1% menthol, and 0.1% peppermint oil). Apart from the solvent peak at 1.84 min, the peak at 2.12 appeared in all the cases, which could only be due to leaching of some indigenous molecules of the skin. Menthol and peppermint oil showed no extra peaks and the peak at 2.12 (for skin ingredients) was sufficiently away from the VH peak at 4.90 min to cause any interference in the quantification of the drug.
Data Analysis
Cumulative amount of the drug permeated was plotted against time, and the slope of the linear portion of the plot was taken to be the steady-state flux. Permeability coefficient was calculated using the following formula:
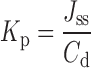 |
Where Kp represents permeability coefficient, Jss steady-state flux, Cd concentration of drug in donor compartment.
Flux enhancement was calculated by dividing iontophoretic steady-state flux with the corresponding passive steady-state flux.
Statistical analysis
Statistical analysis was carried out by one-way analysis of variance (ANOVA) followed by Bonferroni’s test. GraphPad InStat version 3.0 was used for this.
RESULTS AND DISCUSSION
To be successfully delivered through the transdermal route, drug candidates should have adequate skin permeability. Stratum corneum, being a tough membrane, usually allows only small and uncharged molecules to pass through them. Hence physicochemical parameters are of prime importance in the choice of transdermal candidates. Table I lists the physicochemical parameters of VH. Drug candidates for transdermal delivery should have molecular weight around 200–500 Da (29). VH with molecular weight of 313.87 is well within this range. Other parameters, particularly solubility and pKa, which influence the skin permeability, were also found to be suitable. VH, being a hydrochloride salt, has high aqueous solubility, which favored its delivery through aqueous vehicle but its low octanol and water partition coefficient indicated a lesser affinity towards the skin. The experimentally derived pKa value was found to be 9.6, which indicated that the drug was completely ionized at physiological pH. Stratum corneum shows limited permeability towards the ionic species and permeability co-efficients of charged species have been estimated up to 104 times smaller than the uncharged species of same molecular size (30). Hence iontophoresis was thought to be a better option rather than passive diffusion. However for the purpose of comparison, permeation study was carried out by passive diffusion too.
Table I.
Physicochemical Parameters of VH (n = 3)
| Solubility | Partition coefficient (octanol/water) | Molecular weight | pKa | |
|---|---|---|---|---|
| 0.9%NaCl (mg/ml) | Water (mg/ml) | |||
| 572 | 585.5 | 0.525 | 313.87 | 9.6 |
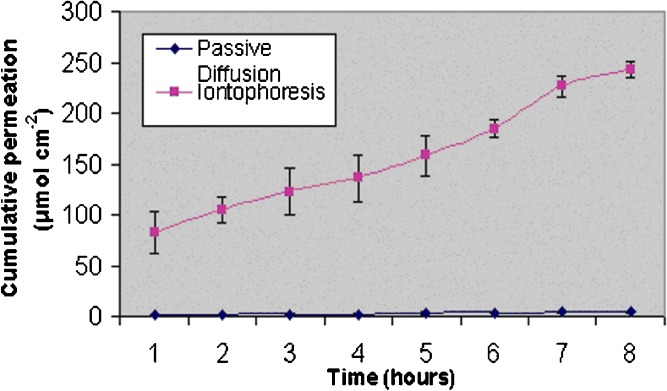
The efficiency of iontophoretic permeation of an ionic species is expressed in terms of transport number. The transport number is the fraction of the total current transported by a specific ion and expresses its efficacy as a charge carrier. To determine this property, constant current iontophoresis (current density 0.5 mA/cm2) was carried out using deionized water in the receiver. To assess the influence of subdermal chloride ion, parallel experiments were conducted using 0.9% NaCl in the receiver too. Figures 1 and 2 depict the permeation profiles of VH in two receiving fluids water and 0.9% NaCl. In both the cases, iontophoresis showed significantly higher permeation rates compared to the corresponding passive diffusion (P < 0.0001). However, the increase was much more pronounced (48.8 fold) when the receiver fluid was deionized water. In contrast, much lesser (3.3 fold) enhancement was found in 0.9% NaCl. According to the Faraday’s law, the transport number (ti) is expressed as:
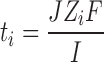 |
Fig. 1.
