Abstract
The aim of this study was to investigate an influence of different types of membrane additives including negative charge (dicetylphosphate, DCP), positive charge (stearylamine, STR) and non-ionic molecule (cholesteryl poly-24-oxyethylene ether, SC24) on the physicochemical properties of drug-free and drug-loaded niosomes. Salicylic acid having different proportions of ionized and unionized species at different pH was selected as a model drug. The niosomes were composed of 1:1 mole ratio of Span 60: cholesterol as vesicle forming agents. The results show that incorporation of salicylic acid to the niosomes did not affect zeta potential values; however, addition of the membrane additives changed the zeta potential depending on the type of the additives. Transmission electron microscopy revealed that niosomes had unilamellar structure. The particle sizes of all developed niosomes were between 217 to 360 nm. The entrapment efficiency (%E.E.) of all salicylic acid niosomes at pH 3 was higher than that of niosomes at pH 5, indicating that salicylic acid in unionized form was preferably incorporated in niosomes. Furthermore, the positively charged niosomes showed the highest %E.E. of salicylic acid owing to electrostatic attraction between STR and salicylic acid. After 3 months of storage at 4°C, the particle size of the niosomes remained in the nanosize range except for DCP salicylic acid niosomes at pH 3 whose size increased due to an instability of DCP at low pH. In addition, all niosomes showed no leakage of the salicylic acid after 3 months of storage indicating the good stability.
Key words: entrapment efficiency, membrane additives, niosomes, salicylic acid, transmission electron microscopy
INTRODUCTION
Niosomes (non-ionic surfactant vesicles) are one of drug delivery systems which have been employed as an alternative to liposomes. These non-ionic surfactant vesicles appear to be similar to liposomes in terms of their physical properties, structures and methods of preparation (1,2). On the other hand, niosomes have more advantages over the liposomes, such as entrapment of more substances, higher stability, needlessness of handling or storing in special conditions, and the availability as well as inexpensiveness of prepared materials (1,2). In addition, an increase in numbers of non-ionic surfactants gives a variety of choices for selection and leads to capability of entrapping both hydrophobic and hydrophilic solutes. Niosome applications, therefore, are now being used both in pharmaceutical and cosmetic industrials (1–5).
Niosomes consist of 2 components, which are the main component and membrane additives (2). The compositions of the main component are mainly non-ionic surfactants. Membrane additives are substances that are added in the formulation in order to stabilize the niosomes. The most common additive found in niosomal systems is cholesterol which is known to abolish the gel to liquid phase transition of liposomal and niosomal systems, resulting in less leakiness of the vesicles. However, it may have effects on membrane permeability, encapsulation efficiency, bilayer rigidity, ease of rehydration of freeze dried niosomes and toxicity. In general, it has been found that a molar ratio of 1:1 between cholesterol and non-ionic surfactants is an optimal ratio for the formulation of physically stable niosomal vesicles (2,6,7). There have been several trials to make an effort of finding other substances in substitution to cholesterol. Devaraj et al. (8) found that stable niosomes of polyglyceryl-3-di-isostearate could be prepared with the use of fatty alcohols and polysorbates instead of cholesterol.
One of the methods used to stabilize niosomes is to add a charged molecule to the bilayer. Dicetylphosphate (DCP) and phosphatidic acid are known as negatively charged molecules, while stearylamine (STR) and cetylpyridinium chloride are positively charged molecules, which are both commonly used for preventing aggregation of niosomes (2). Normally, the charged molecule is added in niosomal formulation in an amount of 2.5–5 mol% because the high concentration of charged molecules can inhibit the formation of niosomes (9). The other additive is non-ionic substances in which cholesteryl poly-24-oxyethylene ether (SC24) is one of the widely used non-ionic surfactants (10). It is added in niosomal formulations at the range of 5–10 mol% to provide the steric barrier to vesicle membrane, which prevents vesicle aggregation and leads to enhance of physical stability of niosomes (11).
Until now, a great deal of attention has been paid on incorporation of a great number of drugs in niosomes such as anticancer, anti-tubercular, anti-leishmanial, anti-inflammatory, hormonal drugs and oral vaccine (12). However, an influence of membrane additives on physicochemical properties and stability of niosomes have not been elucidated completely yet.
The aim of this study, therefore, was to investigate the influence of negatively charged, positively charged and non-ionic membrane additives on physicochemical properties and stability of niosomes at different degrees of salicylic acid ionization. Regarding pKa 3 of salicylic acid, the drug would exist in 50% and 99% ionized forms at pH 3 and 5, respectively. Accordingly, salicylic acid niosomes were prepared at these two pH values using Span 60 and cholesterol as vesicle forming agents (2,6,13). In addition, DCP, STR and SC24 were used as membrane additives, representing negatively charged, positively charged and non-ionic molecules. The effects of these membrane additives on the physicochemical properties and stability of salicylic acid niosomes prepared at pH 3 and 5 were investigated. The obtained niosomes were determined in terms of appearance, morphology, pH values, zeta potential, % entrapment efficiency (%E.E.), and size. Stability of the niosomes was evaluated by means of physical characterization and %E.E. at 4°C for 3 months.
MATERIALS AND METHODS
Materials
Salicylic acid was obtained from Rhodia Organique (Lyon, France). STR and cholesterol were purchased from Sigma-Aldrich (St. Louis, USA). Span 60 was kindly provided by Uniqema (Chicago, USA). SC24 was purchased from Amerchol (New Jersey, USA). DCP was from Fluka (Buchs, Switzerland) and sterile water was from General Hospital Products (Bangkok, Thailand). Methanol HPLC grade and phosphoric acid were purchased from J.T. Baker (New Jersey, USA).
