Abstract
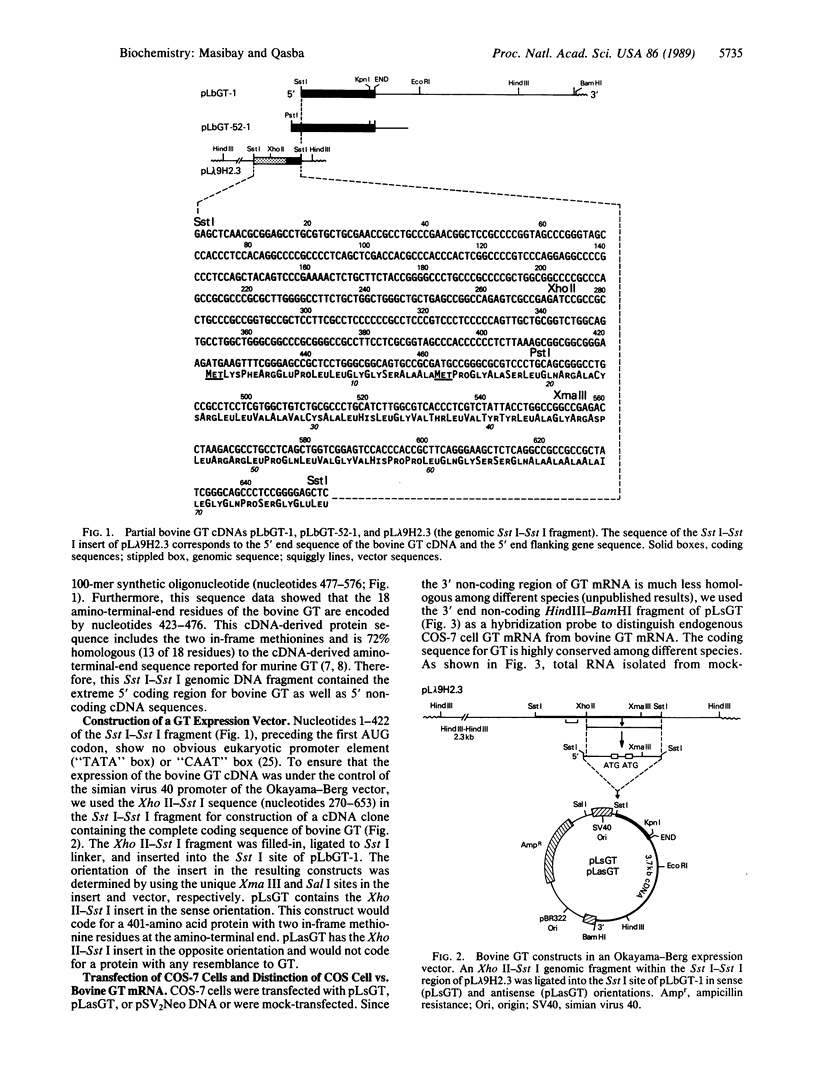
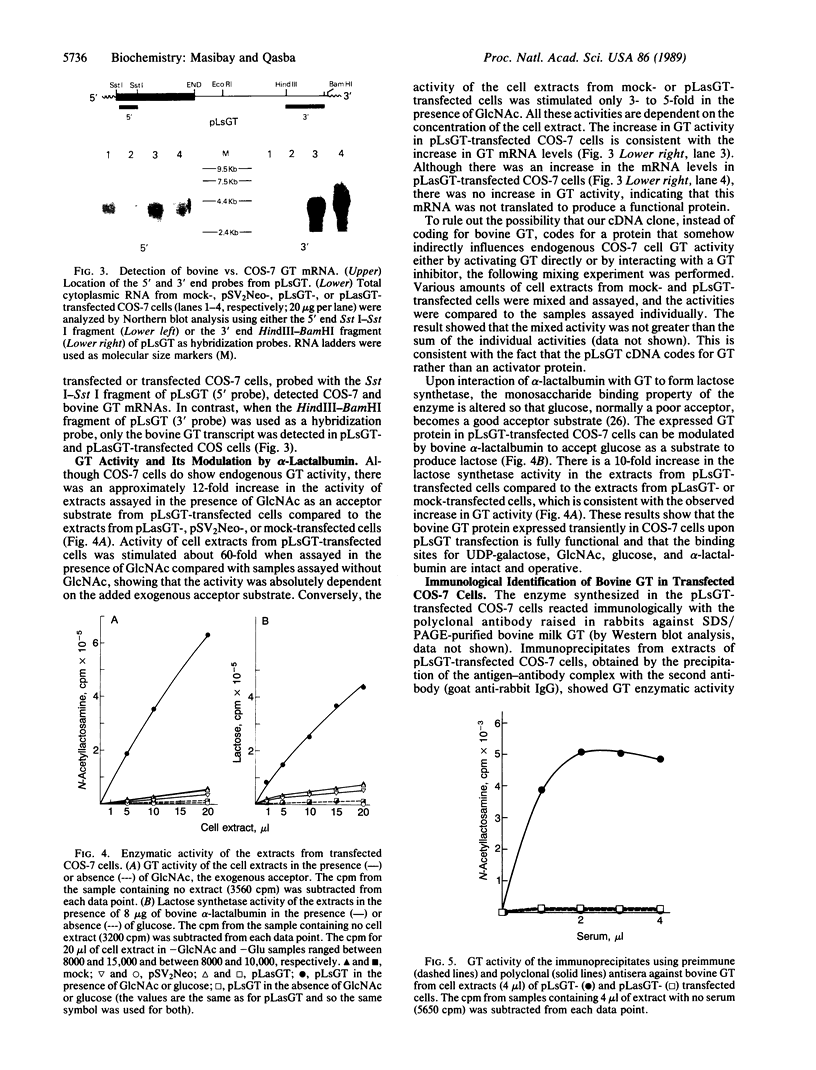
A bovine beta-1,4-galactosyltransferase (GT; EC 2.4.1.90) cDNA in an Okayama-Berg vector, pLsGT, was constructed from a partial cDNA clone and a genomic fragment. We report that the cDNA sequence of pLsGT, in a transient expression assay in COS-7 cells, codes for an enzymatically active GT protein. There is an approximately 12-fold increase in the GT activity in pLsGT-transfected cells compared to cells transfected with the antisense bovine GT construct, pLasGT, or pSV2Neo or mock-transfected cells. The increased activity is correlated with the increase in bovine GT mRNA, which is distinguishable from COS GT mRNA with a 3'-end-specific probe of pLsGT. The expressed GT activity is modulated by alpha-lactalbumin, which changes the acceptor specificity to glucose to synthesize lactose. Polyclonal antibody raised against SDS/PAGE-purified bovine milk GT and a monoclonal antibody (mAb 4-10) directed against a synthetic peptide corresponding to the amino-terminal region of the protein encoded by pLsGT bind the expressed protein, and the resulting immunoprecipitates exhibit GT enzymatic activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appert H. E., Rutherford T. J., Tarr G. E., Wiest J. S., Thomford N. R., McCorquodale D. J. Isolation of a cDNA coding for human galactosyltransferase. Biochem Biophys Res Commun. 1986 Aug 29;139(1):163–168. doi: 10.1016/s0006-291x(86)80094-8. [DOI] [PubMed] [Google Scholar]
- Barker R., Olsen K. W., Shaper J. H., Hill R. L. Agarose derivatives of uridine diphosphate and N-acetylglucosamine for the purification of a galactosyltransferase. J Biol Chem. 1972 Nov 25;247(22):7135–7147. [PubMed] [Google Scholar]
