Abstract
The title compound, [Au2(C26H24P2)2](CF3SO3)2·2CH3CN, comprises a cyclic cation with a short intramolecular aurophilic interaction of 2.9220 (3) Å. The trifluoromethanesulfonate anions and acetonitrile solvent molecules are located in channels formed by the complex cations that run along the crystallographic c axis. Each counter-anion is also engaged in a C—H⋯O contact with one of the methylene H atoms of a 1,2-bis(diphenylphosphino)ethane (dppe) ligand; another C—H⋯O contact involving an aromatic H atom is also observed.
Related literature
For 31P NMR evidence of [Au2(μ-dppe)3]2+, see: Al-Baker et al. (1985 ▶). For [Au2(μ-dppm)2]2+, see: de Jongh et al. (2007 ▶). For a related structure, see: Schuh et al. (2001 ▶).
Experimental
Crystal data
[Au2(C26H24P2)2](CF3SO3)2·2C2H3N
M r = 1571.0
Monoclinic,

a = 11.7888 (9) Å
b = 36.998 (3) Å
c = 14.377 (1) Å
β = 113.011 (1)°
V = 5771.6 (8) Å3
Z = 4
Mo Kα radiation
μ = 5.33 mm−1
T = 100 K
0.21 × 0.15 × 0.07 mm
Data collection
Bruker SMART APEX CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2002 ▶) T min = 0.404, T max = 0.686
36089 measured reflections
13385 independent reflections
10742 reflections with I > 2σ(I)
R int = 0.048
Refinement
R[F 2 > 2σ(F 2)] = 0.042
wR(F 2) = 0.095
S = 1.01
13385 reflections
723 parameters
H-atom parameters constrained
Δρmax = 2.35 e Å−3
Δρmin = −0.75 e Å−3
Data collection: SMART (Bruker, 2002 ▶); cell refinement: SAINT (Bruker, 2003 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶; Atwood & Barbour, 2003 ▶); software used to prepare material for publication: X-SEED.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809025513/im2111sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809025513/im2111Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C212—H212⋯O1i | 0.95 | 2.45 | 3.387 (7) | 171 |
| C21—H21B⋯O1i | 0.99 | 2.34 | 3.268 (7) | 155 |
| C11—H11B⋯O4ii | 0.99 | 2.36 | 3.301 (7) | 158 |
Symmetry codes: (i) 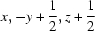 ; (ii)
; (ii)  .
.
Acknowledgments
We would like to thank the National Research Foundation (NRF) of South Africa for financial support.
supplementary crystallographic information
Comment
Ditopic phosphines can form cyclic cations with gold(I) and especially dppm [bis(diphenylphosphino)methane] readily yields the [Au2(µ-dppm)2]2+ cation (de Jongh et al., 2007; and references cited therein). However, the tendency of dppe [1,2-bis(diphenylphosphino)ethane] to form cyclic cations is less apparent than that of dppm since only one structural report of a bis(methanol) solvate, [Au2(µ-dppe)2](CF3SO3)2.CH3OH, has been published (Schuh et al., 2001). The two solvates, however are not isomorphous with (I) being monoclinic and the methanol solvate triclinic.
Compound (I) crystallizes as an asymmetric cation with the trifluoromethanesulfonate anions forming two sets of channels running parallel to the crystallographic a axis (for the anion containing S1) and c axis (for the anion containing S2), respectively. The acetonitrile containing N2 is also found in the former channels while another solvent is embedded between the cations.
Compared to the other example of a crystallographically characterized [Au2(µ-dppe)2]2+ cation in literature, (I) (Figure 1) exhibits a shorter aurophilic interaction and slightly wider P1—Au1···Au2—P2 and P3—Au1···Au2—P4 torsion angles [2.9220 (3) Å, -47.11 (5) and -46.89 (5)° in (I) compared to 2.959 (1) Å, -43.8 (1) and -45.0 (1)°, in the example of Schuh et al.]. The angles at the gold centres in (I) are bent significantly from the linear ideal [P1—Au1—P3 171.77 (5)° and P2—Au2—P4 177.10 (5)°] due to the attractive aurophilic interaction. Other geometric parameters between both structures agree very closely and differences would likely be caused by lattice effects. Another noteworthy feature of (I) is the well defined trifluoromethanesulfonate anions and acetonitrile solvent molecules that do not exhibit disorder despite the fact that the thermal displacement ellipsoids of the acetonitrile containing N1 show higher mobility. Disorder of one trifluoromethanesulfonate anion and methanol molecule each was observed in the crystal structure of the bis(methanol) solvate which may have been enhanced by the higher temperature [223 (2) K] at which data were collected.
The title compound (I) was obtained as the exclusive product in an unsuccessful attempt to structurally characterize the [Au2(µ-dppe)3]2+ cation that has been previously detected by 31P NMR spectroscopy (Al-Baker et al., 1985) and its presence in the mother liquor of (I) can therefore not be completely ruled out.
Experimental
The ditopic phosphine dppe (184 mg, 0.46 mmol) was suspended in 20 ml of acetonitrile, sodium trifluoromethanesulfonate (53 mg, 0.31 mmol) was added and the suspension stirred briefly. [AuCl(tht)] (99 mg, 0.31 mmol; tht = tetrahydrothiophene) and few NaCl crystals (to seed precipitation) were subsequently added. After 1 h the precipitated solids were filtered off, the filtrate was reduced to ca 5 ml and layered with diethyl ether. Colourless blocks of (I) crystallized at 258 K. No other species could be identified in the crystalline phase.
Refinement
All H atoms were positioned geometrically (C—H = 0.95, 0.99 and 0.98 Å for CH, CH2 and CH3 groups, respectively) and constrained to ride on their parent atoms; Uiso(H) values were set at 1.2 times Ueq(C) for CH and CH2 groups and 1.5 times Ueq(C) for CH3 groups.
The maximum residual electron density of 2.35 e Å-3 is located 0.85 Å next to Au1.
Figures
Fig. 1.
The asymmetric unit of (I), ellipsoids are drawn at the 50% probability level.