Cumulative amounts of VH permeated through porcine skin using water as a receiving fluid (donor concentration 585.5 mg/ml; n = 3)
Fig. 2.
Cumulative amounts of VH permeated through porcine skin using 0.9% NaCl as a receiving fluid (donor concentration 585.5 mg/ml; n = 3)
Where J represents flux (mol/s), Zi, the valency, I, current applied (in amperes), F, the Faraday’s constant (coulombs/mol) and ti, the transport number of the permeating ion (31). This indicates that a flow of 0.5 mA/s can theoretically drive a total of 18.6 μmol/h of monovalent ions across an intervening barrier. With water as receiving fluid, flux values obtained were much higher (20.93 μmol/h) indicating a transport efficiency of 112% (ti = 1.125), which is theoretically impossible. However, this apparent anomaly could have resulted from associated factors like electro-osmosis and passive diffusion. The flux of a solute during iontophoresis is the cumulative total of passive, electroosmotic and electro-repulsive contributions (32). Since the drug was mostly in an ionized state, the contribution of the passive diffusion was minimal as was observed in the matching passive experiments. But electroosmotic contribution, a voltage dependent factor, was likely to be considerable. Since deionized water has strong electrical resistance, high voltage (15 ± 1 V) was required to maintain the current. Venlafaxine ions, being positively charged, got the advantage of this voltage-mediated permeation, which resulted in a flux higher than the theoretical maximum of Faraday’s equation. As receiving media reflects body, further studies were carried out using 0.9% NaCl in the receiver. Experiments were performed at two concentration levels, saturated solution (infinite dose) and 100 mg/ml (finite dose). According to the Nernest–Planck electro-diffusion theory, the transport number of a monovalent species M+, present as its chloride salt, when normal saline alone fills subdermal compartment, is given by:
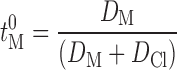 |
Where DM and DCl are the diffusion coefficients of M+ and Cl− in the membrane (31). The concentration of M+ does not appear in the equation as it is assumed that M+ is the only cation in the solution. As VH was delivered as an un-buffered aqueous solution in high concentration, the above condition was approximated and iontophoretic permeation was expected to be independent of concentration. High donor concentration was thought to be beneficial as it helped in vastly outnumbering the intrinsic ions, which were better carriers of electricity by virtue of their smaller size. Surprisingly, the system with lower donor concentration showed higher flux (P < 0.01). This result was found in passive diffusion too, where increased fluxes were recorded at low concentration levels. As concentration gradient was the driving force of mass transfer, this seemed to be a contradictory phenomenon. However, favorable pH conditions of the donor might have been responsible for this. According to the hypothesis of Imanidis and Luetolf, the stratum corneum can be compared as a composite model having distinct aqueous and lipoidal domains and has an isoelectric pH of 4.5 (33). Skin’s permselectivity also depends strongly on the pH of the surrounding media as the overall charge density at these different areas is affected by environmental pH (pH of the donor and receiver system) (34,35). Direction and magnitude of electroosmotic solvent flow is influenced by the surface charge of the barrier membrane and as little as a pH difference of 0.4 units can affect this charge distribution and influence the permeation kinetics (33), Higher flux in pH 7.4 than in pH 4 was reported in case of lysine which was attributed to the neutralization of skin (19). It is also suggested that as pH is decreased towards the value of 4, electroosmotic flow decreases, particularly during continued iontophoresis (37).
VH, a salt of weak base and strong acid with pKa value of 9.6, readily dissolved in water and its aqueous solution was acidic. Lower the VH concentration, higher was the pH. The experimentally determined pH of VH solution (aqueous) were found to be 4.85, 5.63, 5.75 and 6.29 when the concentrations were 585.5, 200, 100 and 50 mg/ml, respectively. Above the pH value of 4.5, the skin carries an average negative charge. Higher the pH, more is the negative charge per pore, and stronger is the attraction for cationic species. For saturated solution (pH 4.85) too, the advantage of positive electroosmotic flux existed but the benefit was minimal. For low concentration donors (100 mg/ml), the pH was higher (5.75), which increased the negative charge density of skin (36,37). This might have been a significant factor that caused greater attraction for the positive venlafaxine ions towards the skin resulting in higher flux. Moreover, at high solute concentrations, activity becomes low. At the level of saturation, concentration of VH was so high that the solution turned viscous. As high viscosity hinders the movement of ions this might also be a contributing factor in reducing the permeation rate (38).
