Abstract
The purpose of this investigation was to determine the mechanism of interaction between ketotifen fumarate and chitosan at different pH values. The specific surface area of chitosan was determined using gas sorption analyzer. The sorption experiments were conducted at pH 7 and 10 using two different particle size ranges of chitosan. The solutions were prepared at constant ionic strength and buffer concentration, with only varying the pH. The rotating bottle method was used for measuring the sorption. The average specific surface areas for the two different particle size ranges of chitosan were found to be 4.56 and 0.74 m2/g. The Langmuir-like equation and a model independent equation were both applied to the sorption experimental data. The extent of ketotifen uptake at pH 7 for small and large particles of chitosan was found to be 1,073 and 2,204 mg/g respectively. While the extent of ketotifen uptake at pH 10 for small and large particles of chitosan was found to be 4 and 11 mg/g respectively. The aforementioned results indicated that sorption of ketotifen fumarate at pH 7 is extremely high compared to pH 10 and that the sorption increases by decreasing the specific surface area of chitosan. Based on the results obtained, the following conclusions were reached. Ketotifen might be absorbed into the bulk structure of chitosan in addition to being adsorbed on the surface and the ability of chitosan to swell at pH 7 has a significant role in increasing its uptake.
Key words: absorption, adsorption, chitosan, ketotifen, sorption
INTRODUCTION
Chitosan is a hydrophilic, cationic polyelectrolyte prepared by deacetylation of chitin. Chitin (1) is the most plentiful natural polymer next to cellulose and it is obtained from crab and shrimp shells. Chitosan is a weak base with a pKa of 6.2–7.0. It is practically insoluble at neutral and alkaline pH values. While in acidic media, the amine groups of chitosan gets protonated resulting in soluble positively charged complex (1–3).
Chitosan seems to gain a lot of interest in the last few years, since it has many interesting applications in pharmaceutical and medical fields. This is a result of having many favorable properties such as being biodegradable, non-toxic, biocompatible and inexpensive (4–6). One of the chitosan’s major and revolutionary applications is its use as a hypolipidimic, hypocholesterolemic agent (a fat magnet). The way it acts is still not known. However, it is postulated that since chitosan has a cationic charge in acidic media, and lipids have anionic charges, ionic interaction at the surface might occur (3).
Previous work (7,8) has demonstrated that ketotifen fumarate interacts with chitosan. That work also concluded that the interaction cannot be due to ionic interaction. This conclusion was based on the fact that ketotifen is a weak base (neutral or positively charged), while chitosan is either positively charged or neutral at the aforementioned experimental conditions. The results also demonstrated that the adsorption capacities at pH 7 and 10 were significantly different. It was not clear at that moment which mechanism is responsible for that difference, since the buffers that were employed and their ionic strengths were different. Therefore, it was of interest to further investigate this phenomenon, and to determine the proper mechanism of interaction between chitosan and ketotifen fumarate.
MATERIALS AND METHODS
Purification of Chitosan Raw Material
Chitosan (>85% degree of deacetylation) was washed with several solvents having different polarity (n-hexane, ethanol and water). After washing, chitosan was filtered using a filtration unit (Sartorious, Gottingen, Germany) and Teflon membranes. The washed chitosan was left in the air to dry and was then divided into different particle sizes by sieving. The desired particle size of chitosan was then dried overnight at 60°C and 50 μm Hg in a vacuum oven (Precision, model 19, USA) which is connected to oil vacuum pump (Welch, 1402, Duo-seal Vacuum Pump, Thomas Industries Inc., Illinois, USA) and the dried chitosan was then used in all of the sorption experiments.
Surface Area Determination of Chitosan
A gas sorption analyzer (Nova 2200, Quantachrome Co. Syosset, NY, USA) was employed to obtain nitrogen vapor adsorption and desorption isotherms at 77K. Chitosan samples were heated to 60°C while being purged under a stream of pure nitrogen. Purging was continued for 24 h prior to analysis and the experiments were done in dublicates.
