Abstract
Preformulation studies were performed on a hemiglutarate ester prodrug of Δ9-tetrahydrocannabinol (THC-HG), to facilitate the development of stable formulations by hot-melt methods. The various studies performed included solid-state thermal characterization, pKa, logP, aqueous and pH dependent solubility, pH stability and effect of moisture, temperature and oxygen on solid-state stability. A hot-melt method was utilized to fabricate THC-HG incorporated poly (ethylene oxide) (PEO) matrices and the bioadhesive properties, release profiles and post-processing stability of these matrices were assessed as a function of the polymer molecular weight. The prodrug exhibited a Tg close to 0°C, indicating its amorphous nature. Thermogravimetric analysis revealed a rapid weight loss after 170°C. The prodrug exhibited a seven-fold higher aqueous solubility as compared to the parent drug (THC). Also, the solubility of the compound increased with increasing pH, being maximum at pH 8. The prodrug exhibited a v-shaped pH-rate profile, with the degradation rate minimum between pH 3 and 4. The moisture uptake and drug degradation increased with an increase in relative humidity. Solid-state stability indicated that the prodrug was stable at −18°C but demonstrated higher degradation at 4°C, 25°C and 40°C (51.6%, 74.5% and 90.1%, respectively) at the end of 3-months. THC-HG was found to be sensitive to the presence of oxygen. The release of the active from the polymeric matrices decreased, while bioadhesion increased, with an increase in molecular weight of PEO.
Key words: molecular weight, PEO, preformulation, solubility, stability, THC
INTRODUCTION
Δ9-Tetrahydrocannabinol (THC), the major pharmacologically active constituent of Cannabis sativa exhibits therapeutic potential in the treatment of nausea and vomiting during cancer chemotherapy, anorexia associated with weight loss in AIDS patients, glaucoma, analgesia, anxiety and other indications (1). Despite the promising clinical potential of THC, an efficient dosage form has not been developed to date. The only commercially available dosage form with a constant THC content is the soft gelatin capsule for oral administration marketed in the US as Marinol®. In this formulation, however, the drug has limited stability and therefore has to be stored at low temperatures (4°C). Moreover, the oral bioavailability of the drug is low (∼6%) and inconsistent which is mainly due to its high first pass metabolism and poor solubility (2). In addition to the pharmacokinetic limitations, the physico-chemical properties of THC present a major challenge in the development of a suitable dosage form. THC is a poorly water-soluble, amorphous substance which is sticky, resin-like and highly viscous which makes it difficult to handle and process. Furthermore, the instability of the THC especially in acidic solutions, and when exposed to heat, air and light has been reported by various researchers (3–5). Δ9-THC-hemiglutarate (THC-HG), a prodrug of THC, has been synthesized by our research group, according to our previously published procedure (6) in an attempt to overcome the pharmacokinetic limitations and improve the physico-chemical properties (solubility and stability) of the parent drug.
In light of the various pharmacokinetic limitations associated with oral delivery of THC, an attempt has been made to deliver the prodrug systemically through the oral transmucosal route. The oral mucosa has a high vasculature enabling easy access to the systemic circulation via the internal jugular vein, thus bypassing the first-pass effect. Prior to the development of a dosage form with a new drug candidate (prodrug in this case), it is important to determine certain fundamental physico-chemical properties of the drug molecule. This information will dictate many of the subsequent events and possible approaches in formulation development (7). An understanding of these properties also provides a rationale for the formulation of a stable, safe and marketable product. Preformulation studies were therefore performed to evaluate the relevant physico-chemical properties of THC-HG in order to aid the development of solid dosage formulations prepared by the hot-melt methods.
For oral transmucosal delivery, a flexible polymeric matrix system that adheres to the mucosa for a pre-determined period of time is desirable. Hot-melt processing has been demonstrated to be a viable method for the preparation of drug-incorporated polymeric matrices (8–12). Hot-melt processing requires a pharmaceutical grade thermoplastic polymer that can be processed at relatively low temperatures (low melting or glass transition temperatures) due to thermal sensitivity of many drugs. Poly (ethylene oxide) (PEO), a low melting (57–73°C), semi-crystalline, hydrophilic polymer has been utilized as the base polymer in the present study. PEO, based on its molecular weight can quickly form a hydrogel and regulate the release of the drug. Hence, while formulating poorly soluble drugs, lower molecular weight PEOs, which do not gel to a greater extent, may be advantageous due to lower diffusion path length for the drug. The bioadhesion and stability of the drugs incorporated in the polymer can also be influenced by the molecular weight (13).
The objective of the present work is two-fold. Firstly, to evaluate the physico-chemical properties of THC-HG, an understanding of which would aid in the development of stable formulations for systemic delivery of THC via the oral transmucosal route. The drug properties studied included solid-state thermal characterization, pKa and log P. The solubility and stability of the compound as a function of pH were also determined. Solid-state stability studies included influence of temperature, moisture and oxygen. A second objective of the study was to investigate the influence of molecular weight of PEO on the bioadhesive properties, release profiles and mechanisms and post-processing stability of the prodrug incorporated in polymeric matrices.
MATERIALS AND METHODS
Materials
PEO [PolyOx® WSR N-10 (PEO N-10), MW 100,000 Daltons (Da); PolyOx® WSR N-80 (PEO N-80), MW 200,000 Da; PolyOx® WSR N-750 (PEO N-750), MW 300,000 Da and PolyOx® WSR 1105 (PEO WSR 1105), MW 900,000 Da] was kindly donated by Dow Chemical Company (Midland, MI). Monobasic potassium phosphate, tetrahydrofuran (HPLC grade) and sodium dodecyl sulfate were purchased from Spectrum Chemical, Inc., (Gardena, CA). Lithium chloride, hydrochloric acid (HCl), sodium hydroxide (NaOH), potassium hydroxide, sodium chloride, potassium sulfate, methanol and acetonitrile (both HPLC grade) were obtained from Fischer Chemicals (Fair Lawn, NJ). Magnesium nitrate was procured from Acros Organics, NJ, potassium biphthalate, boric acid and calcium chloride from Sigma Aldrich (St. Louis, MO) and glacial acetic acid from J.T. Baker (Phillipsburg, NJ). HPLC grade water was freshly prepared in the laboratory (by Nanopure system, Barnstead, Dubuque, IA).