Crystal data
| [Au2(C26H24P2)2](CF3SO3)2·2C2H3N | F(000) = 3072 |
| Mr = 1571.0 | Dx = 1.808 Mg m−3 |
| Monoclinic, P21/c | Melting point: 528 K |
| Hall symbol: -P 2ybc | Mo Kα radiation, λ = 0.71073 Å |
| a = 11.7888 (9) Å | Cell parameters from 6846 reflections |
| b = 36.998 (3) Å | θ = 2.2–27.1° |
| c = 14.377 (1) Å | µ = 5.33 mm−1 |
| β = 113.011 (1)° | T = 100 K |
| V = 5771.6 (8) Å3 | Block, colourless |
| Z = 4 | 0.21 × 0.15 × 0.07 mm |
Data collection
| Bruker SMART APEX CCD area-detector diffractometer | 13385 independent reflections |
| Radiation source: fine-focus sealed tube | 10742 reflections with I > 2σ(I) |
| graphite | Rint = 0.048 |
| ω scans | θmax = 28.2°, θmin = 1.6° |
| Absorption correction: multi-scan (SADABS; Bruker, 2002) | h = −15→14 |
| Tmin = 0.404, Tmax = 0.686 | k = −49→36 |
| 36089 measured reflections | l = −19→19 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.042 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.095 | H-atom parameters constrained |
| S = 1.01 | w = 1/[σ2(Fo2) + (0.0437P)2] where P = (Fo2 + 2Fc2)/3 |
| 13385 reflections | (Δ/σ)max = 0.002 |
| 723 parameters | Δρmax = 2.35 e Å−3 |
| 0 restraints | Δρmin = −0.75 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Au1 | 0.623479 (18) | 0.134499 (5) | 0.336490 (15) | 0.01447 (6) | |
| S1 | 0.51439 (13) | 0.41844 (4) | 0.19532 (10) | 0.0215 (3) | |
| P1 | 0.54886 (12) | 0.18698 (4) | 0.24655 (10) | 0.0140 (3) | |
| F1 | 0.7019 (3) | 0.38763 (10) | 0.3408 (3) | 0.0412 (9) | |
| O1 | 0.5380 (4) | 0.39408 (11) | 0.1268 (3) | 0.0321 (10) | |
| N1 | 0.0693 (7) | 0.4616 (3) | 0.4339 (7) | 0.111 (4) | |
| C1 | 0.6633 (6) | 0.42044 (17) | 0.3017 (5) | 0.0338 (15) | |
| Au2 | 0.415549 (18) | 0.128908 (5) | 0.398184 (15) | 0.01447 (6) | |
| S2 | 0.05080 (14) | 0.32922 (4) | 0.64951 (11) | 0.0270 (3) | |
| P2 | 0.43840 (12) | 0.18914 (4) | 0.44274 (10) | 0.0137 (3) | |
| F2 | 0.7494 (4) | 0.43370 (12) | 0.2719 (3) | 0.0576 (12) | |
| O2 | 0.4945 (4) | 0.45535 (11) | 0.1614 (3) | 0.0310 (10) | |
| N2 | 0.1128 (5) | 0.3048 (2) | 0.3405 (5) | 0.0543 (18) | |
| C2 | 0.0292 (5) | 0.34533 (17) | 0.7605 (5) | 0.0288 (14) | |
| P3 | 0.71663 (12) | 0.08150 (4) | 0.41409 (10) | 0.0147 (3) | |
| F3 | 0.6598 (4) | 0.44111 (12) | 0.3755 (3) | 0.0591 (13) | |
| O3 | 0.4330 (4) | 0.40469 (12) | 0.2393 (3) | 0.0341 (10) | |
| C3 | −0.0048 (7) | 0.4312 (2) | 0.5593 (6) | 0.0466 (18) | |
| H3A | −0.0708 | 0.4143 | 0.5219 | 0.070* | |
| H3B | −0.0361 | 0.4491 | 0.5937 | 0.070* | |
| H3C | 0.0639 | 0.4179 | 0.6094 | 0.070* | |
| P4 | 0.39442 (12) | 0.06958 (4) | 0.34652 (10) | 0.0150 (3) | |
| F4 | 0.1308 (3) | 0.34219 (12) | 0.8437 (3) | 0.0470 (11) | |
| O4 | 0.1513 (4) | 0.35096 (12) | 0.6475 (3) | 0.0370 (11) | |
| C4 | 0.0371 (7) | 0.4493 (2) | 0.4906 (6) | 0.055 (2) | |
| F5 | −0.0018 (4) | 0.38046 (11) | 0.7517 (3) | 0.0530 (12) | |
| O5 | −0.0660 (4) | 0.33678 (14) | 0.5692 (3) | 0.0416 (12) | |
| C5 | 0.0301 (8) | 0.3640 (2) | 0.3840 (6) | 0.058 (2) | |
| H5A | 0.0973 | 0.3815 | 0.4115 | 0.088* | |
| H5B | −0.0045 | 0.3589 | 0.4344 | 0.088* | |
| H5C | −0.0343 | 0.3739 | 0.3229 | 0.088* | |
| F6 | −0.0600 (3) | 0.32773 (11) | 0.7752 (3) | 0.0432 (10) | |
| O6 | 0.0822 (4) | 0.29207 (12) | 0.6734 (4) | 0.0435 (12) | |
| C6 | 0.0770 (7) | 0.3311 (2) | 0.3592 (5) | 0.0416 (17) | |
| C11 | 0.3965 (4) | 0.20169 (14) | 0.2369 (4) | 0.0154 (11) | |
| H11A | 0.3659 | 0.2199 | 0.1823 | 0.018* | |
| H11B | 0.3404 | 0.1806 | 0.2152 | 0.018* | |
| C12 | 0.3855 (5) | 0.21770 (14) | 0.3307 (4) | 0.0172 (11) | |
| H12A | 0.2979 | 0.2238 | 0.3138 | 0.021* | |
| H12B | 0.4328 | 0.2406 | 0.3476 | 0.021* | |