Sorption of Ketotien Fumarate by Chitosan in Aqueous Media (Rotating Bottle Method)
A stock solution was prepared by dissolving ketotifen fumarate (lot no.3100, Jordanian Pharmaceutical Manufacturing Co. (JPM), Amman-Jordan) in 500 ml of 0.2 M buffer (pH 7 or 10). The buffers were prepared by dissolving the appropriate amounts of potassium phophate monobasic and NaOH in water (pH = 7) or disodium hydrogen phosphate and trisodium phosphate in water (pH = 10). Aliquots were then removed from the stock solution and diluted to 50 ml using the same buffer. Five milliliters from each dilution were then removed, and were used as standards for further analysis (9).
Chitosan was vacuum dried at 60°C and 50 μm Hg for 24 h prior to use. After removal from the vacuum oven, chitosan samples (100 mg each) were weighed quickly. Each sample was then placed in a screw cap bottle containing 45 ml of the appropriate solution. The filled bottle was then wrapped with Parafilm to prevent leakage and to avoid direct contact of the suspension with the cap. The screw cap was put on the bottle, and each bottle was then wrapped with a Ziplock bag and an electric tape was placed over it.
The bottles were rotated in a Vankel Sustained Release Apparatus (VanKel Technology Group, VK 7500, model no. 65-3100, NC, USA) for 24 hours (15 rpm and 26°C). The time of the experiment was enough for the equilibrium to be reached. The equilibrium time was determined after several preliminary experiments. Rotation was then stopped, with the bottles in an upright position in the water bath. Chitosan was then allowed to settle to the bottom of the bottles for two hours at 26°C. Aliquots of the supernatant were filtered through 0.45 μm (pore size) Teflon membranes, which were installed in stainless steel filter holders. The filtered solutions were used for the analysis and when required the proper dilutions were made. Experiments were carried out in triplicates.
HPLC Analysis and Methodology
The ketotifen fumarate concentrations both before and after the attainment of equilibrium were determined with the aid of a reversed-phase HPLC system. A 5 μm Spherosorb C18 column (3.9× 100 mm) was used for this separation. The wavelength for detection of ketotifen fumarate was set at 300 nm. The detector sensitivity was set at 0.01 AUFS. The flow rate was 1.0 ml/min and the injection volume was 10 μl.
The HPLC system included the following equipment: liquid pump (model L7150, LaChrom), variable wavelength detector (Diode array L 7455), autosampler model (L7200) and interface model (D 7000) and the software (all Merck, Hitachi, Japan). The mobile phase consisted of 70% acetonitrile and 30% phosphate buffer (0.05 M and pH = 3.4).
Determination of the Solubilities
An excess amount of ketotifen was added to 10 ml of the specified buffer. The samples were rotated in the sustained release dissolution apparatus for 48 h (15 rpm, 26°C). Rotation was then stopped and the supernatant was taken, filtered using stainless steel filter holders and Teflon membranes, diluted and analyzed using HPLC. The time of the experiment was enough for the equilibrium to be reached, and the experiments were done in triplicate.
RESULTS AND DISCUSSION
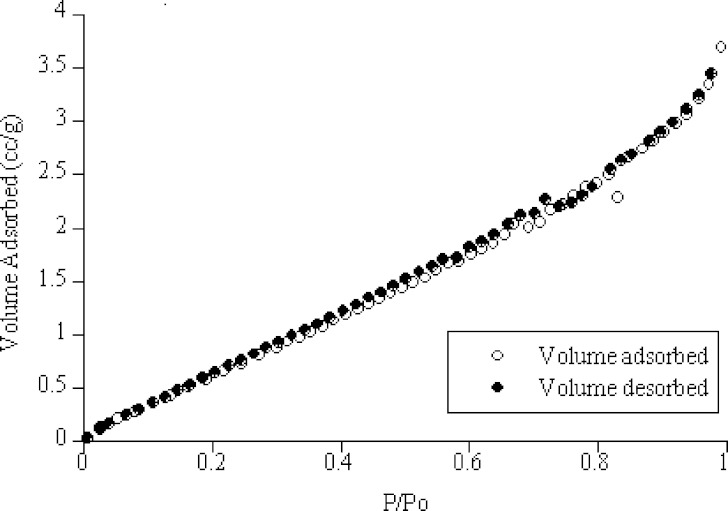
Linear BET plots were used in the calculation of the specific surface areas. The average specific surface area was 4.56 ± 02 m2/g for chitosan with particle size ≤100 μm, while for chitosan with particle size ≥350 μm the average specific surface area was 0.74 m2/g. Typical nitrogen vapor adsorption and desorption isotherms of chitosan are presented in Fig. 1. The isotherms for adsorption and desorption were almost identical and no hysteresis was observed. The value of the constant C in the BET equation was in the range of (2–200) which is a characteristic of a multilayer adsorption (type II isotherm).