Niosome Preparation
In this study, niosomes were prepared by reverse phase evaporation (REV) method (14,15). Briefly, accurate amounts of Span 60 and cholesterol (1:1 mole ratio) and salicylic acid (3 mol% of total lipid) were dissolved in diethyl ether in a stopped cock Erlenmeyer flask. The mixture was shaken until completely dissolved. Hydration medium, citrate buffer 1.13 mM at pH 3 or 5 which had high buffer capacity was subsequently added to the lipid mixture and mixed until a homogeneous w/o emulsion was obtained (the volume ratio of diethyl ether and hydration medium used was 1.5:1). The w/o emulsion was sonicated for 10 min at 7°C (Ultrasonic bath, Mettler Electronics, California, USA). Afterwards, diethyl ether was slowly removed under reduced pressure using a rotary evaporator (60 rpm) and temperature was controlled at 45°C until niosomal suspension was completely formed. The dispersion was evaporated until no smell of diethyl ether was detected. The niosome suspension was finally obtained.
In order to obtain niosomes with homogeneous size, REV vesicles were sonicated for 15 min and subjected to extrusion process by an extruder device (Lipex™, Northern Lipids, British Columbia, Canada), equipped with a 200 nm pore size polycarbonate membrane (Nuclepore®, Whatman, Maidstone, England) (11). All preparations were subjected to extrusion for 3 times. The maximum pressure was set at 800 psi as well as the temperature was controlled at 45°C during the extrusion process.
Appearance and Morphology
Visual Observation
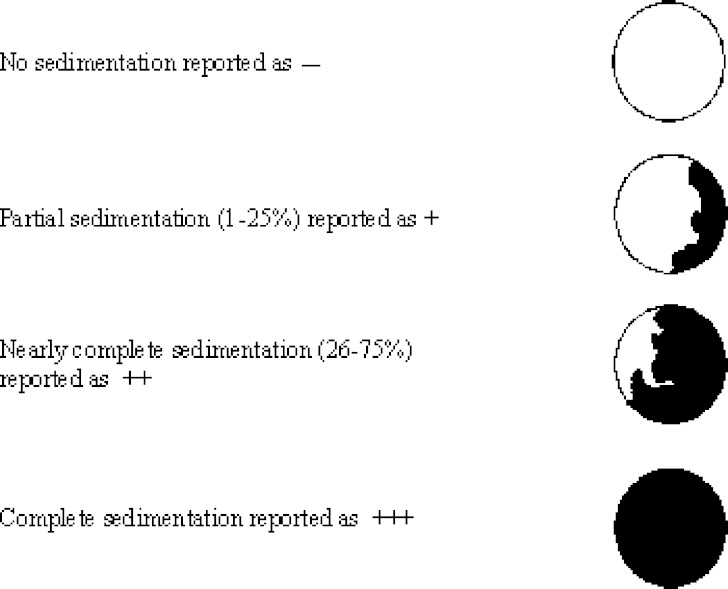
Sedimentation, flocculation and turbidity were visually observed and reported as degrees of sedimentation using the criteria as shown in Fig. 1. The experiment was performed in triplicate.
Fig. 1.
The bottom-view of bottle showed different degrees of sedimentation
Optical Microscopy
Nanoparticulated systems were investigated by means of an Olympus BH-2 microscope (Model BH-2, Olympus, Tokyo, Japan) at magnification of 400× equipped with digital camera. A small droplet of the vesicle suspension was placed on a glass microscope slide, diluted with a few drops of distilled water and covered with a glass cover slip. The samples were examined for vesicle formation, crystal formation and vesicular size.
Transmission Electron Microscopy (TEM)
The vesicle formulations were examined by transmission electron microscopy (Hitachi Model H-7000, Tokyo, Japan) in order to determine size, shape and lamellarity. A 200 mesh formvar copper grid was floated on a droplet of niosome dispersion on parafilm or dental wax for 10 min to allow some vesicles adhere on the formvar. The remaining dispersion was removed by absorbing with the corner of a filter paper. The grid was then transferred onto a nearby drop of negative stain solution (2% uranyl acetate solution) and left for 10 min, blotted excess solution with a filter paper, and air-dried for 30 min. Finally, the sample was observed under a transmission electron microscope.
pH Measurement
The pH of niosomes was measured by a pH meter (Accumet® basic model AB15, Fisher Scientific, Pennsylvania, USA). The pH measurement was performed at 25°C.
Zeta Potential Measurement
Zeta potential of suitably diluted niosome dispersion was determined using zeta potential analyzer based on electrophoretic light scattering and laser Doppler velocimetry method (Zetaplus™, Brookhaven Instrument Corporation, New York, USA). The temperature was set at 25°C. Charge on vesicles and their mean zeta potential values with standard deviation of 5 measurements were obtained directly from the measurement.
Particle Size Measurement
The mean particle size of niosomes was determined by submicron particle analyzer based on photon correlation spectroscopy (PCS) (Coulter counter®, Model N4MD, Coulter Corporate Communication, Florida, USA). A small aliquot of niosome dispersion was dispersed in about 3 mL of deionized-distilled water in a cuvette. The diluted sample was mixed by turning the cuvette up-side down 5 times and immediately measured. The measurement was taken about 120 sec and repeated 6 times for each sample.
Entrapment Efficiency Measurement
Free salicylic acid was separated from niosome-entrapped salicylic acid by ultracentrifugation at 40,000 rpm and 4°C for 30 minutes using an ultracentrifuge model LE-80K with rotor type 70Ti (Beckman, California, USA). Supernatant containing free salicylic acid was collected and analyzed by HPLC method. A 100 μL aliquot of supernatant was diluted with 500 μL of citrate buffer pH 3 or 5 and methanol at the ratio of 1:1 v/v. The diluted solution was mixed by vortex mixer and automatically injected into HPLC column. Briefly, analysis of salicylic acid was performed by HPLC at the wavelength of 235 nm on a Shimadzu-10D (Shimadzu, Kyoto, Japan). The stationary phase was 300 × 3.9 mm i.d. column pack with 10 μm μBondapak® (Water, Ireland). The mobile phase consisted of phosphate buffer pH 2.3 and methanol at the ratio of 65:35 (v/v). The flow rate was 1.2 mL/min. The injection volume was 20 μL. Prior to the analysis, validation of the HPLC method was performed to ensure linearity of the calibration curve between 1.5 to 30 µg/mL and coefficients of variation were less than 5% both intraday and interday. %E.E. of salicylic acid was calculated using the following equation:
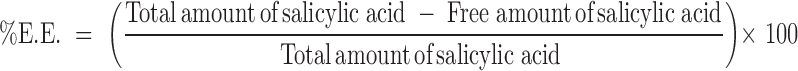 |
1 |
Stability of Salicylic Acid Niosomes
About 5 mL of extruded niosomes were kept in glass-bottles with plastic plug lidded by a screwed cap and kept at 4°C for 3 months. The physical characteristics in terms of zeta potential, mean particle size, and %E.E. at predetermined intervals were evaluated.