Synthesis of Δ9-THC-Hemiglutarate (THC-HG)
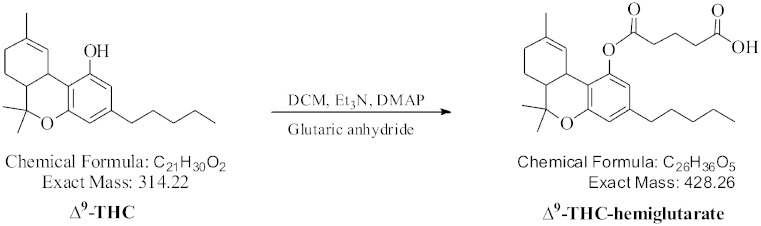
Figure 1 depicts the scheme for THC-HG synthesis. Briefly, THC was dissolved in dichloromethane (DCM) and dimethyl-amino-pyridine (DMAP), in catalytic amount, was added. To this solution was then added glutaric anhydride (1.1 equivalent) along with triethyl amine (catalytic amount). The reaction was allowed to stir overnight at room temperature, where thin layer chromatography (TLC) indicated the completion of the reaction. The reaction mixture was worked up and pure THC-hemigluturate, 95.0% (overall conversion yield of THC-HG from THC) was obtained after performing column chromatography on silica gel. THC-HG structure was confirmed by high resolution electron impact mass spectrometry (HREIMS) and Nuclear magnetic resonance spectroscopy (NMR) experiments (6).
Fig. 1.
Scheme for THC-HG synthesis
Preformulation Studies
Determination of Ionization Constant (pKa)
pKa of the drug was determined by potentiometric titration using an Orion 710A+ pH meter (Thermo Electron Corp., Waltham, MA). A known amount of the dried sample was dissolved in 50 mL of water in a volumetric flask and allowed to equilibrate for 12 h in a shaker bath at 25°C and 50 shakes per minute (spm). The equilibrated drug solution (maintained at 25°C) was titrated with 0.1 N HCl in 0.01 mL increments with adequate stirring until no further increase in pH was observed with addition of the acid. The resulting solution was then back titrated with 0.1 N NaOH. pKa was determined from the intersection of the graph (pH versus volume of titrant added) obtained by the direct and the back titration. The pKa value of the prodrug was also determined using ACD/Chemsketch software from the structure of the molecule.
Partition Coefficient Determination
mlog P (Moriguchi log P) was determined by Molecular Modeling Pro™ 6.27 software (ChemSw, Inc., Fairfield, CA).
Solid-State Thermal Characterization
Thermal stability and degradation temperatures of THC-HG were determined as a function of weight loss utilizing a Perkin-Elmer Pyris-1 thermogravimetric analysis (TGA). Approximately 2–3 mg of the drug sample was heated from 25°C to 500°C at 10°C/min under a constant nitrogen purge. Samples were initially held at 60°C for 5 min to remove the residual solvent.
Hyper-Differential scanning calorimetry (Hyper-DSC) was carried out utilizing a Perkin-Elmer Pyris-1 DSC to determine the glass transition of the prodrug. Samples (∼2–3 mg) were placed in hermetically sealed aluminum pans and heated from −20°C to 100°C at a heating rate of 100°C/min. An empty aluminum pan served as reference. Helium was used as a purge gas at a flow rate of 20 mL/min.
Aqueous and Saturation pH-Solubility
Buffer solutions (0.2 M) ranging from pH 3 to 10 were prepared according to USP procedure. The solubility of the drug was tested in water and in various buffer solutions. THC-HG (in hexane) samples were heated and purged with nitrogen gas, to evaporate the solvent. An excess of the dry sample was added to 10 mL of water or respective buffer solutions in a scintillation vial and allowed to equilibrate in a shaker bath at 25°C and 50 spm for 24 h. One mL sample aliquots (n = 3) were withdrawn every 6 h, until saturation was achieved, centrifuged for 10 min at 4°C and the supernatant was analyzed for the drug concentration using the chromatographic system.
pH Stability Studies
Known concentrations of the drug were prepared in pH 2.4 to 10 buffer solutions (0.2 M), placed at 25°C (in the dark) and analyzed at various time intervals. Due to the unusually low solubility of THC-HG in aqueous media, it was not possible to prepare solutions of appropriate drug concentrations. Thus, a THC-HG solution in ethanol was used to solubilize the drug in aqueous media. For preparation of the drug solutions of known concentration, 0.1 parts of THC-HG (in ethanol) solution was added to 99.9 parts of the pH solutions and vortexed. The resulting solution was centrifuged for 10 min at 4°C, and the supernatant was analyzed using the high performance liquid chromatography (HPLC) system for THC-HG concentration (T0). It has been reported that ethanol has only a slight effect on the aqueous solubility of THC (14).
Hygroscopicity (Moisture Sorption) Studies
The moisture sorption behavior of the prodrug was determined gravimetrically after storage at 25°C under conditions of differing relative humidities (0–97% RH). The various RH conditions were achieved in vacuum desiccators utilizing saturated salt solutions. The relative humidities were 0% RH (silica gel), 13% RH (lithium chloride), 29% RH (calcium chloride), 54% RH (magnesium nitrate), 75% RH (sodium chloride) and 97% RH (potassium sulfate). Samples in triplicate in open glass vials (20 mL) were allowed to equilibrate in the vacuum desiccators and analyzed at various time intervals for the chemical degradation and moisture content utilizing HPLC and TGA, respectively. To determine the moisture content, the samples were heated from 30 to 90°C at a heating rate of 40°C/min and held at 90°C for 20–30 min until no more weight loss was observed. All TGA runs were performed in an open pan with purge utilizing a protective nitrogen gas flow at 40 mL/min.
Solid-State Stability
To investigate the effect of storage temperature on the drug stability, dry samples of the prodrug (n = 3) were stored at four different temperatures (−18°C, 4°C, 25°C and 40°C) and analyzed via HPLC at various time intervals for up to 3 months to determine the amount of THC-HG and the parent drug, THC, present.