- Beyer T. A., Sadler J. E., Rearick J. I., Paulson J. C., Hill R. L. Glycosyltransferases and their use in assessing oligosaccharide structure and structure-function relationships. Adv Enzymol Relat Areas Mol Biol. 1981;52:23–175. doi: 10.1002/9780470122976.ch2. [DOI] [PubMed] [Google Scholar]
- Chen C. A., Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques. 1988 Jul-Aug;6(7):632–638. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Hill R. L., Brew K. Lactose synthetase. Adv Enzymol Relat Areas Mol Biol. 1975;43:411–490. doi: 10.1002/9780470122884.ch5. [DOI] [PubMed] [Google Scholar]
- Humphreys-Beher M. G., Bunnell B., vanTuinen P., Ledbetter D. H., Kidd V. J. Molecular cloning and chromosomal localization of human 4-beta-galactosyltransferase. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8918–8922. doi: 10.1073/pnas.83.23.8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Ando T., Kimura T., Narimatsu H. Cloning and sequencing of a full-length cDNA of mouse N-acetylglucosamine (beta 1-4)galactosyltransferase. J Biochem. 1988 Aug;104(2):165–168. doi: 10.1093/oxfordjournals.jbchem.a122434. [DOI] [PubMed] [Google Scholar]
- Narimatsu H., Sinha S., Brew K., Okayama H., Qasba P. K. Cloning and sequencing of cDNA of bovine N-acetylglucosamine (beta 1-4)galactosyltransferase. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4720–4724. doi: 10.1073/pnas.83.13.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussinov R. Some guidelines for identification of recognition sequences: regulatory sequences frequently contain (T)GTG/CAC(A), TGA/TCA and (T)CTC/GAG(A). Biochim Biophys Acta. 1986 Mar 26;866(2-3):93–108. doi: 10.1016/0167-4781(86)90106-5. [DOI] [PubMed] [Google Scholar]
- Qasba P. K., Chakrabartty P. K. Purification and properties of two forms of rat alpha-lactalbumin. J Biol Chem. 1978 Feb 25;253(4):1167–1173. [PubMed] [Google Scholar]