| C21 | 0.6443 (5) | 0.05786 (14) | 0.4879 (4) | 0.0160 (11) | |
| H21A | 0.7009 | 0.0383 | 0.5256 | 0.019* | |
| H21B | 0.6387 | 0.0751 | 0.5386 | 0.019* | |
| C22 | 0.5162 (5) | 0.04105 (13) | 0.4334 (4) | 0.0176 (11) | |
| H22A | 0.4879 | 0.0320 | 0.4856 | 0.021* | |
| H22B | 0.5253 | 0.0198 | 0.3951 | 0.021* | |
| C111 | 0.5323 (5) | 0.17899 (13) | 0.1181 (4) | 0.0146 (10) | |
| C112 | 0.4219 (5) | 0.18370 (14) | 0.0346 (4) | 0.0170 (11) | |
| H112 | 0.3510 | 0.1921 | 0.0439 | 0.020* | |
| C113 | 0.4150 (5) | 0.17621 (15) | −0.0620 (4) | 0.0231 (12) | |
| H113 | 0.3400 | 0.1800 | −0.1188 | 0.028* | |
| C114 | 0.5184 (5) | 0.16318 (15) | −0.0755 (4) | 0.0237 (12) | |
| H114 | 0.5130 | 0.1571 | −0.1413 | 0.028* | |
| C115 | 0.6284 (5) | 0.15913 (15) | 0.0063 (4) | 0.0240 (12) | |
| H115 | 0.6997 | 0.1513 | −0.0033 | 0.029* | |
| C116 | 0.6350 (5) | 0.16644 (14) | 0.1019 (4) | 0.0203 (12) | |
| H116 | 0.7107 | 0.1629 | 0.1581 | 0.024* | |
| C121 | 0.6486 (5) | 0.22545 (14) | 0.2910 (4) | 0.0157 (11) | |
| C122 | 0.7689 (5) | 0.22112 (17) | 0.3589 (4) | 0.0247 (13) | |
| H122 | 0.8006 | 0.1977 | 0.3814 | 0.030* | |
| C123 | 0.8436 (6) | 0.25158 (19) | 0.3942 (5) | 0.0362 (16) | |
| H123 | 0.9263 | 0.2488 | 0.4411 | 0.043* | |
| C124 | 0.7982 (6) | 0.28533 (18) | 0.3617 (5) | 0.0340 (16) | |
| H124 | 0.8496 | 0.3059 | 0.3865 | 0.041* | |
| C125 | 0.6787 (6) | 0.28986 (16) | 0.2932 (5) | 0.0299 (14) | |
| H125 | 0.6481 | 0.3134 | 0.2707 | 0.036* | |
| C126 | 0.6040 (5) | 0.26026 (15) | 0.2576 (4) | 0.0211 (12) | |
| H126 | 0.5217 | 0.2634 | 0.2101 | 0.025* | |
| C211 | 0.3496 (4) | 0.20220 (13) | 0.5156 (4) | 0.0132 (10) | |
| C212 | 0.3608 (5) | 0.18048 (14) | 0.5987 (4) | 0.0175 (11) | |
| H212 | 0.4141 | 0.1601 | 0.6150 | 0.021* | |
| C213 | 0.2948 (5) | 0.18857 (16) | 0.6568 (4) | 0.0235 (13) | |
| H213 | 0.3047 | 0.1742 | 0.7143 | 0.028* | |
| C214 | 0.2145 (5) | 0.21744 (16) | 0.6315 (4) | 0.0237 (13) | |
| H214 | 0.1678 | 0.2226 | 0.6709 | 0.028* | |
| C215 | 0.2014 (5) | 0.23887 (15) | 0.5494 (4) | 0.0231 (12) | |
| H215 | 0.1468 | 0.2589 | 0.5332 | 0.028* | |
| C216 | 0.2677 (5) | 0.23123 (14) | 0.4904 (4) | 0.0176 (11) | |
| H216 | 0.2573 | 0.2457 | 0.4331 | 0.021* | |
| C221 | 0.5969 (5) | 0.20160 (13) | 0.5168 (4) | 0.0147 (11) | |
| C222 | 0.6842 (5) | 0.17419 (14) | 0.5606 (4) | 0.0178 (11) | |
| H222 | 0.6596 | 0.1496 | 0.5521 | 0.021* | |
| C223 | 0.8067 (5) | 0.18318 (16) | 0.6163 (4) | 0.0224 (12) | |
| H223 | 0.8659 | 0.1647 | 0.6456 | 0.027* | |
| C224 | 0.8423 (5) | 0.21888 (16) | 0.6291 (4) | 0.0233 (12) | |
| H224 | 0.9261 | 0.2249 | 0.6670 | 0.028* | |
| C225 | 0.7564 (5) | 0.24615 (15) | 0.5867 (4) | 0.0224 (12) | |
| H225 | 0.7816 | 0.2707 | 0.5958 | 0.027* | |
| C226 | 0.6345 (5) | 0.23751 (13) | 0.5316 (4) | 0.0166 (11) | |
| H226 | 0.5759 | 0.2562 | 0.5036 | 0.020* | |
| C311 | 0.8709 (5) | 0.09196 (14) | 0.5052 (4) | 0.0171 (11) | |
| C312 | 0.9202 (5) | 0.07739 (15) | 0.6013 (4) | 0.0237 (12) | |
| H312 | 0.8720 | 0.0616 | 0.6232 | 0.028* | |
| C313 | 1.0394 (5) | 0.08574 (16) | 0.6656 (4) | 0.0246 (13) | |
| H313 | 1.0726 | 0.0753 | 0.7312 | 0.029* | |
| C314 | 1.1105 (5) | 0.10878 (16) | 0.6365 (4) | 0.0281 (14) | |
| H314 | 1.1926 | 0.1142 | 0.6811 | 0.034* | |
| C315 | 1.0600 (6) | 0.12436 (16) | 0.5395 (5) | 0.0314 (15) | |
| H315 | 1.1077 | 0.1406 | 0.5183 | 0.038* | |
| C316 | 0.9410 (5) | 0.11595 (15) | 0.4754 (4) | 0.0236 (12) | |
| H316 | 0.9066 | 0.1267 | 0.4103 | 0.028* | |
| C321 | 0.7306 (5) | 0.04977 (14) | 0.3231 (4) | 0.0184 (11) | |
| C322 | 0.7267 (5) | 0.01260 (15) | 0.3364 (4) | 0.0206 (12) | |
| H322 | 0.7189 | 0.0034 | 0.3953 | 0.025* | |