Statistical Analysis
Statistical analysis of differences in the physicochemical properties among predetermined intervals in the same formulation and between formulations was performed by using one-way analysis of variance (one-way ANOVA) and paired-t test, respectively. The level of significance was taken at p value of 0.05.
RESULTS AND DISCUSSION
Appearance and Morphology
Visual Observation
Table I shows the compositions of all developed niosomes. All niosomes except for STR salicylic acid niosomes at pH 5 appeared in translucent white dispersions without sedimentation, indicating that the niosomes were physical stable due to small and uniform vesicle sizes obtained after the extrusion process. The formulations with an addition of the membrane additives showed more turbid and whitish; however, the niosomes could not be formed with the addition of STR at pH 5.
Table I.
The Compositions of All Developed Niosome Formulations in 80 ml Citrate Buffer
| Compositions (mg) | Formulation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Blank pH 3 | Blank pH 5 | Control pH 3 | Control pH 5 | DCPa pH 3 | DCPa pH 5 | STRb pH 3 | STRb pH 5 | SC24c pH 3 | SC24c pH5 | |
| Span 60 | 517.2 | 517.2 | 517.2 | 517.2 | 517.2 | 517.2 | 517.2 | 517.2 | 517.2 | 517.2 |
| Cholesterol | 464.4 | 464.4 | 464.4 | 464.4 | 464.4 | 464.4 | 464.4 | 464.4 | 464.4 | 464.4 |
| Salicylic acid | – | – | 9.94 | 9.94 | 9.94 | 9.94 | 9.94 | 9.94 | 9.94 | 9.94 |
| Dicetylphosphate | – | – | – | – | 65.6 | 65.6 | – | – | – | – |
| Stearylamine | – | – | – | – | – | – | 32.4 | 32.4 | – | – |
| Cholesteryl poly-24-oxyethylene ether | – | – | – | – | – | – | – | – | 175.2 | 175.2 |
| Final pH | 3.0 | 5.0 | 3.0 | 5.0 | 3.0 | 5.0 | 3.0 | 5.0 | 3.0 | 5.0 |
aDCP was a formulation which added dicetylphosphate.
bSTR was a formulation which added stearylamine.
cSC24 was a formulation which added cholesteryl poly-24-oxyethylene ether.
Optical Microscopy
Under the optical microscope, aggregation of the vesicles could be observed for all niosomes before size reduction. After submitted to the extrusion process, nonaggregated niosomes were obtained. Unfortunately, information concerning microstructure of niosomes could not be visualized by the low-magnification power of optical microscope (lower of detection ∼200 nm), therefore TEM was employed to elucidate niosome morphology.
Transmission Electron Microscopy (TEM)
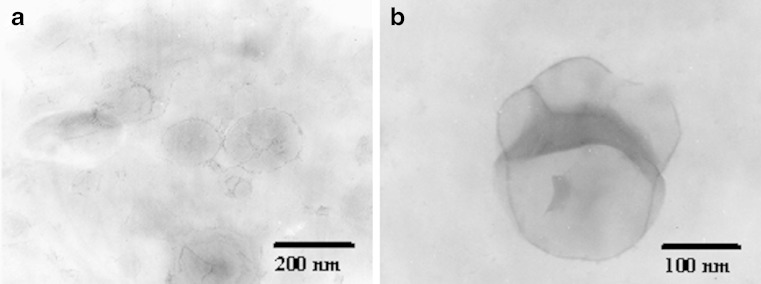
From Fig. 2, the STR and SC24 salicylic acid niosomes were spherical large unilamellar vesicles which were a vesicular structure of the REV niosomes (16). Similar results were observed in other formulations (data not shown). Moreover, the fusion of niosome was occasionally observed as depicted in Fig. 2b.
Fig. 2.
Transmission electron photomicrographs of salicylic acid niosomes prepared with (a) stearylamine (STR) at pH 3 and (b) cholesteryl poly-24-oxyethylene ether (SC24) at pH 5
pH Measurement
The pH values of all niosome formulations are listed in Table II. Salicylic acid is a weak acid (pKa 3) which can be dissolved and ionized in a hydration medium as illustrated by the following equation:
| 2 |
Table II.
The pH Values, the Zeta Potential and the Percentage of Entrapment Efficiency (%E.E.) of Freshly Prepared Salicylic Acid Niosomes
| Formulation | pH valuea | Zeta potentialb (mV) | Particle sizec (nm) | %E.E.a |
|---|---|---|---|---|
| Blank pH 3 | 3.00 ± 0.00 | −16.34 ± 0.23 | 284.39 ± 2.51 | – |
| Blank pH 5 | 5.02 ± 0.00 | −20.38 ± 0.26 | 281.94 ± 0.42 | – |
| Control pH 3 | 2.91 ± 0.00 | −15.70 ± 0.29 | 314.28 ± 15.94 | 14.53 ± 0.61 |
| Control pH 5 | 4.28 ± 0.01 | −19.06 ± 0.47 | 266.33 ± 13.13 | 3.06 ± 0.89 |
| DCP pH 3 | 2.84 ± 0.01 | −22.16 ± 0.32 | 360.33 ± 9.07 | 9.54 ± 1.33 |
| DCP pH 5 | 3.67 ± 0.03 | −49.19 ± 1.23 | 324.17 ± 2.47 | 1.94 ± 0.84 |
| STR pH 3 | 3.14 ± 0.02 | 23.66 ± 0.52 | 223.06 ± 3.60 | 34.99 ± 0.39 |
| SC24 pH 3 | 2.91 ± 0.02 | −2.37 ± 0.17 | 216.72 ± 9.45 | 14.76 ± 0.39 |
| SC24 pH 5 | 4.29 ± 0.03 | −7.55 ± 0.16 | 265.44 ± 5.58 | 4.60 ± 0.59 |
aThe data were reported as an average of 3 measurements (mean±S.D.).
bThe data were reported as an average of 5 measurements (mean±S.D.).
cThe data were reported as an average of 6 measurements (mean±S.D.).