Oxidative Stability
To assess the stability of the drug to oxidation, dry samples (∼2 mg) of THC-HG (n = 3) were taken in glass vials and low pressure oxygen gas was bubbled through them for 3–4 min, using a needle attached to the oxygen tank through Teflon tubing. The glass vials were then sealed using a Teflon-lined screw cap and stored (in the dark) at −18°C and analyzed at various time intervals for the drug remaining for up to 3 months. A sample flushed with nitrogen and stored similarly was used as a control.
Effect of Polymer Molecular Weight
Preparation of Polymeric Matrices by Hot-Melt Method
PEO polymeric matrices incorporated with THC-HG at 5% w/w were fabricated utilizing a hot-melt method. The various molecular weights of PEO investigated included PEO N-10, PEO N-80, PEO N-750 and PEO WSR 1105. Briefly, a die containing a 13 mm diameter opening was placed on top of a brass sheet and heated at 110–150°C (based on the polymer molecular weight). Approximately 200 mg of physical mixture of drug and polymer in the ratio of 5:95, respectively was positioned in the orifice of the die, and compressed using a punch. This compressed mixture was heated for 5–10 min to form a melt, followed by cooling under room conditions to form a thin polymeric patch.
Bioadhesion Studies
Bioadhesive measurements were performed on both placebo polymeric films and those containing the drug utilizing a TA.XT2i Texture Analyzer (Texture Technologies Corp., Scarsdale, NY/Stable Micro Systems, Godalming, Surrey, UK) equipped with Texture Expert™ software. Rabbit buccal mucosa was used as a biological substrate. The samples (n = 5) were wetted with artificial saliva (13) (adjusted to a pH of 6.8 ± 0.05) for approximately 60 s and placed on the lower base of the instrument. The mucosal substrate was attached to the probe with a cyano-acrylate adhesive and equilibrated with the artificial saliva before the bioadhesion testing. The probe lined with mucosa was set to approach the sample with a predetermined speed of 0.5 mm/s and applied a force of 3.5 N. The test speed was 0.1 mm/s and contact time was 60 s. The probe was then withdrawn at a speed of 1 mm/s following the application of force. These parameters were chosen based on our previous studies (10,15,16). The parameters measured were peak adhesive force (PAF) and work of adhesion (WA).
Determination of Release Profiles and Release Mechanisms
Dissolution studies (n = 3) were performed utilizing a Hanson SR8-Plus dissolution test system according to USP 31 apparatus 5, paddle over disk method. The polymeric patch was sandwiched between the watch glass and a mesh so that the release was unidirectional. Nine hundred mL of 1% w/v SLS at 37°C was used as the dissolution medium and the paddle rotation speed was 100 rpm. Samples were collected at predetermined time intervals and analyzed by HPLC. The average of the three replicates was calculated and used to perform the data analysis. The release data was fitted to three models; first order, square root, and zero-order to describe the drug release kinetics from the polymeric matrices. The data was also fitted to Kopcha et al. (17) and Peppas (18) models to determine the mechanism of drug release from the polymeric matrices.
Chromatographic Analysis and Sample Preparation
The chromatographic system consisted of a Waters 600 pump and a dual wavelength Waters 2487 UV detector (Waters Corp, Milford, MA). A Luna 5 μ C-18 (2), 150 × 4.60 mm column (Phenomenex, Torrance, CA), was used for the detection of the drug. The mobile phase consisted of 52% methanol, 20% acetonitrile and 18% water with 0.75 mL acetic acid added per 1,000 mL solvent. The flow rate was maintained at 1.8 mL/min, with THC-HG, THC and cannabinol (CBN) (oxidative degradant of THC) eluting at 13.8, 10.0 and 6.2 min, respectively. The injection volume was 20 μL, and the column effluent was monitored by UV absorption at 228 nm. The temperature of the column was maintained at 25°C.
A weighed portion of the THC-HG or drug-incorporated polymeric matrix was dissolved in a known volume of methanol by sonicating it for 10–15 min. The resulting solution was filtered, transferred into vials and 20 μL was injected into the HPLC column for drug analysis.
Statistical Analysis
Statistical analysis was performed using Microsoft Excel® and a Students t-test was used to analyze the results. A p < 0.05 was considered statistically significant. Statistica (version 5.5, Stat Soft, Tulsa, OK) was used to fit the dissolution data to the Kopcha model.
RESULTS AND DISCUSSION
Determination of Ionization Constant (pKa)
A dry sample of THC-HG was added to water to obtain a concentration of 30 μg/mL. However, owing to its hydrophobic nature, the prodrug was not completely soluble in water and therefore, after equilibrating for 12 h, the solution was centrifuged and the supernatant was used for the assay. To ensure the presence of drug in the assay solution, 20 μL of the supernatant was injected into the HPLC system. A very low concentration of 7.3 μg/mL was detected after 12 h, indicating that the drug is poorly soluble. The pKa value obtained from the intersection of the graph obtained by the direct and the back titration was found to be 3.56 ± 0.36 at 25°C. This value was in close agreement with that obtained using ACD/Chemsketch software (4.59 ± 0.1).
Partition Coefficient Determination
Log octanol/water partition coefficient calculated by Moriguchi’s method (mlog P) was found to be 3.92, indicating the lipophilic nature of the drug.