| C323 | 0.7340 (5) | −0.01093 (16) | 0.2645 (5) | 0.0310 (15) | |
| H323 | 0.7294 | −0.0363 | 0.2729 | 0.037* | |
| C324 | 0.7480 (5) | 0.00253 (18) | 0.1808 (5) | 0.0328 (15) | |
| H324 | 0.7550 | −0.0137 | 0.1320 | 0.039* | |
| C325 | 0.7521 (5) | 0.03941 (17) | 0.1665 (4) | 0.0294 (14) | |
| H325 | 0.7619 | 0.0484 | 0.1082 | 0.035* | |
| C326 | 0.7420 (5) | 0.06317 (15) | 0.2373 (4) | 0.0227 (12) | |
| H326 | 0.7429 | 0.0885 | 0.2270 | 0.027* | |
| C411 | 0.2566 (5) | 0.04865 (14) | 0.3496 (4) | 0.0169 (11) | |
| C412 | 0.2230 (5) | 0.05834 (15) | 0.4281 (4) | 0.0231 (12) | |
| H412 | 0.2696 | 0.0760 | 0.4757 | 0.028* | |
| C413 | 0.1218 (5) | 0.04255 (16) | 0.4376 (5) | 0.0269 (13) | |
| H413 | 0.0996 | 0.0490 | 0.4922 | 0.032* | |
| C414 | 0.0529 (5) | 0.01721 (16) | 0.3673 (4) | 0.0265 (13) | |
| H414 | −0.0174 | 0.0066 | 0.3731 | 0.032* | |
| C415 | 0.0858 (5) | 0.00731 (16) | 0.2890 (4) | 0.0266 (13) | |
| H415 | 0.0388 | −0.0103 | 0.2413 | 0.032* | |
| C416 | 0.1883 (5) | 0.02315 (15) | 0.2798 (4) | 0.0236 (12) | |
| H416 | 0.2112 | 0.0164 | 0.2258 | 0.028* | |
| C421 | 0.3955 (4) | 0.06367 (13) | 0.2221 (4) | 0.0151 (11) | |
| C422 | 0.3721 (5) | 0.09313 (15) | 0.1579 (4) | 0.0216 (12) | |
| H422 | 0.3519 | 0.1158 | 0.1782 | 0.026* | |
| C423 | 0.3780 (5) | 0.08951 (16) | 0.0631 (4) | 0.0282 (14) | |
| H423 | 0.3601 | 0.1096 | 0.0186 | 0.034* | |
| C424 | 0.4097 (5) | 0.05691 (16) | 0.0344 (4) | 0.0277 (14) | |
| H424 | 0.4172 | 0.0548 | −0.0288 | 0.033* | |
| C425 | 0.4304 (5) | 0.02744 (16) | 0.0964 (4) | 0.0264 (13) | |
| H425 | 0.4491 | 0.0048 | 0.0746 | 0.032* | |
| C426 | 0.4246 (5) | 0.03023 (14) | 0.1899 (4) | 0.0201 (12) | |
| H426 | 0.4401 | 0.0097 | 0.2326 | 0.024* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Au1 | 0.01467 (11) | 0.01186 (10) | 0.01630 (11) | 0.00198 (7) | 0.00542 (8) | 0.00180 (8) |
| S1 | 0.0256 (8) | 0.0168 (7) | 0.0209 (7) | −0.0008 (5) | 0.0078 (6) | 0.0022 (6) |
| P1 | 0.0143 (7) | 0.0131 (6) | 0.0138 (6) | 0.0013 (5) | 0.0046 (6) | 0.0010 (5) |
| F1 | 0.039 (2) | 0.031 (2) | 0.046 (2) | 0.0108 (17) | 0.0080 (19) | 0.0172 (18) |
| O1 | 0.049 (3) | 0.020 (2) | 0.031 (2) | −0.0057 (19) | 0.019 (2) | −0.0058 (19) |
| N1 | 0.067 (6) | 0.153 (9) | 0.103 (7) | −0.006 (6) | 0.023 (5) | 0.088 (7) |
| C1 | 0.035 (4) | 0.027 (3) | 0.032 (4) | 0.000 (3) | 0.006 (3) | 0.006 (3) |
| Au2 | 0.01510 (11) | 0.01046 (10) | 0.01807 (11) | −0.00068 (7) | 0.00673 (8) | −0.00238 (8) |
| S2 | 0.0228 (8) | 0.0291 (8) | 0.0316 (8) | 0.0023 (6) | 0.0132 (7) | −0.0049 (7) |
| P2 | 0.0140 (7) | 0.0121 (6) | 0.0146 (6) | −0.0006 (5) | 0.0050 (6) | −0.0010 (5) |
| F2 | 0.036 (2) | 0.057 (3) | 0.068 (3) | −0.012 (2) | 0.008 (2) | 0.023 (2) |
| O2 | 0.040 (3) | 0.019 (2) | 0.029 (2) | 0.0025 (18) | 0.008 (2) | 0.0015 (18) |
| N2 | 0.032 (4) | 0.064 (5) | 0.055 (4) | 0.000 (3) | 0.004 (3) | −0.007 (4) |
| C2 | 0.021 (3) | 0.032 (4) | 0.029 (3) | 0.000 (3) | 0.005 (3) | −0.002 (3) |
| P3 | 0.0134 (7) | 0.0118 (6) | 0.0178 (7) | 0.0015 (5) | 0.0047 (6) | 0.0009 (5) |
| F3 | 0.075 (3) | 0.052 (3) | 0.027 (2) | 0.009 (2) | −0.006 (2) | −0.012 (2) |
| O3 | 0.031 (2) | 0.034 (3) | 0.041 (3) | 0.0042 (19) | 0.017 (2) | 0.011 (2) |
| C3 | 0.052 (5) | 0.041 (4) | 0.055 (5) | −0.006 (3) | 0.030 (4) | −0.005 (4) |
| P4 | 0.0146 (7) | 0.0111 (6) | 0.0192 (7) | −0.0010 (5) | 0.0066 (6) | −0.0024 (5) |
| F4 | 0.027 (2) | 0.077 (3) | 0.027 (2) | −0.003 (2) | −0.0003 (17) | −0.001 (2) |
| O4 | 0.025 (2) | 0.042 (3) | 0.047 (3) | 0.001 (2) | 0.017 (2) | 0.007 (2) |
| C4 | 0.035 (4) | 0.065 (6) | 0.058 (5) | 0.004 (4) | 0.011 (4) | 0.026 (4) |
| F5 | 0.077 (3) | 0.036 (2) | 0.053 (3) | 0.017 (2) | 0.033 (3) | −0.004 (2) |