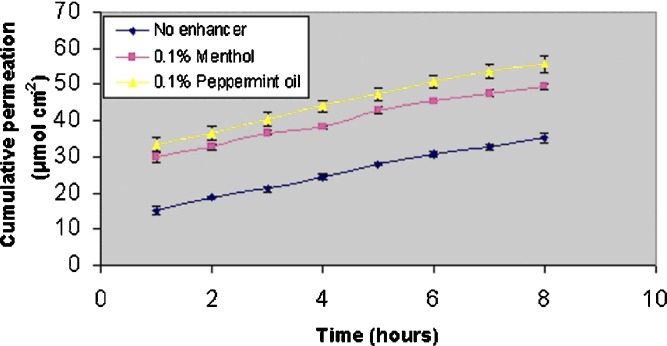
To improve the skin permeation further, experiments were carried out incorporating menthol (0.1%) and peppermint oil (0.1%) into the donor system (Figs. 3 and 4). It is apparent that the enhancers had increased the first hour flux in both passive and iontophoretic permeation, but increment of steady-state flux was moderate. To investigate the possibility of any synergistic or antagonistic effect, matching experiments were performed by passive diffusion too. Cumulative permeation was enhanced with both the enhancers (Table II). However, the same was not observed in case of steady-state flux. Of the two enhancers, steady state flux was significantly increased only in the peppermint oil (P < 0.05; Table III). Control experiments in which VH was delivered from a vehicle containing 1% alcohol did not show any permeation enhancement over that of VH delivered from aqueous vehicle (data not shown).
Fig. 3.
Enhancer mediated permeation (passive) of VH from an unsaturated donor (donor concentration 100 mg/ml; n = 3)
Fig. 4.
Enhancer mediated permeation (iontophoresis) of VH from an unsaturated donor (donor concentration 100 mg/ml; n = 3)
Table II.
Cumulative Permeation of VH from Different Donor Systems (n = 3)
| Donor system | Cumulative permeation at Eighth Hour (μmol/cm2) (Mean ± SE) | Percent permeation |
|---|---|---|
| VH 100 mg/ml in water (passive) | 10.68 ± 0.2864 | 3.35 |
| VH 100 mg/ml in water (iontophoresis) | 35.12 ± 0.7889 | 11.01 |
| VH 100 mg/ml in water + 0.1% menthol (passive) | 12.39 ± 0.2588 | 3.88 |
| VH 100 mg/ml in water + 0.1% menthol (iontophoresis) | 49.39 ± 0.8772 | 15.48 |
| VH 100 mg/ml in water + 0.1% peppermint oil (passive) | 14.33 ± 0.2864 | 4.49 |
| VH 100 mg/ml in water + 0.1% peppermint oil (iontophoresis) | 55.69 ± 1.3138 | 17.46 |
Table III.