Solid-State Thermal Characterization
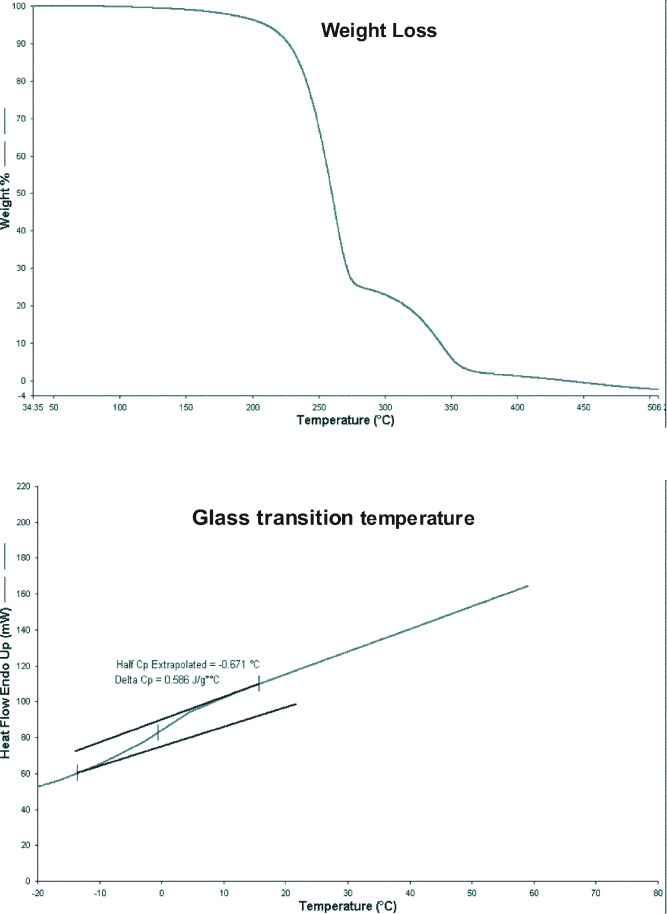
The thermal stability (as a function of temperature) and glass transition temperature of THC-HG are depicted in Fig. 2. Thermal analysis curve of the prodrug depicted two stages of weight loss corresponding to either volatilization or sublimation. The initial drop that initiated in the temperature region of 170°C resulted in a rapid loss in the weight of the sample (∼75%) and the second drop that occurred between 275°C and 360°C resulted in a weight loss of ∼23%. Hence, temperatures below 170°C must be employed for formulation purposes to avoid chemical and/or thermal degradation of the prodrug.
Fig. 2.
Weight loss (as a function of temperature) and glass transition temperature of THC-HG
Since the prodrug was amorphous in nature, the glass transition temperature (Tg) was determined utilizing Hyper-DSC. The Tg was found to be well below room temperature (0.586°C). A low Tg value indicates that THC-HG has very high molecular mobility and hence high reactivity even when stored at 4°C.
Aqueous and Saturation pH-Solubility
The aqueous solubility of the prodrug was determined to be 18.1 μg/mL at saturation (24 h), which is seven-fold higher than that of the parent drug (THC) (14). pH dependent solubility studies demonstrated an increase in the solubility of THC-HG upon increasing the pH of the medium (Table I). No detectable THC-HG peak could be seen in buffer solutions at pH’s 3.0 and 4.0. This might be due to the fact that the concentration of the drug was below the detection level of the analytical system (poor solubility). The prodrug was poorly soluble at acidic pH, with no significant change in solubility between pH 5.0 and 7.0. However, a significant increase in solubility of the drug was observed as the pH of the solution shifted towards the basic range with pH 8.0 exhibiting the maximum solubility (539.6 μg/mL). This solubility behavior could be expected since the drug is acidic in nature with a pKa of 4.59 (ACD/Chemsketch software). Increasing the pH of the solution above the pKa would lead to ionization of the molecule. Ionized forms of a drug generally exhibit higher solubility than the unionized form.
Table I.
First-Order Degradation Rate Constants (k), Degradation Half-Lives (t 1/2) and Solubility of THC-HG at 25°C in Buffers of Various pH Values
| pH | k (h−1) | t 1/2 (days) | Solubility (μg/mL) |
|---|---|---|---|
| 2.4 | 0.0051 | 5.66 | − |
| 3.0 | 0.0014 | 20.63 | + |
| 4.0 | 0.0016 | 18.05 | + |
| 5.0 | − | − | 2.52 |
| 6.0 | 0.0069 | 4.18 | 6.12 |
| 7.0 | 0.0094 | 3.07 | 17.29 |
| 8.0 | 0.0121 | 2.39 | 539.56 |
| 10.0 | − | − | 411.34 |
− Study not performed at that condition, + No detectable THC-HG peak observed
pH Stability Studies
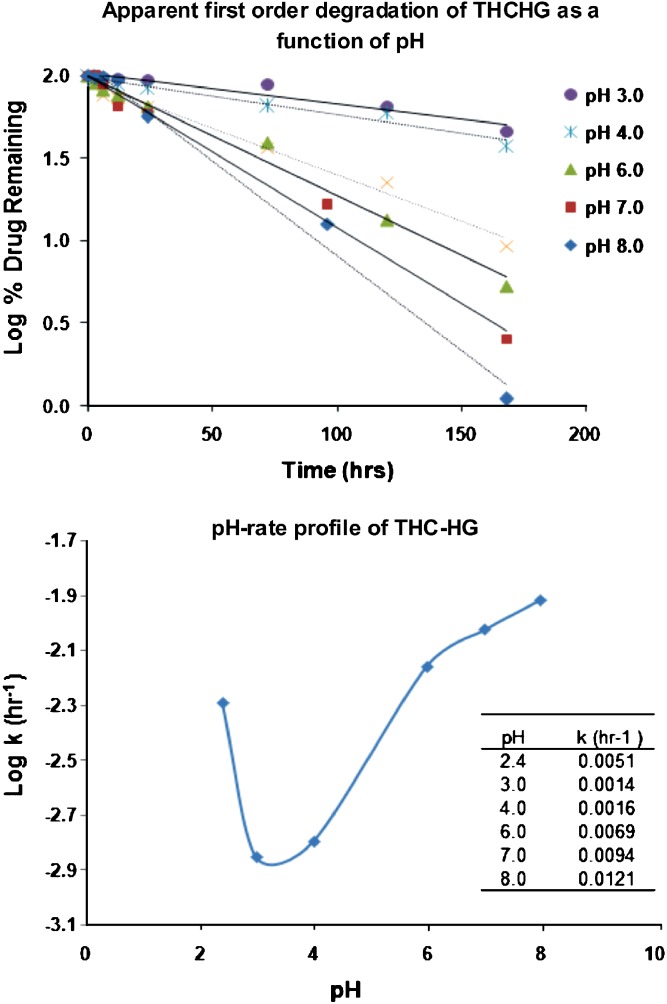
The degradation rate of many pharmaceutical compounds in solution is a function of solution pH. The degradation of drugs in solution is mainly attributed to the catalytic effect of either the hydrogen ions or hydroxide ions. An understanding of the pH-rate profile can aid in the development of a stable formulation by formulating it at the pH of maximum stability of the drug. The availability of pH-rate profile is also useful in predicting solid-state stability of a drug in the presence of acidic or basic excipients. To assess the stability of THC-HG as a function of pH, the log % THC-HG remaining versus time profiles were initially constructed (Fig. 3). The linear curves of the plots at all pH values indicated first-order degradation. Degradation rate constants obtained from the slopes of the curves were used to determine the half-lives at various pH values (Table I). The pH-stability profile was constructed by plotting rate constants versus pH (Fig. 3).