From Table II, the pH values of both blank niosomes at pH 3 and 5 were remained at 3.00 and 5.02, respectively. After adding salicylic acid, the pH of niosomal dispersions decreased due to the presence of hydronium ion from the ionization process. At pH 5, salicylic acid was fully ionized which gave rise in more hydronium ion concentration. Therefore, the decrease of pH of solution at pH 5 was more pronounced than that of salicylic acid niosomes at pH 3 (Table II).
Concerning an effect of membrane additives on the pH of solution, DCP and STR are negative and positive charges which could be ionized and gave rise in hydronium and hydroxyl ion concentrations, respectively, as follows:
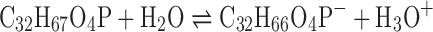 |
3 |
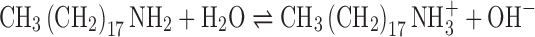 |
4 |
As a result, the addition of DCP into salicylic acid niosomes promoted a decrease of pH of such dispersions, as indicated by Eq. 3. On the other hand, the pH value of salicylic acid niosomes was raised after addition of STR according to Eq. 4.
For non-ionic charged membrane additive (SC24), the pH of salicylic acid niosomes at pH 3 and 5 did not change significantly as compared to the control formulations (p > 0.05), due to the fact that SC24 does not provide an ion into the dispersion medium.
Zeta Potential Measurement
Zeta potential values of all niosome formulations are provided in Table II. The blank niosomes exhibited negative values of the zeta potential which might be attributed to the adsorption of counter ions or the preferential adsorption of hydroxyl ions at the vesicle surface. In comparison, the zeta potential values of the blank niosomes at pH 5 was higher than those at pH 3 since the high concentration of hydroxyl ion at higher pH could adsorb on the surface of niosomes, leading to the higher zeta potential.
After incorporation of salicylic acid (the control formulations), the final pH of niosomes both at pH 3 and pH 5 slightly reduced as compared to the blank niosomes (Table II), resulting in slightly reduction of the zeta potential values. However, no significant difference in the zeta potential values between the blank and the control formulations was found (p > 0.05). On the other hand, the addition of the membrane additives affected the zeta potential values depending on the type of the membrane additives. The zeta potential of DCP salicylic acid niosomes at pH 5 was much higher than that at pH 3 due to the fact that DCP introduced the negative charge onto the surface of niosomes and the extent of ionization increased at higher pH (pKa of DCP 4.5). At pH 5, the process of dissociation reached completion, leading to large increase in zeta potential of DCP salicylic acid niosomes as compared to that at pH 3. Conversely, STR introduced positive charge via the protonation of basic -NH2 group. Subsequently, it adsorbed on the surface of the niosomes leading to the positive zeta potential values of 23.66 mV.
The decrease in zeta potential after inclusion of SC24 in the salicylic niosomes as compared to the control formulations was due to the absorption of polyoxyethylene chain of SC24 on the surface of salicylic acid niosomes, leading to the shift of shear plane and subsequently reduction of zeta potential values. The degree of reduction of zeta potential values from the absorption of chemical like polymer was different depending on surface density and the length of polymer chain (17–19).
Particle Size Analysis
After production, the mean particle sizes determined by PCS of all developed niosomes were in the range of 217 to 360 nm, as seen in Table II. The average particle sizes of the blank formulations at pH 3 and 5 were comparable meaning that the pH of the dispersions did not affect the particle size. However, the particle sizes of the control formulations at pH 3 and 5 were different. At pH 3, salicylic acid was in 50% ionized form, and unionized salicylic acid might be incorporated within the bilayer of vesicles resulting in increasing particle size. In contrast, the particle size of the control formulation at pH 5 was of the same magnitude as the blank at pH 5 because the drug was present in almost 100% ionized form which could not be incorporated within the bilayer. However, it should be kept in mind that PCS results reflect overall particle size measurement and cannot discern whether these reflect primary or secondary particle sizes, e.g. aggregation of particles.
The inclusion of charged inducing agents into niosomes was found to have strong influence on their particle size as shown in Table II. It could be described on the basis of different mechanisms in forming charged vesicles, which spontaneously occurred during hydration process. When hydrating with a certain pH of buffer, certain types of charge developed on the charge-inducing agents and the drug molecules followed by the orientation of non-ionic surfactant molecules into bilayers membrane. The bilayer membrane would subsequently curve and split up to form closed vesicles so as to reduce its free energy. The types of charge developed within the bilayer membrane just before the formation of vesicles may likely modify not only the rigidity of the membrane but also the rate of curving, splitting, and vesicle forming, which in turn determined the size and size distribution of vesicles (20). In general, an incorporation of charged molecules into the bilayer increases the volume of the aqueous compartment by separating adjacent bilayers in the MLV due to charge repulsion, resulting in increase in the particle size (21–24). In contrast, some studies revealed that the inclusion of the charged molecules tended to reduce the size of vesicles (25–27). As a result from Table II, the inclusion of DCP into salicylic acid niosomes showed relatively large in the average particle sizes in comparison to the control formulations both at pH 3 and 5 whereas the inclusion of STR into salicylic acid niosomes showed much smaller in the particle size as compared to the control at pH 3. The increase in the particle size of DCP niosomes could be explained by the different in chemical structures of Span 60 and DCP. DCP is composed of two cetyl chains whereas Span 60 comprises of stearyl chain. During the formation of vesicle tightly packed bilayers could not be formed resulting in reduction of membrane curvature and obtaining the larger particle size. In case of STR niosomes, both Span 60 and STR are composed of stearyl chain in the molecule. Consequently, the closely packed bilayers could be achieved during the formation of vesicle leading to increase of membrane curvature and reduction in the particle size.