Steady State Fluxes and Enhancement Ratio of VH Obtained From Various Donor Systems (n = 3)
| Experimental system | Steady-state flux (μmol cm−2 h−1) | Enhancement ratio (R) | Net benefit of iontophoresis (μmol cm−2 h−1) | ||
|---|---|---|---|---|---|
| Donor | Receiver | Passive | Iontophoresis | In/p | In-P |
| VH sat. sol. 585.5 mg/ml | Water | 0.472 ± 0.083 | 23.03 ± 4.201 | 48.79 | 22.50 ± 4.180 |
| VH sat. sol. 585.5 mg/ml | 0.9% NaCl | 0.577 ± 0.032 | 1.92 ± 0.651 | 3.32 | 1.34 ± 0.043 |
| VH 100 mg/ml in water | 0.9% NaCl | 1.119 ± 0.127 | 2.87 ± 0.069 | 2.39 | 1.69 ± 0.605 |
| VH 100 mg/ml in water + 0.1% menthol | 0.9% NaCl | 1.172 ± 0.102 | 2.86 ± 0.184 | 2.44 | 1.69 ± 0.175 |
| VH 100 mg/ml in water + 0.1% peppermint oil | 0.9% NaCl | 1.303 ± 0.057 | 3.28 ± 0.103 | 2.51 | 1.98 ± 0.488 |
Menthol is considered to be a suitable enhancer for the hydrophilic drugs and is reported to improve the skin permeation better than other terpenes (39). Below the limit of 8%, it has shown to enhance the permeation of nicardipene hydrochloride in a concentration dependent manner and at 8% w/w level it enhanced the permeation by more than seven folds (40). It is suggested that within the stratum corneum, the long chain lipids (ceramides) are arranged in a bilayer, strongly bonded through hydrogen bonding. This high degree of hydrogen bonding, which provides strength and stability to the stratum corneum, opposes the permeation of hydrophilic moieties. In presence of alcoholic terpenes like menthol, this network of hydrogen bond gets stressed up. As the alcoholic –OH group (menthol) is more electronegative than the –NH of amide (ceramides), it can lead to disruption of existing hydrogen bonding between ceramide head groups (5). In our study too the permeation was enhanced but the benefit was minimal. The passive flux was enhanced by 5% (P > 0.1). It appears that the low concentration of menthol (0.1%) was not sufficient to exert a significant enhancement in permeation. However, in terms of net benefit, the combination of enhancer and iontophoresis seemed to have a mild synergistic effect when peppermint oil was used as an enhancer. The steady-state flux was increased by about 16% (P < 0.05). Though menthol is the main ingredient of peppermint oil, it also contains other terpenes like d and l-menthone, l-limonene, l-alpha pinine or phellandrene etc. (41). The combined action of all these moieties might have been responsible for the enhancement of permeation when peppermint oil was used. In case of nicotine too, peppermint oil was found to be more potent permeation enhancer than menthol (42).
Due to the presence of undissolved drug in saturated donor, the cumulative percent permeated could not be calculated. However, for the low concentration donors, it varied between 3% and 4.5%, when passive diffusion was the permeation process. The same was much higher (11–17.5%) in case of iontophoresis (Table II).
Table III shows the net benefit of iontophoresis in terms of steady-state fluxes and enhancement ratios. Highest benefit in terms of steady-state flux was observed when deionized water was used as the receiving fluid. Permeability parameters are listed in Table IV. As expected, highest permeability was found when peppermint oil was used as an enhancer.
Table IV.
Permeability and Diffusion Coefficients of VH in Different Systems for Passive Diffusion and Iontophoresis (n = 3)
| Donor system | Permeability coefficient (cm/h) × 104 | |
|---|---|---|
| Passive | Iontophoresis | |
| VH saturated (585.5 mg/ml) | 3.09 ± 0.176 | 10.29 ± 0.352 |
| VH (100 mg/ml) | 36.79 ± 0.176 | 90.05 ± 2.128 |
| VH (100 mg/ml) + 0.1% menthol | 36.79 ± 0.332 | 89.90 ± 5.790 |
| VH (100 mg/ml) + 0.1% peppermint oil | 40.91 ± 1.790 | 102.93 ± 3.247 |
VH is available as extended release formulations in dose strengths of 25, 37.5 and 75 mg intended for thrice, twice and once daily administrations, respectively. Using the formula of Abdou for these dose strengths, the maintenance requirement was calculated to be 3.1 mg/h (9.87 μmol/h) (43). In patients with hepatic dysfunction, the metabolism of VH in liver is delayed (44). As transdermal route avoids the first pass metabolism, it is expected to have delayed elimination and higher half-life, which is likely to decrease the maintenance requirement. The highest steady-state flux obtained was 3.279 μmol/cm2/h when iontophoresis was combined with peppermint oil as an enhancer. As the patches available in market use much greater area (10 cm2 and above), the results indicate that drug is a promising candidate for iontophoretic delivery.