Fig. 1.
Adsorption/desorption isotherms of chitosan
The solubility of the adsorbate in the solution phase plays an important role in the extent of its interaction with the adsorbent at fixed Ceq, since the extent of adsorption is a balance between the forces favoring interaction of the molecule with the surface and the forces favoring interaction of the molecule with the solvent. In general, higher solubility results in lower adsorption at fixed Ceq. This generalization assumes that the adsorption mechanism remains the same (10). Therefore, it was necessary to determine the solubility of ketotifen fumarate in solution at pH 7 and 10 in the absence of chitosan. The solubilities of ketotifen fumarate at pH 7 and 10 were found to be 10.50 and 0.02 mg/ml, respectively. The difference in the solubilities at the two different pH values was expected, since ketotifen is a weak base, and the ionization of weak bases increases by decreasing the pH.
Sorption of ketotifen fumarate by chitosan was studied at pH 7 and 10 using two different particle size ranges (<100 and >350 μm). The ketotifen fumarate concentrations, both before the addition of chitosan and after the attainment of equilibrium were determined with the aid of an HPLC system and the nonlinear Langmuir-like equation was applied to the experimental data. The Langmuir-like treatment is summarized by the following equation (11,12):
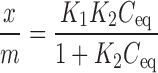 |
1 |
Where x is the amount of solute adsorbed, m is the mass of adsorbent, Ceq is the concentration of the unadsorbed solute at equilibrium, k1 is the capacity constant and k2 is the affinity constant. The derivation of the Langmuir-like equation is dependent upon the following assumptions. The heat of adsorption is independent of surface coverage (all of the sites available for adsorption are energetically equivalent), the adsorbed phase is confined to a monolayer, there are no lateral interactions between adsorbate molecules, the adsorbate solution is very dilute and there is no mixed film formation at maximum solute adsorption. It is necessary to emphasize that if the adsorption mechanism remains the same at different pH values, then the adsorption capacity constants are expected to be similar, while the affinity constants are expected to be different. This is important, since the number of adsorption sites is usually constant, while the affinity to these sites depends on the solute-solvent interaction.
The parameters of the Langmuir-like equation (the capacity and affinity constants) for ketotifen fumarate are presented in Table I. A typical plot of the nonlinear Langmuir-like isotherm is also shown in Fig. 2. The results of the application of the Langmuir-like equation (in Table I) show that the 95% confidence limit at pH 7 is extremely large. The poor fit of the data to the Langmuir-like equation, at pH 7, indicates that the mechanism of interaction at pH 7 and 10 might not be the same.
Table I.
The Parameters of Ketotifen Fumarate Uptake by Chitosan According to the Nonlinear Langmuir-Like Equation
| Particle size of chitosan <100 μm | Particle size of chitosan >350 μm | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | pH 7 | 95% conf. limit | pH 10 | 95% conf. limit | pH 7 | 95% conf. limit | pH 10 | 95% conf. limit |
| Capacity (mg/g) | 6629 | −2,140–15,398 | 5 | 4–6 | 3,236 | 1,688–4,787 | 14 | 11–18 |
| Affinity (ml/mg) | 0.0 | – | 147.3 | 103.6–188.3 | 0.1 | – | 79.3 | 53.4–105.1 |
| Area/molecule (A2) | 0.1 | 65.0 | 0.0 | 3.5 | ||||
| Specific surface area (m2/g) | 4.6 | 0.7 | ||||||
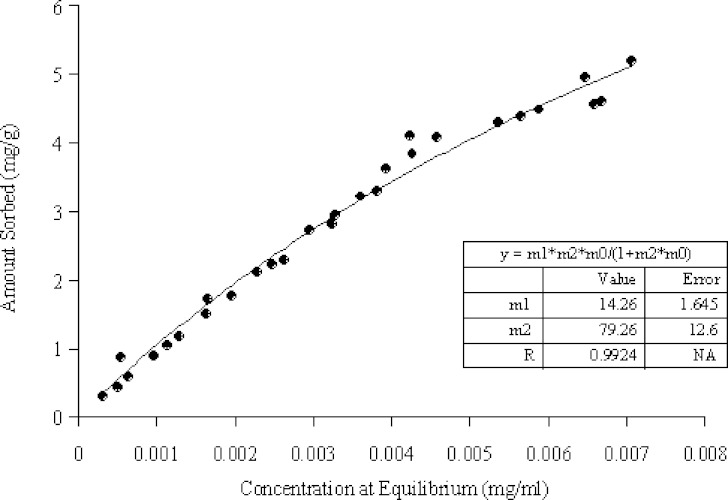
Fig. 2.