| O5 | 0.028 (3) | 0.066 (4) | 0.025 (2) | −0.003 (2) | 0.005 (2) | −0.012 (2) |
| C5 | 0.087 (7) | 0.045 (5) | 0.054 (5) | −0.002 (4) | 0.040 (5) | 0.007 (4) |
| F6 | 0.026 (2) | 0.070 (3) | 0.040 (2) | −0.0067 (19) | 0.0208 (18) | −0.004 (2) |
| O6 | 0.049 (3) | 0.026 (2) | 0.064 (3) | 0.006 (2) | 0.032 (3) | −0.006 (2) |
| C6 | 0.036 (4) | 0.049 (5) | 0.040 (4) | −0.010 (3) | 0.015 (3) | −0.002 (4) |
| C11 | 0.013 (3) | 0.016 (3) | 0.014 (2) | 0.002 (2) | 0.002 (2) | 0.002 (2) |
| C12 | 0.016 (3) | 0.016 (3) | 0.017 (3) | 0.003 (2) | 0.004 (2) | −0.005 (2) |
| C21 | 0.015 (3) | 0.016 (3) | 0.017 (3) | 0.002 (2) | 0.005 (2) | 0.003 (2) |
| C22 | 0.021 (3) | 0.010 (2) | 0.023 (3) | −0.002 (2) | 0.009 (2) | −0.001 (2) |
| C111 | 0.020 (3) | 0.011 (2) | 0.011 (2) | −0.002 (2) | 0.005 (2) | 0.001 (2) |
| C112 | 0.016 (3) | 0.015 (3) | 0.019 (3) | 0.002 (2) | 0.005 (2) | 0.001 (2) |
| C113 | 0.022 (3) | 0.028 (3) | 0.014 (3) | 0.001 (2) | 0.001 (2) | −0.002 (2) |
| C114 | 0.032 (3) | 0.022 (3) | 0.020 (3) | −0.004 (2) | 0.013 (3) | −0.004 (2) |
| C115 | 0.021 (3) | 0.024 (3) | 0.029 (3) | 0.000 (2) | 0.012 (3) | −0.002 (3) |
| C116 | 0.017 (3) | 0.022 (3) | 0.023 (3) | 0.002 (2) | 0.009 (2) | −0.001 (2) |
| C121 | 0.018 (3) | 0.015 (3) | 0.015 (3) | −0.002 (2) | 0.007 (2) | −0.002 (2) |
| C122 | 0.016 (3) | 0.034 (3) | 0.023 (3) | −0.001 (2) | 0.006 (2) | 0.001 (3) |
| C123 | 0.023 (3) | 0.053 (5) | 0.026 (3) | −0.020 (3) | 0.003 (3) | −0.004 (3) |
| C124 | 0.045 (4) | 0.032 (4) | 0.031 (3) | −0.021 (3) | 0.022 (3) | −0.016 (3) |
| C125 | 0.047 (4) | 0.017 (3) | 0.033 (3) | 0.000 (3) | 0.024 (3) | −0.006 (3) |
| C126 | 0.023 (3) | 0.019 (3) | 0.021 (3) | 0.002 (2) | 0.009 (2) | 0.001 (2) |
| C211 | 0.013 (3) | 0.013 (2) | 0.013 (2) | −0.0059 (19) | 0.005 (2) | −0.006 (2) |
| C212 | 0.016 (3) | 0.017 (3) | 0.015 (3) | −0.002 (2) | 0.001 (2) | −0.003 (2) |
| C213 | 0.023 (3) | 0.025 (3) | 0.021 (3) | −0.011 (2) | 0.008 (3) | −0.004 (2) |
| C214 | 0.019 (3) | 0.033 (3) | 0.026 (3) | −0.013 (2) | 0.015 (3) | −0.011 (3) |
| C215 | 0.017 (3) | 0.024 (3) | 0.029 (3) | −0.002 (2) | 0.009 (3) | −0.012 (3) |
| C216 | 0.018 (3) | 0.017 (3) | 0.017 (3) | 0.000 (2) | 0.006 (2) | −0.003 (2) |
| C221 | 0.014 (3) | 0.014 (3) | 0.015 (2) | −0.002 (2) | 0.004 (2) | −0.003 (2) |
| C222 | 0.018 (3) | 0.016 (3) | 0.017 (3) | 0.000 (2) | 0.005 (2) | −0.002 (2) |
| C223 | 0.018 (3) | 0.026 (3) | 0.020 (3) | 0.006 (2) | 0.003 (2) | 0.001 (2) |
| C224 | 0.019 (3) | 0.031 (3) | 0.018 (3) | −0.002 (2) | 0.006 (2) | −0.002 (2) |
| C225 | 0.022 (3) | 0.023 (3) | 0.021 (3) | −0.008 (2) | 0.007 (3) | −0.007 (2) |
| C226 | 0.019 (3) | 0.012 (3) | 0.020 (3) | 0.001 (2) | 0.008 (2) | 0.000 (2) |
| C311 | 0.010 (3) | 0.018 (3) | 0.020 (3) | 0.005 (2) | 0.002 (2) | −0.003 (2) |
| C312 | 0.022 (3) | 0.017 (3) | 0.032 (3) | 0.000 (2) | 0.011 (3) | −0.001 (2) |
| C313 | 0.022 (3) | 0.026 (3) | 0.023 (3) | 0.005 (2) | 0.005 (3) | 0.002 (2) |
| C314 | 0.017 (3) | 0.028 (3) | 0.032 (3) | −0.004 (2) | 0.002 (3) | −0.011 (3) |
| C315 | 0.028 (3) | 0.026 (3) | 0.042 (4) | −0.008 (3) | 0.015 (3) | −0.001 (3) |
| C316 | 0.019 (3) | 0.026 (3) | 0.023 (3) | 0.002 (2) | 0.005 (2) | 0.004 (2) |
| C321 | 0.014 (3) | 0.018 (3) | 0.019 (3) | 0.004 (2) | 0.003 (2) | 0.000 (2) |
| C322 | 0.013 (3) | 0.022 (3) | 0.030 (3) | 0.004 (2) | 0.012 (2) | 0.002 (2) |
| C323 | 0.018 (3) | 0.022 (3) | 0.049 (4) | 0.000 (2) | 0.008 (3) | −0.009 (3) |
| C324 | 0.023 (3) | 0.042 (4) | 0.029 (3) | −0.001 (3) | 0.006 (3) | −0.020 (3) |
| C325 | 0.028 (3) | 0.037 (4) | 0.025 (3) | −0.004 (3) | 0.013 (3) | −0.005 (3) |