Fig. 3.
Apparent first-order degradation and pH-rate profile of THC-HG in 0.2 M buffer solutions at various pH values, stored at 25°C
The hemiglutarate ester followed a v-shaped pH-rate profile, typical of ester prodrugs with degradation being the lowest between pH 3 and 4 in the pH range studied. The v-shaped profile suggests that the degradation of the ester is catalyzed by specific acid (hydronium ion) and specific base (hydroxide ion) (19,20). The degradation was extremely rapid under highly acidic pH (pH 2.4) and alkaline conditions. The degradation half-lives were 5.66, 4.18, 3.07 and 2.39 days at pH 2.4, 6.0, 7.0 and 8.0, respectively, while the pH range 3.0–4.0 had degradation half-lives of 20.63 and 18.05 days, respectively. A significant curvature obtained in the pH-rate profile of THC-HG in the pH region of 3 to 4 suggests the presence of a pKa value close to these pH’s (21). As has already been mentioned, the pKa value for the prodrug was calculated to be 3.56 using the potentiometric titration.
Hygroscopicity Studies
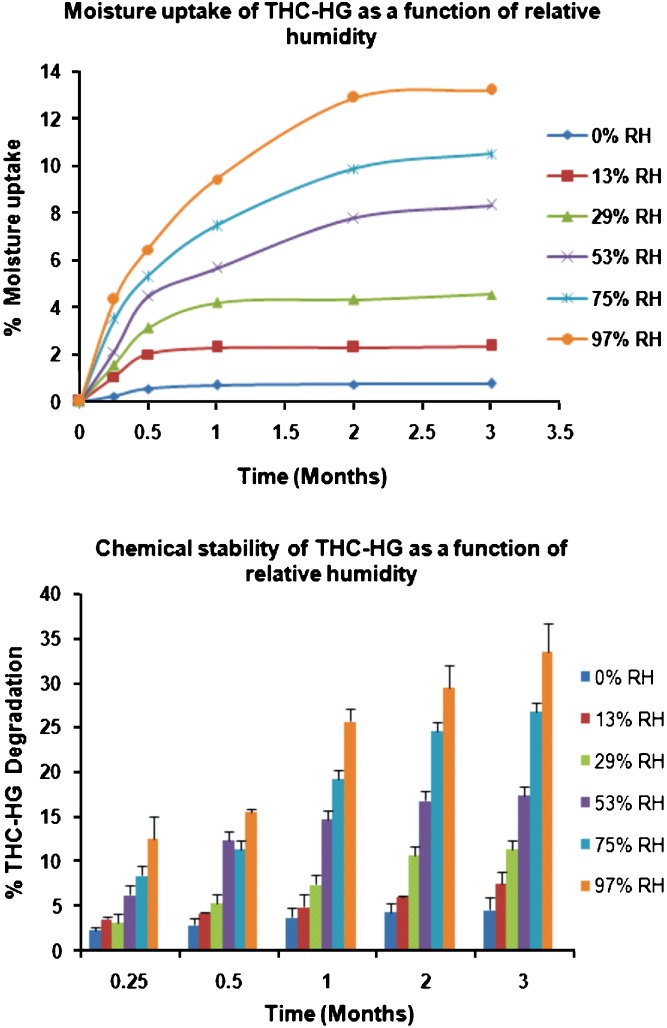
Moisture is an important factor that can affect the stability of drugs and their formulations. Sorption of water molecules onto a drug (or excipient) can often induce hydrolysis (22). The moisture uptake behavior and chemical stability of THC-HG as a function of RH are depicted in Fig. 4. The results demonstrated that the moisture uptake by the drug increased with increasing relative humidity. The samples stored at lower RH’s (0%, 13% and 29%) exhibited relatively lower moisture uptake while those stored at higher RH’s (53%, 75% and 97%) exhibited higher moisture uptake (8.3%, 10.5% and 13.2% at 53%, 75% and 97%, respectively) at the end of the study.
Fig. 4.
Moisture uptake behavior and chemical stability of THC-HG as a function of relative humidity (RH). The samples (n = 3) were stored at 25°C
An examination of the HPLC chromatograms of the samples exposed to various humidities indicated THC as the major peak, besides the THC-HG peak, indicating hydrolysis of the ester bond to yield the parent drug, as the major mechanism of degradation. An increase in moisture generally increases the chemical degradation of a drug, since water has the capacity to act both as a chemical reactant for hydrolysis and as a reaction medium (19,23). A trend supporting this statement was obtained as illustrated in Fig. 4. The samples stored at lower humidities (0% and 13% RH) were found to be relatively stable, while those stored at higher humidities continued to degrade with time. Approximately, 26.7% and 33.5% of the drug degradation was observed from the samples stored at 75% and 97% RH, respectively at the end of 3 months. The results of this study indicated the susceptibility of the prodrug to moisture. Hence, the drug should be stored at lower RH’s to prevent its degradation.