A typical plot for ketotifen fumarate sorption by chitosan. The nonlinear Langmuir-like equation was applied to the experimental data, where m 1 and m 2 are the capacity and the affinity constants respectively (particle size >350 μm, pH = 10)
In order to confirm the inability of the Langmuir-like equation to address the equilibrium data in this system, the apparent areas occupied per ketotifen fumarate on chitosan surface at different pH values and different particle sizes of chitosan were calculated according to the following equation (13):
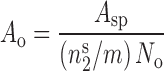 |
2 |
Where, Asp is the specific surface area of chitosan obtained from BET analysis of nitrogen vapor adsorption data, 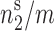 is the number of moles of adsorbate per gram of adsorbent at maximum surface coverage (obtained from the nonlinear Langmuir-like equation) and N0 is the Avogadro’s number. The results of these calculations are presented in Table I.
is the number of moles of adsorbate per gram of adsorbent at maximum surface coverage (obtained from the nonlinear Langmuir-like equation) and N0 is the Avogadro’s number. The results of these calculations are presented in Table I.
From the results it was noticed that the areas calculated according to the Langmuir-like equation were extremely small, since the expected area for ketotifen fumarate is 200 A2/molecule (based on the chemical structure). These results confirm that the Langmuir-like equation is not capable of addressing the equilibrium data in this system and that adsorption might not be the only mechanism responsible for the interaction between ketotifen fumarate and chitosan.
This result was confirmed with the swelling that occurs to chitosan present in phosphate buffer at pH 7 compared with that present at pH 10 as seen under the microscope and by the naked eye. During swelling, chitosan macromolecules might uncoil due to the repulsive forces between the multiple positive charges located on the amino groups. The ability of chitosan to swell at pH 7 has a significant role in increasing the uptake. Based on the aforementioned results it was concluded that ketotifen might be absorbed into the bulk structure of chitosan in addition to being adsorbed on the surface. The amount of ketotifen sorbed depends on the pH of the solution and the ionization of chitosan.
As the adsorption mechanism was not the only one responsible for the uptake of ketotifen fumarate by chitosan, the application of the nonlinear Langmuir-like equation was considered invalid and another equation had to be chosen. Therefore, it was decided to find an equation that can predict, statistically, the correlation between the amount adsorbed and Ceq. No physicochemical significance was ascribed to the relationship. Hence, the term “model independent” is used to refer to a model, which has no basis in a fundamental adsorption theory. An automated curve-fitting program (Table Curve 2D V3, Jandel Scientific, San Rafael, CA, USA) was used. This specialized program uses automated statistical methods to process the X–Y data table. The program offers up to 3,456 linear and nonlinear equations that can be tried and then sorted according to the goodness of the fit.
The equation that was most frequently selected by the program to fit the experimental results was the following:
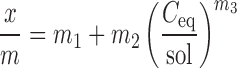 |
3 |
Where, x/m is the amount of ketotifen fumarate sorbed per mass of chitosan, Ceq is the concentration of the unsorbed ketotifen fumarate in phosphate buffer at equilibrium, sol is the solubility of ketotifen fumarate in phosphate buffer, and m1, m2 and m3 are constants having no assumptions related to the sorption process. A typical plot of the sorption isotherms is shown in Fig. 3. The tabulated data (shown in Table II) revealed that the sorption of ketotifen at pH 7 is extremely high compared to pH 10 (Ceq/Sol = 1). It also revealed that the sorption increases by decreasing the surface area of chitosan. This was not expected, since the adsorption increases by increasing the surface area of the adsorbent.
Fig. 3.