| C326 | 0.022 (3) | 0.021 (3) | 0.026 (3) | −0.004 (2) | 0.010 (3) | −0.003 (2) |
| C411 | 0.013 (3) | 0.012 (3) | 0.022 (3) | −0.002 (2) | 0.003 (2) | 0.001 (2) |
| C412 | 0.024 (3) | 0.018 (3) | 0.024 (3) | 0.000 (2) | 0.006 (3) | −0.005 (2) |
| C413 | 0.024 (3) | 0.027 (3) | 0.033 (3) | 0.006 (2) | 0.015 (3) | 0.003 (3) |
| C414 | 0.018 (3) | 0.025 (3) | 0.036 (3) | −0.003 (2) | 0.009 (3) | 0.006 (3) |
| C415 | 0.020 (3) | 0.023 (3) | 0.028 (3) | −0.009 (2) | −0.001 (3) | −0.004 (3) |
| C416 | 0.028 (3) | 0.019 (3) | 0.026 (3) | −0.003 (2) | 0.013 (3) | −0.004 (2) |
| C421 | 0.011 (3) | 0.013 (2) | 0.017 (3) | −0.0030 (19) | 0.001 (2) | −0.004 (2) |
| C422 | 0.021 (3) | 0.020 (3) | 0.020 (3) | −0.001 (2) | 0.003 (2) | −0.002 (2) |
| C423 | 0.035 (4) | 0.025 (3) | 0.019 (3) | −0.008 (3) | 0.004 (3) | −0.003 (2) |
| C424 | 0.029 (3) | 0.032 (3) | 0.022 (3) | −0.015 (3) | 0.009 (3) | −0.009 (3) |
| C425 | 0.022 (3) | 0.026 (3) | 0.029 (3) | −0.001 (2) | 0.008 (3) | −0.008 (3) |
| C426 | 0.020 (3) | 0.013 (3) | 0.028 (3) | 0.002 (2) | 0.009 (2) | 0.000 (2) |
Geometric parameters (Å, °)
| Au1—P1 | 2.3079 (13) | C125—C126 | 1.373 (8) |
| Au1—P3 | 2.3105 (13) | C125—H125 | 0.9500 |
| Au1—Au2 | 2.9220 (3) | C126—H126 | 0.9500 |
| S1—O3 | 1.433 (4) | C211—C216 | 1.394 (7) |
| S1—O2 | 1.438 (4) | C211—C212 | 1.402 (7) |
| S1—O1 | 1.440 (4) | C212—C213 | 1.379 (7) |
| S1—C1 | 1.824 (6) | C212—H212 | 0.9500 |
| P1—C121 | 1.797 (5) | C213—C214 | 1.379 (8) |
| P1—C111 | 1.804 (5) | C213—H213 | 0.9500 |
| P1—C11 | 1.829 (5) | C214—C215 | 1.380 (8) |
| F1—C1 | 1.340 (7) | C214—H214 | 0.9500 |
| N1—C4 | 1.121 (10) | C215—C216 | 1.390 (7) |
| C1—F3 | 1.323 (7) | C215—H215 | 0.9500 |
| C1—F2 | 1.338 (7) | C216—H216 | 0.9500 |
| Au2—P4 | 2.2993 (13) | C221—C226 | 1.390 (7) |
| Au2—P2 | 2.3052 (13) | C221—C222 | 1.407 (7) |
| S2—O6 | 1.430 (5) | C222—C223 | 1.390 (7) |
| S2—O5 | 1.437 (4) | C222—H222 | 0.9500 |
| S2—O4 | 1.442 (4) | C223—C224 | 1.376 (8) |
| S2—C2 | 1.813 (6) | C223—H223 | 0.9500 |
| P2—C221 | 1.811 (5) | C224—C225 | 1.390 (8) |
| P2—C211 | 1.812 (5) | C224—H224 | 0.9500 |
| P2—C12 | 1.820 (5) | C225—C226 | 1.380 (7) |
| N2—C6 | 1.134 (9) | C225—H225 | 0.9500 |
| C2—F6 | 1.322 (7) | C226—H226 | 0.9500 |
| C2—F4 | 1.326 (6) | C311—C312 | 1.382 (7) |
| C2—F5 | 1.343 (7) | C311—C316 | 1.389 (7) |
| P3—C321 | 1.812 (5) | C312—C313 | 1.382 (8) |
| P3—C311 | 1.819 (5) | C312—H312 | 0.9500 |
| P3—C21 | 1.823 (5) | C313—C314 | 1.371 (8) |
| C3—C4 | 1.430 (10) | C313—H313 | 0.9500 |
| C3—H3A | 0.9800 | C314—C315 | 1.408 (8) |
| C3—H3B | 0.9800 | C314—H314 | 0.9500 |
| C3—H3C | 0.9800 | C315—C316 | 1.380 (8) |
| P4—C421 | 1.807 (5) | C315—H315 | 0.9500 |
| P4—C411 | 1.816 (5) | C316—H316 | 0.9500 |
| P4—C22 | 1.826 (5) | C321—C326 | 1.384 (7) |
| C5—C6 | 1.437 (10) | C321—C322 | 1.392 (7) |
| C5—H5A | 0.9800 | C322—C323 | 1.380 (8) |
| C5—H5B | 0.9800 | C322—H322 | 0.9500 |
| C5—H5C | 0.9800 | C323—C324 | 1.371 (9) |
| C11—C12 | 1.525 (7) | C323—H323 | 0.9500 |
| C11—H11A | 0.9900 | C324—C325 | 1.383 (9) |
| C11—H11B | 0.9900 | C324—H324 | 0.9500 |
| C12—H12A | 0.9900 | C325—C326 | 1.385 (8) |
| C12—H12B | 0.9900 | C325—H325 | 0.9500 |
| C21—C22 | 1.536 (7) | C326—H326 | 0.9500 |
| C21—H21A | 0.9900 | C411—C412 | 1.382 (7) |
| C21—H21B | 0.9900 | C411—C416 | 1.383 (7) |
| C22—H22A | 0.9900 | C412—C413 | 1.382 (8) |
| C22—H22B | 0.9900 | C412—H412 | 0.9500 |
| C111—C112 | 1.394 (7) | C413—C414 | 1.385 (8) |
| C111—C116 | 1.398 (7) | C413—H413 | 0.9500 |
| C112—C113 | 1.387 (7) | C414—C415 | 1.375 (8) |
| C112—H112 | 0.9500 | C414—H414 | 0.9500 |
| C113—C114 | 1.393 (8) | C415—C416 | 1.395 (8) |
| C113—H113 | 0.9500 | C415—H415 | 0.9500 |