Solid-State Stability
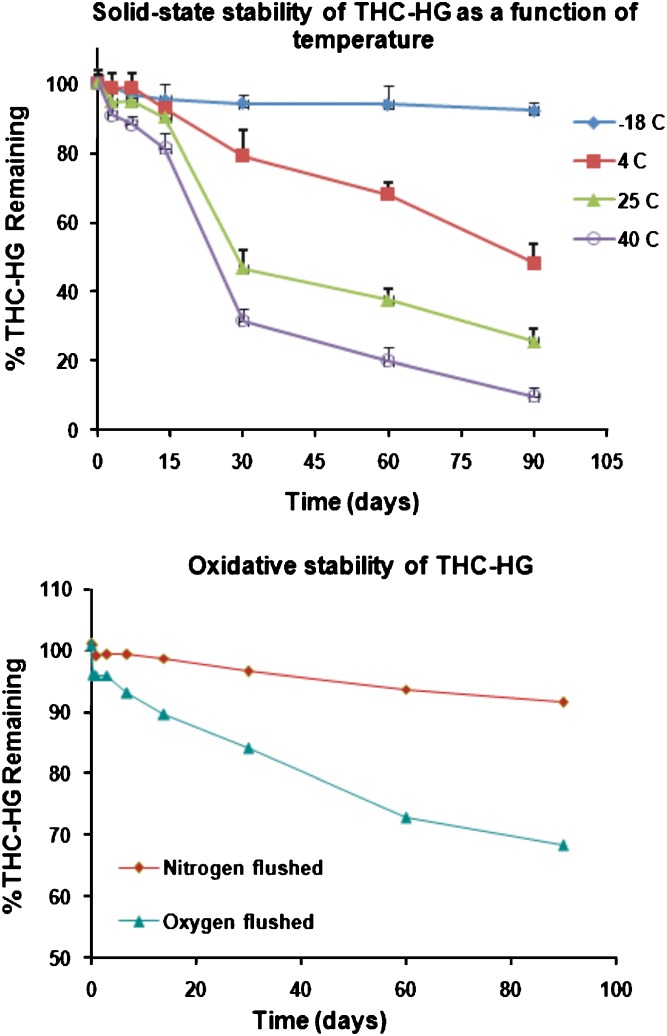
The solid-state stability of THC-HG was assessed as a function of temperature as depicted in Fig. 5. The first-order degradation rate constants (k) were calculated by a linear regression from the slope of linear plots of the logarithm of the % drug remaining versus time. Solid state stability indicated that the prodrug was stable at −18°C as indicated by a low k value (0.001 day−1) after 3-months storage. However, higher degradation was observed when stored at 4°C, 25°C and 40°C (51.6%, 74.5% and 90.1%, respectively) during the same period as indicated by higher k values at these temperatures (0.0032, 0.0066 and 0.0117 day−1, respectively at 4°C, 25°C and 40°C). This may be attributed to the fact that these storage temperatures were above the Tg of THC-HG (0.586°C), where the drug has higher molecular mobility and hence higher reactivity.
Fig. 5.
Solid-state stability (as a function of temperature) and oxidative stability (samples were stored at −18°C) of THC-HG. Each point represents the mean of three observations
Oxidative Stability
Next to hydrolysis and solvolysis reactions, oxidation is the primary cause for drug instability (24). The stability data of the prodrug to oxidation is also presented in Fig. 5. THC-HG exhibited a higher degradation in presence of oxygen (29.1%) (k = 0.0006 day−1), while the corresponding sample stored similarly in the presence of nitrogen was found to be relatively stable (∼8.4%) (k = 0.0028 day−1) after 3-month storage at −18°C. This indicates that oxidation is one of the mechanisms (besides hydrolysis) by which the prodrug degrades. This observation is further substantiated by the presence of cannabinol (CBN) (oxidative degradant of THC) as the main peak, besides the THC peak in the HPLC chromatograms of samples flushed with oxygen.
Effect of Polymer Molecular Weight
Bioadhesion Studies
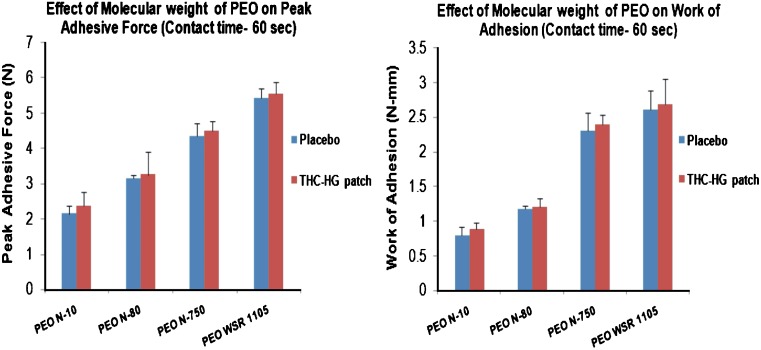
The results of the effect of PEO molecular weight on the PAF and WA of placebo and drug incorporated polymeric matrices are depicted in Fig. 6. It was observed that both the parameters studied increased with an increase in polymer molecular weight (p < 0.05). The higher molecular weight PEO, PEO WSR-1105 demonstrated higher peak adhesive force and work of adhesion values when compared to the lower molecular weight PEO, PEO N-10 which exhibited the lowest value. The bioadhesive strength of the films was found to be in the following order: PEO WSR 1105 > PEO N-750 > PEO N-80 > PEO N-10. The process of mucoadhesion has been proposed to begin with the establishment of an intimate contact between the polymer and the mucosal surface followed by penetration of the polymer into the mucosal surface to form secondary chemical bonds (25). The bond strength however, depends on the depth of penetration of the polymer chains which in turn is influenced by the molecular chain length (molecular weight). The higher bioadhesion observed with PEO WSR 1105 may be attributed to its ability to form a greater number of entanglements and hence its penetration to a greater extent into the mucus glycoprotein network as compared to the shorter chain length (lower molecular weight) PEO N-10. A similar trend of increasing peak adhesive force and work of adhesion values as a function of molecular weight was obtained with the drug incorporated PEO matrices. Further, it was observed that the presence of drug in the PEO matrices had only an insignificant effect (p > 0.05) on the bioadhesive strength at the concentration employed (5% w/w).
Fig. 6.