CONCLUSION
Initial studies on transport number determination indicated that VH is a favorable candidate for iontophoretic permeation. Comparative studies on passive permeation and iontophoresis at two donor concentrations (585.5 and 100 mg/ml) also confirmed this finding. Iontophoresis increased the permeation rate at both concentration levels over their passive counterparts (P < 0.01). Surprisingly, higher steady-state flux was obtained from lower donor drug load (P < 0.01) and favorable pH of the donor system is suggested to be the cause. Iontophoresis in conjunction with peppermint oil showed mild synergistic effect which was not obtained in menthol.
References
- 1.Keller M.B. Rationale and options for the long-term treatment of depression. Hum. Psychopharmacol. Clin. Exp. 2002;17:S43–S46. doi: 10.1002/hup.400. [DOI] [PubMed] [Google Scholar]
- 2.Thompson C., Wilkinson G., Angst J., Kind P., Wade A. Effective management of depression today: report from an interactive workshop. Int. Clin. Psychopharmacol. 1996;11:45–50. doi: 10.1097/00004850-199603001-00009. [DOI] [PubMed] [Google Scholar]
- 3.Keller M.B., Hirschfeld R.M., Demyttenaere K. Optimizing outcomes in depression: focus on antidepressant compliance. Int. Clin. Psychopharmacol. 2002;17:265–271. doi: 10.1097/00004850-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Kilts C.D. Potential new drug delivery system for antidepressants: an overview. J. Clin. Psychiatry. 2003;64:31–33. [PubMed] [Google Scholar]
- 5.Jain A.K., Thomas N.S., Panchagnula R. Transdermal drug delivery of imipramine hydrochloride. I. Effect of terpenes. J. Control Release. 2002;79:93–101. doi: 10.1016/S0168-3659(01)00524-7. [DOI] [PubMed] [Google Scholar]
- 6.Jain A.K., Panchagnula R. Transdermal drug delivery of tricyclic antidepressants: effect of fatty acids. Exp. Clin. Pharmacol. 2003;25:413–421. doi: 10.1358/mf.2003.25.6.769645. [DOI] [PubMed] [Google Scholar]
- 7.Bodkin J.A., Amsterdam J.D. Transdermal selegiline in major depression: a double-blind placebo-controlled, parallel-group study in outpatients. Am. J. Psychiatry. 2002;159:1869–1875. doi: 10.1176/appi.ajp.159.11.1869. [DOI] [PubMed] [Google Scholar]
- 8.Wilson A.D., Howell C., Waring W.S. Venlafaxine ingestion is associated with rhabdomyolysis in adults: a case series. J. Toxicol. Sci. 2007;32:97–101. doi: 10.2131/jts.32.97. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez A.M., Stimmel L.G., Aiso Y.J. Venlafaxine: a 2003 update. Clin. Ther. 2003;25:2138–2154. doi: 10.1016/S0149-2918(03)80210-2. [DOI] [PubMed] [Google Scholar]
- 10.Nemeroff C.B., Entsuah R., Benattia I., Demitrack M., Sloan D.M., Thase M.E. Comprehensive analysis of remission with venlafaxine versus SSRIs. Biol. Psychiatry. 2008;63:424–434. doi: 10.1016/j.biopsych.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 11.Sindrup S.H., Bach F.W., Madsen C., Gram L.F., Jensen T.S. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology. 2003;60:1284–1289. doi: 10.1212/01.wnl.0000058749.49264.bd. [DOI] [PubMed] [Google Scholar]
- 12.Tasmuth T., Hartel B., Kalso E. Venlafaxine in neuropathic pain following treatment of breast cancer. Eur. J. Pain. 2002;6:17–24. doi: 10.1053/eujp.2001.0266. [DOI] [PubMed] [Google Scholar]
- 13.Rowbotham M.C., Golib V., Kunzc N.R., Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004;110:697–704. doi: 10.1016/j.pain.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Freeman E.W., Rickels K., Yonkers K.A., Kunz N.R., Pherson M.M., Upton G.V. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet. Gynecol. 2001;98:737–744. doi: 10.1016/S0029-7844(01)01530-7. [DOI] [PubMed] [Google Scholar]