| C114—C115 | 1.377 (7) | C416—H416 | 0.9500 |
| C114—H114 | 0.9500 | C421—C422 | 1.385 (7) |
| C115—C116 | 1.373 (7) | C421—C426 | 1.409 (7) |
| C115—H115 | 0.9500 | C422—C423 | 1.397 (7) |
| C116—H116 | 0.9500 | C422—H422 | 0.9500 |
| C121—C122 | 1.381 (7) | C423—C424 | 1.373 (8) |
| C121—C126 | 1.403 (7) | C423—H423 | 0.9500 |
| C122—C123 | 1.398 (8) | C424—C425 | 1.368 (8) |
| C122—H122 | 0.9500 | C424—H424 | 0.9500 |
| C123—C124 | 1.367 (9) | C425—C426 | 1.377 (7) |
| C123—H123 | 0.9500 | C425—H425 | 0.9500 |
| C124—C125 | 1.377 (9) | C426—H426 | 0.9500 |
| C124—H124 | 0.9500 | ||
| P1—Au1—P3 | 171.77 (5) | C122—C123—H123 | 119.8 |
| P1—Au1—Au2 | 92.77 (3) | C123—C124—C125 | 120.6 (6) |
| P3—Au1—Au2 | 95.17 (3) | C123—C124—H124 | 119.7 |
| O3—S1—O2 | 116.0 (3) | C125—C124—H124 | 119.7 |
| O3—S1—O1 | 114.9 (3) | C126—C125—C124 | 119.8 (6) |
| O2—S1—O1 | 114.3 (2) | C126—C125—H125 | 120.1 |
| O3—S1—C1 | 103.7 (3) | C124—C125—H125 | 120.1 |
| O2—S1—C1 | 102.8 (3) | C125—C126—C121 | 120.3 (5) |
| O1—S1—C1 | 102.6 (3) | C125—C126—H126 | 119.9 |
| C121—P1—C111 | 106.7 (2) | C121—C126—H126 | 119.9 |
| C121—P1—C11 | 106.2 (2) | C216—C211—C212 | 119.2 (5) |
| C111—P1—C11 | 105.1 (2) | C216—C211—P2 | 123.6 (4) |
| C121—P1—Au1 | 114.46 (18) | C212—C211—P2 | 117.1 (4) |
| C111—P1—Au1 | 107.50 (17) | C213—C212—C211 | 120.3 (5) |
| C11—P1—Au1 | 116.15 (17) | C213—C212—H212 | 119.9 |
| F3—C1—F2 | 108.1 (5) | C211—C212—H212 | 119.9 |
| F3—C1—F1 | 107.4 (5) | C214—C213—C212 | 120.1 (5) |
| F2—C1—F1 | 106.9 (5) | C214—C213—H213 | 120.0 |
| F3—C1—S1 | 112.0 (5) | C212—C213—H213 | 120.0 |
| F2—C1—S1 | 110.4 (4) | C213—C214—C215 | 120.4 (5) |
| F1—C1—S1 | 111.9 (4) | C213—C214—H214 | 119.8 |
| P4—Au2—P2 | 177.10 (5) | C215—C214—H214 | 119.8 |
| P4—Au2—Au1 | 88.13 (3) | C214—C215—C216 | 120.3 (5) |
| P2—Au2—Au1 | 89.72 (3) | C214—C215—H215 | 119.8 |
| O6—S2—O5 | 117.2 (3) | C216—C215—H215 | 119.8 |
| O6—S2—O4 | 113.8 (3) | C215—C216—C211 | 119.7 (5) |
| O5—S2—O4 | 114.3 (3) | C215—C216—H216 | 120.1 |
| O6—S2—C2 | 102.5 (3) | C211—C216—H216 | 120.1 |
| O5—S2—C2 | 102.9 (3) | C226—C221—C222 | 119.1 (5) |
| O4—S2—C2 | 103.5 (3) | C226—C221—P2 | 121.8 (4) |
| C221—P2—C211 | 106.8 (2) | C222—C221—P2 | 119.1 (4) |
| C221—P2—C12 | 106.9 (2) | C223—C222—C221 | 119.9 (5) |
| C211—P2—C12 | 106.3 (2) | C223—C222—H222 | 120.0 |
| C221—P2—Au2 | 113.02 (17) | C221—C222—H222 | 120.0 |
| C211—P2—Au2 | 112.79 (16) | C224—C223—C222 | 120.0 (5) |
| C12—P2—Au2 | 110.66 (17) | C224—C223—H223 | 120.0 |
| F6—C2—F4 | 107.9 (5) | C222—C223—H223 | 120.0 |
| F6—C2—F5 | 106.8 (5) | C223—C224—C225 | 120.4 (5) |
| F4—C2—F5 | 106.7 (5) | C223—C224—H224 | 119.8 |
| F6—C2—S2 | 111.9 (4) | C225—C224—H224 | 119.8 |
| F4—C2—S2 | 112.1 (4) | C226—C225—C224 | 120.0 (5) |
| F5—C2—S2 | 111.2 (4) | C226—C225—H225 | 120.0 |
| C321—P3—C311 | 108.1 (2) | C224—C225—H225 | 120.0 |
| C321—P3—C21 | 107.7 (2) | C225—C226—C221 | 120.5 (5) |
| C311—P3—C21 | 104.3 (2) | C225—C226—H226 | 119.7 |
| C321—P3—Au1 | 111.19 (17) | C221—C226—H226 | 119.7 |
| C311—P3—Au1 | 108.64 (17) | C312—C311—C316 | 119.2 (5) |
| C21—P3—Au1 | 116.49 (17) | C312—C311—P3 | 123.2 (4) |
| C4—C3—H3A | 109.5 | C316—C311—P3 | 117.5 (4) |
| C4—C3—H3B | 109.5 | C313—C312—C311 | 120.2 (5) |
| H3A—C3—H3B | 109.5 | C313—C312—H312 | 119.9 |
| C4—C3—H3C | 109.5 | C311—C312—H312 | 119.9 |
| H3A—C3—H3C | 109.5 | C314—C313—C312 | 121.2 (5) |
| H3B—C3—H3C | 109.5 | C314—C313—H313 | 119.4 |
| C421—P4—C411 | 109.0 (2) | C312—C313—H313 | 119.4 |
| C421—P4—C22 | 107.4 (2) | C313—C314—C315 | 119.0 (5) |
| C411—P4—C22 | 102.0 (2) | C313—C314—H314 | 120.5 |
| C421—P4—Au2 | 113.18 (17) | C315—C314—H314 | 120.5 |