Peak adhesive force (PAF) and work of adhesion (WA) of hot-melt fabricated PEO matrices (n = 5) as a function of polymer molecular weight
Determination of Release Profiles and Release Mechanisms
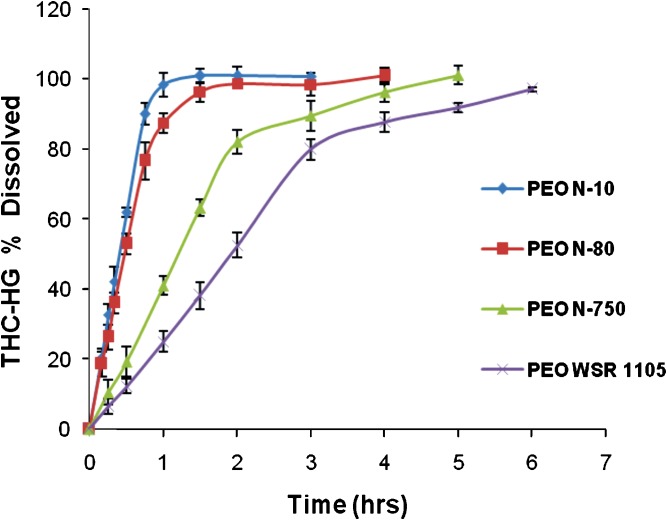
The release profiles of THC-HG as a function of molecular weight of PEO is depicted in Fig. 7. An increase in molecular weight of the polymer resulted in a decrease in drug release from these matrices. The lower molecular weight PEO’s exhibited a faster release (100% drug released from PEO N-10 and PEO N-80 in 1.5 and 2 h, respectively), while the higher molecular weight PEO’s demonstrated a sustained-release of the drug (100% drug released from PEO N-750 and PEO WSR 1105 in 5 and 6 h, respectively). The kinetics of drug release was determined to be zero-order from all of the polymeric matrices when the dissolution data was fitted to the three aforementioned models. The coefficients obtained by fitting the dissolution data to the Kopcha model (17) and Peppas model (18) indicated that the drug release occurs predominantly by erosion irrespective of the polymer molecular weight. The ratio A/B (Kopcha model coefficients) was less than 1 and the release exponent, n (Peppas model) was 1 for all of the polymeric matrices investigated (Table II). The decrease in dissolution rate observed with increasing molecular weight may be explained by the fact that the higher molecular weight PEO’s possess a longer chain length and hence stronger chain entanglements, which lead to slower polymer erosion.
Fig. 7.
Release profiles of THC-HG from hot-melt fabricated PEO matrices as a function of polymer molecular weight in 1% SLS (100 rpm, n = 3)
Table II.
Model Coefficients for the Release of THC-HG from PEO Matrices as a Function of Polymer Molecular Weight in 1% SLS at 37°C and a Paddle Rotation Speed of 100 rpm
| Formulation | Kopcha model 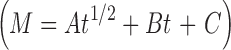
|
Peppas model 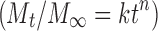
|
|||
|---|---|---|---|---|---|
| A (mgh−1/2) | B (mgh−1) | C (mg) | A/B | n (release exponent) | |
| PEO N-10 | 8.89 | 130.73 | −0.20 | 0.06 | 0.98 |
| PEO N-80 | 7.41 | 94.39 | −0.08 | 0.08 | 0.95 |
| PEO N-750 | 4.05 | 42.16 | −0.01 | 0.11 | 1.00 |
| PEO WSR 1105 | 3.24 | 21.34 | 0.30 | 0.15 | 1.02 |
A/B < 1; erosion predominates over diffusion [Kopcha Model (17)]. For swellable matrices, n = 1; case II transport mechanism (erosion) with zero-order kinetics [Peppas Model (18)]
A Diffusion term, B erosion term, C related to physical parameters, M t/M ∞ fraction of drug released, t release time, k kinetic constant, n release exponent indicative of the release mechanism of the drug
Post-Processing Drug Content
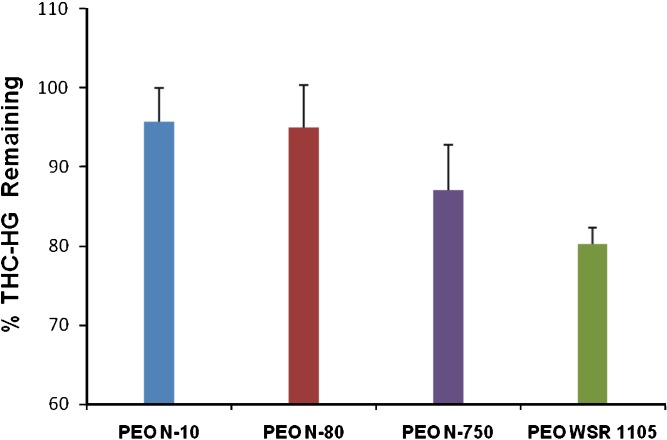
The post-processing content of THC-HG was assessed as a function of the molecular weights of PEO investigated, the results of which are presented in Fig. 8. Less than 5% of the drug degradation was observed in lower molecular weight PEO’s (PEO N-10 and PEO N-80) as opposed to significantly higher degradation in the higher molecular weight PEO’s, post-processing (12.9% and 19.8% in PEO N-750 and PEO WSR 1105, respectively). This most likely can be attributed to the higher processing temperatures (140–150°C) required to lower the melt viscosity of the higher molecular weight PEO’s during hot-melt fabrication. The lower molecular PEO’s, however required only 110°C for processing. These results demonstrated that lower molecular PEO’s may be suitable for fabricating polymeric matrices incorporated with thermo-labile drugs such as THC-HG by the hot-melt technique.
Fig. 8.
Post-processing stability of THC-HG in PEO matrices as a function of polymer molecular weight. The matrices (n = 3) were fabricated by a hot-melt technique
CONCLUSIONS
The sensitivity of the prodrug to oxygen and higher temperature and relative humidity conditions (based on the results of this study) suggests that one should provide considerations for temperature and moisture during formulation and packaging. However, the higher aqueous solubility of the prodrug (seven-fold higher as compared to THC) is very promising for improving the bioavailability of a poorly soluble drug such as THC. The solid-state stability of the prodrug can be enhanced by employing suitable formulation approaches and by adjusting the microenvironmental pH of the dosage form to correspond with the pH of the maximum stability of the drug. The results of the present investigation also demonstrated that the molecular weight of the polymer influenced the bioadhesion properties and release of the drug from PEO matrices and hence can be tailored to obtain the optimum formulations for oral transmucosal delivery of the prodrug.