Interestingly, the addition of SC24, non-charged molecule, into salicylic acid niosomes at pH 3 showed the smaller particle sizes as compared to those of the control formulations. The similar finding was reported by Levchenko et al. (17) who indicated that the inclusion of PEG 750-PE and PEG 5000-PE into liposomes resulted in the reduction of liposomal size. Concerning the chemical structure of SC24, it is composed of cholesterol conjugated with polyoxyethylene chain. Hence, incorporation of SC24 into salicylic acid niosomes resulted in the closely packed bilayers due to the strong interaction of SC24 with cholesterol and Span 60. Moreover, the steric shielding from SC24 might lead to the reduction of particle aggregation tendency of salicylic acid niosomes at pH 3. As the results, the smaller particle sizes were obtained.
Entrapment Efficiency Measurement
% E.E. of all developed niosomes is shown in Table II. In general, REV method produces a large unilamellar vesicle with a large internal aqueous core (16). It is suitable for encapsulating water soluble compounds. At pH 5, salicylic acid appeared in ionized form almost 100%, therefore the control at pH 5 was expected to show the higher %E.E. than the control at pH 3. However, %E.E. of the control at pH 3 was found to be almost five times greater than that at pH 5 (Table II). It could be described in terms of a partition-like equilibrium (19). An increase in pH from 3 to 5 induced a decrease in %E.E. from 14.53% to 3.06% (Table II), indicating the preference of entrapment of unionized salicylic acid. It was assumed that uptake of ionized and unionized species by vesicles occurred independently and that the process can be represented as a simple partitioning between the vesicle and the aqueous phase, in which the distribution coefficients of salicylic acid are defined by Eqs. 5 and 6.
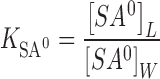 |
5 |
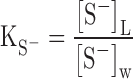 |
6 |
where K is the distribution coefficient, SA0 refers to unionized salicylic acid, S− is salicylate, L is lipid phase, and W is water. The brackets indicate concentrations.
The amount entrapped (E) is the sum of the amounts of the two species associated with the vesicles, which can be written as
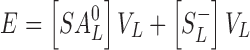 |
7 |
where VL is the volume of the lipid (vesicle) phase. Substitution of Eqs. 5 and 6 into Eq. 7 and rearrangement lead to
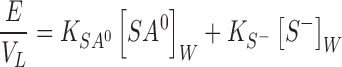 |
8 |
where E is obtained from the experiment, [SA0]W and [S−]W can be calculated at each value of pH from the definition of ionization constant. The pKa of salicylic acid is 3. By inserting appropriate values for pH 3 and 5 and solving for the distribution coefficients in Eq. 8 simultaneously, the values of KS-of the control, DCP and SC24 formulations were found to be 0.0298, 0.0187 and 0.0461, respectively, while those of KSA0 were 0.2753, 0.1813 and 0.2640, respectively. Consequently, the ratios of KSA0 to KS-of the control, DCP and SC 24 formulation were turned out to be 9.24, 9.69 and 5.73. The large values of the ratios of KSA0 to KS-indicate that salicylic acid in unionized form distributes into lipid bilayer of niosome vesicles greater than the ionized species. Accordingly, the results from Table II show that %E.E. of all formulations at pH 3 with half ionization increased as compared to that at pH 5 of fully ionization, which agreed with the discussion above.
Another possible reason to decrease in %E.E. at pH 5 was due to the fact that the leakage of entrapped ionized salicylic acid may occur during the size reduction by the extrusion process. In contrast, salicylic acid appears equally in ionized and unionized forms at pH 3, which are capable to be entrapped in the internal aqueous core or intercalated within the bilayers, respective. The extrusion process did not affect the embedded drug in bilayers, therefore %E.E. of all niosomes at pH 3 was higher than that at pH 5. In addition, the inclusion of cholesterol in the bilayers was also found to increase an entrapment efficiency of hydrophobic drug as previously reported by Bernsdorff et al. (28)
The inclusion of DCP into salicylic acid niosomes was found to decrease %E.E. with respect to the control formulations at both pHs, which was presumably due to the electrostatic repulsion forces between the carboxyl group of salicylic acid and an anionic head group of DCP. In contrast, the inclusion of STR into salicylic acid niosomes at pH 3 appeared to increase %E.E. of salicylic acid by 2 times greater than the control at the same pH, owing to the electrostatic attraction between the positively charged head group of STR and the carboxyl group of dissociated salicylic acid (29). In case of non-charged niosomes, %E.E. of SC24 niosomes at pH 3 was not significantly different than the control at pH 3 (p > 0.05), while %E.E. of SC24 niosomes at pH 5 was higher than the control at pH 5 (p < 0.05). It had been shown elsewhere that polymers can interact with drug molecules via electrostatic bonds (i.e. ion–ion, ion–dipole, dipole–dipole) but other types of forces, such as van der Waals forces and hydration bridges, may frequently participate in the complex formation (30). The results from Table II indicate that SC24 tended to interact mainly with ionized salicylic acid through dipole–ion interaction between polyoxyethylene chain of SC24 and carboxyl group of ionized salicylic acid resulting in increasing %E.E. of SC24 niosomes at pH 5. On the other hand, there was less ionized salicylic acid in SC24 niosomes at pH 3, and the inclusion of SC24 did not affect the encapsulated unionized salicylic acid, leading to no distinctive difference in %E.E. as compared to the control at pH 3.
Long Term Physical Stability of Developed Niosomes
To investigate the long-term physical stability of all developed niosomes, they were stored at 4°C for 3 months.