- 15.Troy S.M., Parekar V.D., Fruncillo R.J., Chiang S.T. The pharmacokinetics of venlafaxine when given in a twice daily regimen. J. Clin. Pharmacol. 1995;35:404–409. doi: 10.1002/j.1552-4604.1995.tb04081.x. [DOI] [PubMed] [Google Scholar]
- 16.Sweetman S.C. Martindale—the complete drug reference. 34. London: PhP Pharmaceutical; 2005. pp. 321–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stahl S.M., Grady M.M., Moret C., Briley M. SNRIs: their pharmacology, clinical efficacy and tolerability in comparison with other classes of antidepressants. CNS Spectr. 2005;10:732–747. doi: 10.1017/s1092852900019726. [DOI] [PubMed] [Google Scholar]
- 18.Buckley N.A., McManus P.R. Fatal toxicity of serotoninergic and other antidepressant drugs: analysis of United Kingdom mortality data. BMJ. 2002;325:1332–1333. doi: 10.1136/bmj.325.7376.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin R.Y., Ou Y.C., Chen W.Y. The role of electroosmotic flow on in vitro transdermal iontophoresis. J. Control. Release. 1997;43:23–33. doi: 10.1016/S0168-3659(96)01468-X. [DOI] [Google Scholar]
- 20.Al-Khalili M., Meidan M.V., Michniak B.B. Iontophoretic transdermal delivery of buspirone hydrochloride in hairless mouse skin. AAPS PharmSci. 2003;5:E14. doi: 10.1208/ps050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh B., Reddy L.H. Effect of physicochemical parameters on skin permeability of antihypertensives. Ind. J. Exp Biol. 2001;39:710–714. [PubMed] [Google Scholar]
- 22.Anroop B., Ghosh B., Parcha V., Kumar A., Khanam J. Synthesis and comparative skin permeability of atenolol and propranolol esters. J. Drug Deliv. Sci. Technol. 2005;15:187–190. [Google Scholar]
- 23.C.G Silcocks. In Physical Chemistry, 3rd edition, ELBS and Macdonald and Evans, London, 1980, p. 239.
- 24.Hawkins G.S., Reifenrath W.G. Influence of skin source, penetration cell fluid, and partition coefficient on in vitro skin penetration. J. Pharm. Sci. 1986;75:378–381. doi: 10.1002/jps.2600750411. [DOI] [PubMed] [Google Scholar]
- 25.A. Jain, B. Ghosh, N. Rajgor, B.G. Desai. Passive and iontophoretic permeation of glipizide. Eur J Pharm Biopharm. (2008) Jan 19; 18291633 [DOI] [PubMed]
- 26.Suwanpidokkul N., Thongnopnua P., Umprayn K. Transdermal delivery of zidovudine: the effects of vehicles, enhancers and polymer membranes on permeation across cadaver pigskin. AAPS PharmSciTech. 2004;5:48. doi: 10.1208/pt050348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glikfield P., Cullander C., Hinz R., Guy R. A new system for in vitro studies of iontophoresis. Pharm. Res. 1988;5:443–446. doi: 10.1023/A:1015944619348. [DOI] [PubMed] [Google Scholar]
- 28.Matoga M., Pehourcq F., Titier K., Dumora F., Jarry C. Rapid high-performance liquid chromatographic measurement of venlafaxine and O-desmethyl venlafaxine in human plasma: application to management of acute intoxications. J. Chromatogr. B. 2001;760:213–218. doi: 10.1016/S0378-4347(01)00270-5. [DOI] [PubMed] [Google Scholar]
- 29.Doh J.H., Cho J.W., Yong S.C., Choi G.H., Kim S.J., Lee H.C., Kim D.D. Synthesis and evaluation of ketorolac ester prodrugs for transdermal delivery. J. Pharm. Sci. 2003;92:1008–1017. doi: 10.1002/jps.10353. [DOI] [PubMed] [Google Scholar]