- Qasba P. K., Safaya S. K. Similarity of the nucleotide sequences of rat alpha-lactalbumin and chicken lysozyme genes. Nature. 1984 Mar 22;308(5957):377–380. doi: 10.1038/308377a0. [DOI] [PubMed] [Google Scholar]
- Roth S. Are glycosyltransferases the evolutionary antecedents of the immunoglobulins? Q Rev Biol. 1985 Jun;60(2):145–153. doi: 10.1086/414313. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaper N. L., Hollis G. F., Douglas J. G., Kirsch I. R., Shaper J. H. Characterization of the full length cDNA for murine beta-1,4-galactosyltransferase. Novel features at the 5'-end predict two translational start sites at two in-frame AUGs. J Biol Chem. 1988 Jul 25;263(21):10420–10428. [PubMed] [Google Scholar]
- Shaper N. L., Shaper J. H., Meuth J. L., Fox J. L., Chang H., Kirsch I. R., Hollis G. F. Bovine galactosyltransferase: identification of a clone by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1573–1577. doi: 10.1073/pnas.83.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Brew K. Isolation and characteristics of galactosyltransferase from Golgi membranes of lactating sheep mammary glands. J Biol Chem. 1977 Oct 25;252(20):7294–7299. [PubMed] [Google Scholar]
- Smith C. A., Powell J. T., Brew K. Puromycin does not inactivate the galactrosyltransferase of Golgi membranes. Biochem Biophys Res Commun. 1975 Feb 3;62(3):621–626. doi: 10.1016/0006-291x(75)90444-1. [DOI] [PubMed] [Google Scholar]
- Weinstein J., Lee E. U., McEntee K., Lai P. H., Paulson J. C. Primary structure of beta-galactoside alpha 2,6-sialyltransferase. Conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J Biol Chem. 1987 Dec 25;262(36):17735–17743. [PubMed] [Google Scholar]
- Woychik R. P., Camper S. A., Lyons R. H., Horowitz S., Goodwin E. C., Rottman F. M. Cloning and nucleotide sequencing of the bovine growth hormone gene. Nucleic Acids Res. 1982 Nov 25;10(22):7197–7210. doi: 10.1093/nar/10.22.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]