Physical Appearance
The degrees of sedimentation of all niosomes were recorded at 0, 7, 14, 28, 56 and 84 days, as shown in Table III. After storage at 4°C for 14 days, the formulation without membrane additives showed complete sedimentation, while the formulations with membrane additives showed only partial sedimentation which attributed to the presence of charged species and steric stabilizer reducing the likelihood of vesicle aggregation. After 84 days, the formulations with membrane additives were partial and almost sedimentation with ease of redispersion except for DCP at pH 3 which was complete sedimentation which might be due to the instability of DCP at low pH (31).
Table III.
Degrees of Sedimentation of Salicylic Acid Niosomes After Being Stored at 4°C for 3 months
| Formulation | 0 day | 7 days | 14 days | 28 days | 56 days | 84 days |
|---|---|---|---|---|---|---|
| Blank pH 3 | − | ++ | +++ | +++ | +++ | +++ |
| Blank pH 5 | − | ++ | +++ | +++ | +++ | +++ |
| Control pH 3 | − | +++ | +++ | +++ | +++ | +++ |
| Control pH 5 | − | + | ++ | ++ | ++ | ++ |
| DCP pH 3 | − | ++ | ++ | +++ | +++ | +++ |
| DCP pH 5 | − | + | + | + | ++ | ++ |
| STR pH 3 | − | + | + | ++ | ++ | ++ |
| SC24 pH 3 | − | + | + | ++ | ++ | ++ |
| SC24 pH 5 | − | + | + | + | ++ | ++ |
− no sedimentation, + (1–25%) partial sedimentation, ++ (26–75%) nearly complete sedimentation,
+++ complete sedimentation
Zeta Potential Measurement
Table IV shows the zeta potential of all niosomes which gradually decreased during storage time, due to the low zeta potential of the niosomes after production (<|30 mV|), therefore the aggregation of niosomes was likely to occur as indicated by the increase in the particle size of niosomes. Given the fact that the zeta potential depends on the movement of particles (electrophoretic velocities), the aggregated particles show the slow movement resulting in the decrease of zeta potential during storage. In this experiment, DCP niosomes at pH 5 were found to be the most stable formulation due to the highest zeta potential. In case of SC24 niosomes at pH 3 and 5, the low zeta potential values caused by the masking electrostatic charge ability of the polymer could not be used to determine the niosome stability. The main mechanism enhancing the physical stability of niosomes stabilized by non-ionic surfactant is steric stabilization. It can be stated that not only electrostatic stabilization but also steric stabilization should be considered as the most important indicators of niosomal stability indicated by the mean particle size (cf. next section).
Table IV.
The Zeta Potential Values of Salicylic Acid Niosomes after Being Stored at 4°C for 3 months
| Formulation | Zeta potentiala | ||
|---|---|---|---|
| 0 day | 28 days | 84 days | |
| Blank pH 3 | −16.34 ± 0.23 | −3.51 ± 0.15 | −3.29 ± 0.32 |
| Blank pH 5 | −20.38 ± 0.26 | −10.95 ± 0.33 | −12.33 ± 0.19 |
| Control pH 3 | −15.70 ± 0.29 | −2.73 ± 0.05 | −2.94 ± 0.42 |
| Control pH 5 | −19.06 ± 0.47 | −10.12 ± 0.10 | −9.56 ± 0.84 |
| DCP pH 3 | −22.16 ± 0.32 | −4.47 ± 0.18 | −5.53 ± 0.72 |
| DCP pH 5 | −49.19 ± 1.23 | −29.74 ± 1.81 | −31.73 ± 1.09 |
| STR pH 3 | 23.66 ± 0.52 | 10.91 ± 0.57 | 6.81 ± 0.45 |
| SC24 pH 3 | −2.37 ± 0.17 | 3.06 ± 0.08 | 1.38 ± 0.36 |
| SC24 pH 5 | −7.55 ± 0.16 | −2.91 ± 0.28 | −2.78 ± 0.30 |
aThe reported data were the average of five measurements (mean±S.D.).
Particle Size Measurement
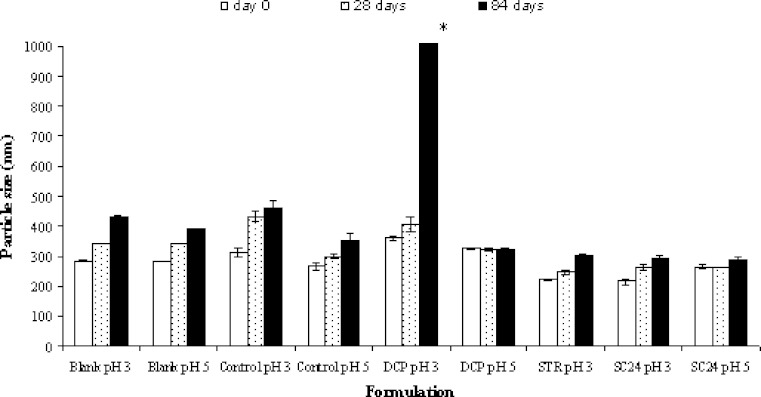
Figure 3 shows the particle sizes of all niosomes over 3 months of storage. The results demonstrated that the blank and the control formulations exhibited an increase in mean particle as compared to those at production day. The addition of membrane additives increased the long-term physical stability of the salicylic acid niosomes as seen by the reduction in particle growth, except for DCP salicylic acid niosomes at pH 3. The increase in the particle size of DCP at pH 3 is due to the instability of DCP as previously mentioned.
Fig. 3.