- 30.Stott P.W., Williams A.C., Barry B.W. Characterization of complex coacervates of some tricyclic antidepressants and evaluation of their potential for enhancing transdermal flux. J. Control Release. 1996;41:215–227. doi: 10.1016/0168-3659(96)01328-4. [DOI] [Google Scholar]
- 31.Mudry B., Guy R.H., Delgado-Charro M.B. Transport numbers in transdermal iontophoresis. Biophys. J. 2006;90:2822–2830. doi: 10.1529/biophysj.105.074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sieg A., Guy R.H., Delgado-Charro M.B. Electro-osmosis in transdermal iontophoresis: implications for non-invasive and calibration-free glucose monitoring. Biophys. J. 2004;87:3344–3350. doi: 10.1529/biophysj.104.044792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imanidis G., Luetolf P. An extended model based on the modified Nernst–Planck equation for describing transdermal iontophoresis of weak electrolytes. J. Pharm. Sci. 2006;95:1434–1447. doi: 10.1002/jps.20551. [DOI] [PubMed] [Google Scholar]
- 34.Marro D., Guy R.H., Delgado-Charro M.B. Characterization of the iontophoretic permeation selectivity properties of human and pigskin. J. Control Release. 2001;70:213–217. doi: 10.1016/S0168-3659(00)00350-3. [DOI] [PubMed] [Google Scholar]
- 35.Nugroho A.K., Li G.L., Danhof M., Bouwstra J.A. Transdermal Iontophoresis of rotigotine across human stratum corneum in vitro: influence of pH and NaCl concentration. Pharm Res. 2004;21:844–850. doi: 10.1023/B:PHAM.0000026438.57787.10. [DOI] [PubMed] [Google Scholar]
- 36.Panchagnula R., Bokalial R., Sharma P., Khandavilli S. Transdermal delivery of naloxone: skin permeation, pharmacokinetic, irritancy and stability studies. Int. J. Pharm. 2005;293:213–223. doi: 10.1016/j.ijpharm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Pikal M.J. The role of electroosmotic flow in transdermal iontophoresis. Adv. Drug Deliv. Rev. 2001;46:281–305. doi: 10.1016/S0169-409X(00)00138-1. [DOI] [PubMed] [Google Scholar]
- 38.Huang J.F., Sung K.C., Hu O.Y.P., Wang J.J., Lin Y.H., Fang J.Y. The effects of electrically assisted methods on transdermal delivery of nalbuphine benzoate and sebacoyl dinalbuphine ester from solutions and hydrogels. Int. J. Pharm. 2005;297:1162–171. doi: 10.1016/j.ijpharm.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Amnuaikit C., Ikeuchi I., Ogawara K., Higaki K., Toshikiro K. Skin permeation of propanolol from polymeric film containing terpene enhancers for transdermal use. Int. J. Pharm. 2005;289:167–178. doi: 10.1016/j.ijpharm.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Krishnaiah Y.S.R., Satyanarayana V., Bhaskar P. Influence of menthol and pressure-sensitive adhesives on the in vivo performance of membrane-moderated transdermal therapeutic system of nicardipine hydrchloride in human volunteers. Eur. J. Pharm. Biopharm. 2003;55:329–337. doi: 10.1016/S0939-6411(03)00027-4. [DOI] [PubMed] [Google Scholar]
- 41.Ale S.A., Hostynek J.J., Maibach H.I. Menthol: a review of its sensitization potential. Exog Dermatol. 2002;1:74–80. doi: 10.1159/000058336. [DOI] [Google Scholar]
- 42.Tiwary A.K., Sapra B., Jain S. Innovations in transdermal drug delivery: formulations and techniques. Recent Patents on Drug Delivery & Formulation. 2007;1:23–36. doi: 10.2174/187221107779814087. [DOI] [PubMed] [Google Scholar]
- 43.Abdou H.M. Dissolution, Bioavailability & Bioequivalence. Easton: Mack; 1989. [Google Scholar]
- 44.Beliles K., Stoudemire A. Psychopharmacologic treatment of depression in medically ill. Psychosomatics. 1998;39:S2–S19. doi: 10.1016/S0033-3182(98)71339-8. [DOI] [PubMed] [Google Scholar]