Acknowledgements
This project was supported by Grant Number P20RR021929 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
References
- 1.Voth E. A., Schwartz R. H. Medicinal applications of delta-9-tetrahydrocannabinol and marijuana. Ann. Intern. Med. 1997;126:791–798. doi: 10.7326/0003-4819-126-10-199705150-00008. [DOI] [PubMed] [Google Scholar]
- 2.Ohlsson A., Lindgren J. E., Wahlen A., et al. Plasma delta-9-tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin. Pharmacol. Ther. 1980;28:409–416. doi: 10.1038/clpt.1980.181. [DOI] [PubMed] [Google Scholar]
- 3.Mechoulam R. Marihuana chemistry. Science. 1970;168:1159–1166. doi: 10.1126/science.168.3936.1159. [DOI] [PubMed] [Google Scholar]
- 4.Fairbairn J. W., Liebmann J. A., Rowan M. G. The stability of cannabis and its preparations on storage. J. Pharm. Pharmacol. 1976;28:1–7. doi: 10.1111/j.2042-7158.1976.tb04014.x. [DOI] [PubMed] [Google Scholar]
- 5.Mechoulam R., Hanus L. A historical overview of chemical research on cannabinoids. Chem. Phys. Lipids. 2000;108:1–13. doi: 10.1016/S0009-3084(00)00184-5. [DOI] [PubMed] [Google Scholar]
- 6.M. A. ElSohly, S. A. Ross, and S. Feng. Method of preparing delta-9-tetrahydrocannabinol esters. In: US patent 6008383; 1999
- 7.Lachman L., Lieberman H. A., Kanig J. L. The theory and practice of industrial pharmacy. Philadelphia: Lea & Febiger; 1986. [Google Scholar]
- 8.Munjal M., ElSohly M. A., Repka M. A. Chemical stabilization of a delta-9-tetrahydrocannabinol prodrug in polymeric matrix systems produced by a hot-melt method: role of microenvironment pH. AAPS PharmSciTech. 2006;7:71. doi: 10.1208/pt070371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang F., McGinity J. W. Properties of sustained-release tablets prepared by hot-melt extrusion. Pharm. Dev. Technol. 1999;4:241–250. doi: 10.1081/PDT-100101358. [DOI] [PubMed] [Google Scholar]
- 10.Repka M. A., McGinity J. W. Influence of vitamin E TPGS on the properties of hydrophilic films produced by hot-melt extrusion. Int. J. Pharm. 2000;202:63–70. doi: 10.1016/S0378-5173(00)00418-X. [DOI] [PubMed] [Google Scholar]
- 11.Repka M. A., Gutta K., Prodduturi S., Munjal M., Stodghill S. P. Characterization of cellulosic hot-melt extruded films containing lidocaine. Eur. J. Pharm. Biopharm. 2005;59:189–196. doi: 10.1016/j.ejpb.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Repka M. A., Gerding T. G., Repka S. L., McGinity J. W. Influence of plasticizers and drugs on the physical–mechanical properties of hydroxypropylcellulose films prepared by hot-melt extrusion. Drug Dev. Ind. Pharm. 1999;25:625–633. doi: 10.1081/DDC-100102218. [DOI] [PubMed] [Google Scholar]
- 13.Prodduturi S., Manek R. V., Kolling W. M., Stodghill S. P., Repka M. A. Solid-state stability and characterization of hot-melt extruded poly(ethylene oxide) films. J. Pharm. Sci. 2005;94:2232–2245. doi: 10.1002/jps.20437. [DOI] [PubMed] [Google Scholar]
- 14.Garrett E. R., Hunt C. A. Physiochemical properties, solubility, and protein binding of delta 9-tetrahydrocannabinol. J. Pharm. Sci. 1974;63:1056–1064. doi: 10.1002/jps.2600630705. [DOI] [PubMed] [Google Scholar]
- 15.Repka M. A., McGinity J. W. Physical–mechanical, moisture absorption and bioadhesive properties of hydroxypropylcellulose hot-melt extruded films. Biomaterials. 2000;21:1509–1517. doi: 10.1016/S0142-9612(00)00046-6. [DOI] [PubMed] [Google Scholar]
- 16.Repka M. A., McGinity J. W. Bioadhesive properties of hydroxypropylcellulose topical films produced by hot-melt extrusion. J. Control. Release. 2001;70:341–351. doi: 10.1016/S0168-3659(00)00365-5. [DOI] [PubMed] [Google Scholar]
- 17.Kopcha M., Tojo K. J., Lordi N.G. Evaluation of methodology for assessing release characteristics of thermosoftening vehicles. J. Pharm. Pharmacol. 1990;42:745–751. doi: 10.1111/j.2042-7158.1990.tb07014.x. [DOI] [PubMed] [Google Scholar]
- 18.Peppas N. A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985;60:110–111. [PubMed] [Google Scholar]
- 19.Martin A. Physical Pharmacy. 4. Philadelphia: Lea & Febiger; 1993. [Google Scholar]
- 20.Ni N., Sanghvi T., Yalkowsky S. H. Stabilization and preformulation of anticancer drug—SarCNU. Int. J. Pharm. 2002;249:257–264. doi: 10.1016/S0378-5173(02)00522-7. [DOI] [PubMed] [Google Scholar]
- 21.Drustrup J., Fullerton A., Christrup L., Bundgaard H. Utilization of prodrugs to enhance the transdermal absorption of morphine. Int. J. Pharm. 1991;71:105–116. doi: 10.1016/0378-5173(91)90072-V. [DOI] [Google Scholar]
- 22.Yoshioka S., Carstensen J. T. Nonlinear estimation of kinetic parameters for solid-state hydrolysis of water-soluble drugs. II: Rational presentation mode below the critical moisture content. J. Pharm. Sci. 1990;79:799–801. doi: 10.1002/jps.2600790911. [DOI] [PubMed] [Google Scholar]
- 23.Ahlneck C., Zografi G. The molecular basis of moisture effects on the physical and chemical stability of drugs in the solid state. Int. J. Pharm. 1990;62:87–95. doi: 10.1016/0378-5173(90)90221-O. [DOI] [Google Scholar]
- 24.Connors K. A., Amidon G. L., Stella V. J. Chemical Stability of Pharmaceuticals: A handbook for pharmacists. 2. New York: Wiley; 1986. [Google Scholar]
- 25.Duchene D., Touchard F., Peppas N. A. Pharmaceutical and medical aspects of bioadhesive systems for drug administration. Drug Dev. Ind. Pharm. 1988;14:283–318. doi: 10.3109/03639048809151972. [DOI] [Google Scholar]