The particle size measurement of the niosome formulations as a function of time. The data represent the mean ± S.D. of six measurements. Asterisk The mean particle size was higher than 3,500 nm
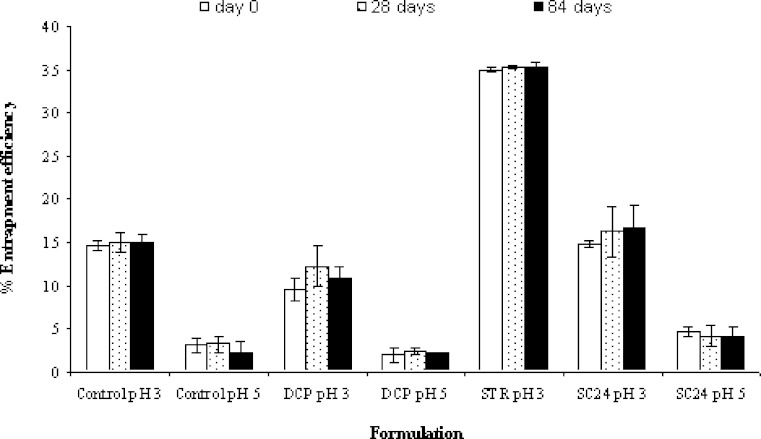
% Entrapment efficiency measurement
Figure 4 shows %E.E. of the niosomes stored at 4°C for 84 days. It was found that %E.E. of all formulations did not change significantly during 3 months (p > 0.05). The results indicated that all niosomes showed relatively good stability in %E.E. whether membrane additives were added or not. Considering the compositions of each formulation, it was found that 1:1 ratio of Span 60 and cholesterol was used as vesicle forming agents in all formulations. The beneficial role of cholesterol within vesicular drug carriers is well established, for example, increasing the stability and reducing the permeability of bilayers (32–34). Therefore, it was clearly supported that the use of cholesterol at equimolar ratio with lipid could stabilize the entrapment efficiency of niosomes. In addition, the rigidifying effect at this amount of cholesterol in the bilayers was able to prevent the leakage of the drug upon storage (20).
Fig. 4.
Percent entrapment efficiency of the niosome formulations as a function of time. The data represent the mean ± S.D. of three measurements
CONCLUSION
In conclusion, the high %E.E. of salicylic acid niosomes at pH 3 indicated that unionized salicylic acid was favored to be incorporated in the niosome vesicles. The addition of the membrane additives affected the particle size, zeta potential, and %E.E. depending on the interaction between the additives and salicylic acid. With respect to the long-term physical stability, the addition of the membrane additives increased physical stability but did not affect %E.E. of the niosomes due to the rigid bilayer membrane composed of 1:1 Span 60 and cholesterol.
Footnotes
All the listed authors have read and approved this manuscript.
References
- 1.Baillie A. J., Florence A. T., Hume L. R., Muirhead G. T., Rogerson A. The preparation and properties of niosomes-non-ionic surfactant vesicles. J. Pharm. Pharmacol. 1985;37:863–868. doi: 10.1111/j.2042-7158.1985.tb04990.x. [DOI] [PubMed] [Google Scholar]
- 2.Uchegbu I. F., Vyas S. P. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int. J. Pharm. 1998;172:33–70. doi: 10.1016/S0378-5173(98)00169-0. [DOI] [Google Scholar]
- 3.Uchegbu I. F., Turton J. A., Double J. A., Florence A. T. Drug distribution and a pulmonary adverse effect of intraperitoneally administered doxorubicin niosomes in the mouse. Biopharm. Drug Dispos. 1994;15:691–707. doi: 10.1002/bdd.2510150807. [DOI] [PubMed] [Google Scholar]
- 4.Weiner N., Lieb L., Niemiec S., Ramachandran C., Hu Z., Egbaria K. Liposomes: a novel topical delivery system for pharmaceutical and cosmetic applications. J. Drug Target. 1994;2:405–410. doi: 10.3109/10611869408996816. [DOI] [PubMed] [Google Scholar]
- 5.Choi M. J., Maibach H. I. Liposomes and niosomes as topical drug delivery systems. Skin Pharmacol. Physiol. 2005;18:209–219. doi: 10.1159/000086666. [DOI] [PubMed] [Google Scholar]
- 6.Hao Y., Zhao F., Li N., Yang Y., Li K. Studies on a high encapsulation of colchicines by niosome system. Int. J. Pharm. 2002;244:73–80. doi: 10.1016/S0378-5173(02)00301-0. [DOI] [PubMed] [Google Scholar]
- 7.Nasseri B. Effect of cholesterol and temperature on the elastic properties of niosomal membranes. Int. J. Pharm. 2005;300:95–101. doi: 10.1016/j.ijpharm.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Devaraj G. N., Parakh S. R., Devraj R., Apte S. S., Rao B. R., Rambhau D. Release studies on niosomes containing fatty alcohols as bilayer stabilizers instead of cholesterol. J. Colloid. Interface. Sci. 2002;251:360–365. doi: 10.1006/jcis.2002.8399. [DOI] [PubMed] [Google Scholar]
- 9.Hu C., Rhodes D. G. Proniosomes: a novel drug carrier preparation. Int. J. Pharm. 1999;185:23–35. doi: 10.1016/S0378-5173(99)00122-2. [DOI] [PubMed] [Google Scholar]
- 10.Dimitrijevic D., Lamandin C., Uchegbu I. F., Shaw A. J., Florence A. T. The effect of monomers and of micellar and vesicular forms of non-ionic surfactants (Solulan C24 and Solulan 16) on Caco-2 cell monolayers. J. Pharm. Pharmacol. 1997;49:611–616. doi: 10.1111/j.2042-7158.1997.tb06854.x. [DOI] [PubMed] [Google Scholar]
- 11.Arunothayanun P., Sooksawate T., Florence A. T. Extrusion of niosomes from capillaries: approaches to a pulsed delivery device. J. Control Release. 1999;60:391–397. doi: 10.1016/S0168-3659(99)00095-4. [DOI] [PubMed] [Google Scholar]
- 12.Manosroi A., Wongtrakul P., Manosroi J., Sakai H., Sugawara F., Yuasa M. Characterization of vesicles prepared with various non-ionic surfactants mixed with cholesterol. Colloids Surf B: Biointerfaces. 2003;30:129–138. doi: 10.1016/S0927-7765(03)00080-8. [DOI] [Google Scholar]
- 13.Yoshioka T., Sternberg B., Florence A. T. Preparation and properties of vesicles (niosomes) of sorbitan monoesters (Span 20, 40, 60 and 80) and a sorbitan triester (Span 85) Int. J. Pharm. 1994;105:1–6. doi: 10.1016/0378-5173(94)90228-3. [DOI] [Google Scholar]