A typical plot for ketotifen fumarate sorption by chitosan. The model independent equation was applied to the experimental data (particle size > 350 μm, pH = 7)
Table II.
The Amount of Ketotifen Fumarate Sorbed by Chitosan at the Solubility Limit (Ceq/Sol = 1) According to the Model Independent Equation
| Extent of uptake at pH 7 (mg/g) | Extent of uptake at pH 10 (mg/g) | |
|---|---|---|
| <100 μm | 1,073 | 4 |
| >350 μm | 2,204 | 11 |
The aforementioned results indicate that the mechanism of interaction is likely to be due to absorption and not adsorption and that the ability of chitosan to swell at pH 7 might have a significant role in increasing the sorption of ketotifen fumarate by chitosan (not shown, but observed under the microscope). Therefore, precautions need to be taken when chitosan and medications are given simultaneously.
CONCLUSIONS
The results obtained from this study showed that sorption of ketotifen at pH 7 is extremely high compared to pH 10 and that sorption increases by decreasing the surface area of chitosan. Based on that result, it was concluded that ketotifen might be absorbed into the bulk structure of chitosan in addition to being adsorbed on the surface and that ketotifen absorption into the bulk structure is affected by the pH of the solution.
References
- 1.Bodmeier R., Oh K.-H., Pramar Y. Preparation and evaluation of drug-containing chitosan beads. Drug. Dev. Ind. Pharm. 1989;15(9):1475–1494. doi: 10.3109/03639048909062758. [DOI] [Google Scholar]
- 2.Felt O., Buri P., Gunny R. Chitosan a unique polysaccharide for drug delivery. Drug. Dev. Ind. Pharm. 1998;24(11):979–993. doi: 10.3109/03639049809089942. [DOI] [PubMed] [Google Scholar]
- 3.Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998;15(9):1326–1331. doi: 10.1023/A:1011929016601. [DOI] [PubMed] [Google Scholar]
- 4.Henriksen I., Skaugrud Ø., Karlsen J. Use of chitosan and chitosan malate as an excipient in wet granulation of three water soluble drugs. Int. J. of Pharm. 1993;98:181–188. doi: 10.1016/0378-5173(93)90055-K. [DOI] [Google Scholar]
- 5.Imai T., Shiraishi S., Saito H., Otagiri M. Interaction of indomethacin with low molecular weight chitosan, and improvements of some pharmaceutical properties of indomethacin by low molecular weight chitosans. Int. J. of Pharm. 1991;67:11–20. doi: 10.1016/0378-5173(91)90260-U. [DOI] [Google Scholar]
- 6.Thacharodi D., Panduranga R. K. Release of nifedipine through crosslinked chitosan membranes. Int. J. Pharm. 1993;96:33–39. doi: 10.1016/0378-5173(93)90209-X. [DOI] [Google Scholar]
- 7.W. Obeidat. In vitro interaction of chitosan with allopurinol and ketotifen. M. Sc. Thesis, Jordan University of Science and Technology, Irbid, Jordan (1999).
- 8.K. Alkhamis, W. Obeidat, N. Najib. The adsorption of ketotifen and allopurinol by chitosan. PharmSciTech, 2(1) article 3. http://www.pharmscitech.com (2001) [DOI] [PubMed]
- 9.G. M. Burke. Adsorptivity and surface characterization of activated charcoals. Ph.D. Thesis; University of Iowa, Iowa City (1991).
- 10.Wurster D. E., Alkhamis K. A., Matheson L. E. Prediction of the adsorption of diazepam by activated carbon in aqueous media. J. Pharm. Sci. 2003;92(10):2008–2016. doi: 10.1002/jps.10454. [DOI] [PubMed] [Google Scholar]
- 11.Kipling J. Adsorption from Solutions of Non-electrolytes. New York: Academic; 1965. pp. 92–93. [Google Scholar]
- 12.Adamson A. W. Physical Chemistry of Surfaces. 6. New York: Wiley; 1997. pp. 391–392. [Google Scholar]
- 13.Burke G., Wurster D. E., Buraphacheep V., Berg M. J., Veng-Pedersen P., Schottelius D. Model selection for the adsorption of phenobarbital by activated carbon. Pharm. Res. 1991;8(2):228–231. doi: 10.1023/A:1015800322286. [DOI] [PubMed] [Google Scholar]