- 14.Perugini P., Genta I., Pavanetto F., Conti B., Scalia S., Baruffini A. Study on glycolic acid delivery by liposomes and microspheres. Int. J. Pharm. 2000;196:51–61. doi: 10.1016/S0378-5173(99)00439-1. [DOI] [PubMed] [Google Scholar]
- 15.Fresta M., Puglisi G., Panico A. M., Marco S. D., Mazzone G. CDP-choline entrapment and release from multilamellar and reverse-phase evaporation liposomes. Drug Dev. Ind. Pharm. 1993;19:559–585. doi: 10.3109/03639049309062967. [DOI] [Google Scholar]
- 16.Guinedi A. S., Mortada N. D., Mansour S., Hathout R. M. Preparation and evaluation of reverse-phase evaporation and multilamellar niosomes as ophthalmic carriers of acetazolamide. Int. J. Pharm. 2005;306:71–82. doi: 10.1016/j.ijpharm.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Levchenko T. S., Rammohan R., Lukyanov A. N., Whiteman K. R., Torchilin V. P. Liposome clearance in mice: the effect of a separate and combined presence of surface charge and polymer coating. Int. J. Pharm. 2002;240:95–102. doi: 10.1016/S0378-5173(02)00129-1. [DOI] [PubMed] [Google Scholar]
- 18.Vyas S. P., Venkatesan N. Poly(phthaloyl-L-lysine)-coated multilamellar vesicles for controlled drug delivery: in vitro and in vivo performance evaluation. Pharm. Acta Helv. 1999;74:51–58. doi: 10.1016/S0031-6865(99)00016-3. [DOI] [PubMed] [Google Scholar]
- 19.Carrion F. J., De La Maza A., Parra J. L. The influence of ionic strength and lipid bilayer charge on the stability of liposomes. J. Colloid Interface Sci. 1994;164:78–87. doi: 10.1006/jcis.1994.1145. [DOI] [Google Scholar]
- 20.I. Nooprasit. Influence of lipid composition, liposome charge and pH of hydration medium on the physicochemical properties and stability of amphotericin B liposomes. Master Thesis in Pharmaceutics, Faculty of Pharmacy, Mahidol University; Bangkok, Thailand, 2000.
- 21.Weiner N., Martin F. J., Riaz M. Liposomes as a drug delivery system. Drug Dev. Ind. Pharm. 1989;15:1523–1554. doi: 10.3109/03639048909052502. [DOI] [Google Scholar]
- 22.Carafa M., Santucci E., Lucania G. Lidocaine-loaded non-ionic surfactant vesicles: characterization and in vitro permeation studies. Int. J. Pharm. 2002;231:21–32. doi: 10.1016/S0378-5173(01)00828-6. [DOI] [PubMed] [Google Scholar]
- 23.Fang J. Y., Hong C. T., Chiu W. T., Wang Y. Y. Effect of liposomes and niosomes on skin permeation of enoxacin. Int. J. Pharm. 2001;219:61–72. doi: 10.1016/S0378-5173(01)00627-5. [DOI] [PubMed] [Google Scholar]
- 24.Alpar O. H., Bamford J. B., Walters V. The in vitro incorporation and release of hydroxocobalamin by liposomes. Int. J. Pharm. 1981;7:349–351. doi: 10.1016/0378-5173(81)90062-4. [DOI] [Google Scholar]
- 25.Namdeo A., Jain N. K. Niosomal delivery of 5-fluorouracil. J. Microencapsul. 1999;16:731–740. doi: 10.1080/026520499288672. [DOI] [PubMed] [Google Scholar]
- 26.Perrett S., Golding M., Williams W. P. A simple method for the preparation of liposomes for pharmaceutical applications: characterization of the liposomes. J. Pharm. Pharmacol. 1991;43:154–161. doi: 10.1111/j.2042-7158.1991.tb06657.x. [DOI] [PubMed] [Google Scholar]
- 27.Hal D. A. V., Bouwstra J. A., Rensen A. V., Jeremiasse E., Vringer T. D., Junginger H. E. Preparation and characterization of non-ionic surfactant vesicles. J. Colloid Interface Sci. 1996;178:263–273. doi: 10.1006/jcis.1996.0114. [DOI] [Google Scholar]
- 28.Bernsdorff C., Wolf A., Winter R., Gratton E. Effect of hydrostatic pressure on water penetration and rotational dynamics in phospholipid-cholesterol bilayers. Biophys. J. 1997;72:1264–1277. doi: 10.1016/S0006-3495(97)78773-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammed A. R., Weston N., Coombes A. G., Fitzgerald M., Perrie Y. Liposome formulation of poorly water soluble drugs: optimisation of drug loading and ESEM analysis of stability. Int. J. Pharm. 2004;285:23–34. doi: 10.1016/j.ijpharm.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Loftsson T., Fri∂riksdóttir H., Gu∂mundsdóttir T. K. The effect of water-soluble polymers on aqueous solubility of drugs. Int. J. Pharm. 1996;127:293–296. doi: 10.1016/0378-5173(95)04207-5. [DOI] [Google Scholar]
- 31.Rowland R. N., Woodley J. F. The stability of liposomes in vitro to pH, bile salts and pancreatic lipase. Biochim. Biophys. Acta. 1980;620:400–409. doi: 10.1016/0005-2760(80)90131-9. [DOI] [PubMed] [Google Scholar]
- 32.Gregoriadis G., Davis C. Stability of liposomes in vivo and in vitro is promoted by their cholesterol content and the presence of blood cells. Biochem. Biophys. Res. Commun. 1979;89:1287–1293. doi: 10.1016/0006-291X(79)92148-X. [DOI] [PubMed] [Google Scholar]
- 33.Semple S. C., Chonn A., Cullis P. R. Influence of cholesterol on the association of plasma proteins with liposomes. Biochemistry. 1996;35:2521–2525. doi: 10.1021/bi950414i. [DOI] [PubMed] [Google Scholar]
- 34.Papahadjopoulos D., Jacobson K., Nir S., Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim. Biophys. Acta. 1973;311:330–348. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]