| C411—P4—Au2 | 112.60 (18) | C316—C315—C314 | 119.6 (6) |
| C22—P4—Au2 | 111.96 (17) | C316—C315—H315 | 120.2 |
| N1—C4—C3 | 176.0 (11) | C314—C315—H315 | 120.2 |
| C6—C5—H5A | 109.5 | C315—C316—C311 | 120.7 (5) |
| C6—C5—H5B | 109.5 | C315—C316—H316 | 119.6 |
| H5A—C5—H5B | 109.5 | C311—C316—H316 | 119.6 |
| C6—C5—H5C | 109.5 | C326—C321—C322 | 119.7 (5) |
| H5A—C5—H5C | 109.5 | C326—C321—P3 | 118.6 (4) |
| H5B—C5—H5C | 109.5 | C322—C321—P3 | 121.6 (4) |
| N2—C6—C5 | 178.7 (9) | C323—C322—C321 | 120.3 (5) |
| C12—C11—P1 | 118.0 (3) | C323—C322—H322 | 119.8 |
| C12—C11—H11A | 107.8 | C321—C322—H322 | 119.8 |
| P1—C11—H11A | 107.8 | C324—C323—C322 | 119.6 (6) |
| C12—C11—H11B | 107.8 | C324—C323—H323 | 120.2 |
| P1—C11—H11B | 107.8 | C322—C323—H323 | 120.2 |
| H11A—C11—H11B | 107.1 | C323—C324—C325 | 120.8 (6) |
| C11—C12—P2 | 116.0 (3) | C323—C324—H324 | 119.6 |
| C11—C12—H12A | 108.3 | C325—C324—H324 | 119.6 |
| P2—C12—H12A | 108.3 | C324—C325—C326 | 119.9 (6) |
| C11—C12—H12B | 108.3 | C324—C325—H325 | 120.0 |
| P2—C12—H12B | 108.3 | C326—C325—H325 | 120.0 |
| H12A—C12—H12B | 107.4 | C321—C326—C325 | 119.6 (5) |
| C22—C21—P3 | 119.2 (4) | C321—C326—H326 | 120.2 |
| C22—C21—H21A | 107.5 | C325—C326—H326 | 120.2 |
| P3—C21—H21A | 107.5 | C412—C411—C416 | 119.9 (5) |
| C22—C21—H21B | 107.5 | C412—C411—P4 | 117.0 (4) |
| P3—C21—H21B | 107.5 | C416—C411—P4 | 123.1 (4) |
| H21A—C21—H21B | 107.0 | C411—C412—C413 | 120.3 (5) |
| C21—C22—P4 | 117.9 (3) | C411—C412—H412 | 119.8 |
| C21—C22—H22A | 107.8 | C413—C412—H412 | 119.8 |
| P4—C22—H22A | 107.8 | C412—C413—C414 | 119.7 (5) |
| C21—C22—H22B | 107.8 | C412—C413—H413 | 120.1 |
| P4—C22—H22B | 107.8 | C414—C413—H413 | 120.1 |
| H22A—C22—H22B | 107.2 | C415—C414—C413 | 120.4 (5) |
| C112—C111—C116 | 118.4 (5) | C415—C414—H414 | 119.8 |
| C112—C111—P1 | 123.5 (4) | C413—C414—H414 | 119.8 |
| C116—C111—P1 | 118.0 (4) | C414—C415—C416 | 119.8 (5) |
| C113—C112—C111 | 120.5 (5) | C414—C415—H415 | 120.1 |
| C113—C112—H112 | 119.8 | C416—C415—H415 | 120.1 |
| C111—C112—H112 | 119.8 | C411—C416—C415 | 119.9 (5) |
| C112—C113—C114 | 119.8 (5) | C411—C416—H416 | 120.1 |
| C112—C113—H113 | 120.1 | C415—C416—H416 | 120.1 |
| C114—C113—H113 | 120.1 | C422—C421—C426 | 118.9 (5) |
| C115—C114—C113 | 120.1 (5) | C422—C421—P4 | 119.2 (4) |
| C115—C114—H114 | 119.9 | C426—C421—P4 | 121.9 (4) |
| C113—C114—H114 | 119.9 | C421—C422—C423 | 120.1 (5) |
| C116—C115—C114 | 120.0 (5) | C421—C422—H422 | 120.0 |
| C116—C115—H115 | 120.0 | C423—C422—H422 | 120.0 |
| C114—C115—H115 | 120.0 | C424—C423—C422 | 120.0 (6) |
| C115—C116—C111 | 121.1 (5) | C424—C423—H423 | 120.0 |
| C115—C116—H116 | 119.4 | C422—C423—H423 | 120.0 |
| C111—C116—H116 | 119.4 | C425—C424—C423 | 120.3 (6) |
| C122—C121—C126 | 119.5 (5) | C425—C424—H424 | 119.8 |
| C122—C121—P1 | 120.5 (4) | C423—C424—H424 | 119.8 |
| C126—C121—P1 | 120.0 (4) | C424—C425—C426 | 120.8 (6) |
| C121—C122—C123 | 119.3 (6) | C424—C425—H425 | 119.6 |
| C121—C122—H122 | 120.3 | C426—C425—H425 | 119.6 |
| C123—C122—H122 | 120.3 | C425—C426—C421 | 119.9 (5) |
| C124—C123—C122 | 120.4 (6) | C425—C426—H426 | 120.1 |
| C124—C123—H123 | 119.8 | C421—C426—H426 | 120.1 |
| Au2—Au1—P1—C121 | 118.83 (19) | C12—P2—C211—C212 | 171.0 (4) |
| Au2—Au1—P1—C111 | −122.84 (18) | Au2—P2—C211—C212 | 49.6 (4) |
| Au2—Au1—P1—C11 | −5.49 (18) | C216—C211—C212—C213 | −2.3 (7) |
| O3—S1—C1—F3 | −58.3 (5) | P2—C211—C212—C213 | −179.2 (4) |
| O2—S1—C1—F3 | 62.9 (5) | C211—C212—C213—C214 | 2.0 (8) |
| O1—S1—C1—F3 | −178.2 (4) | C212—C213—C214—C215 | −1.4 (8) |
| O3—S1—C1—F2 | −178.7 (4) | C213—C214—C215—C216 | 1.0 (8) |
| O2—S1—C1—F2 | −57.6 (5) | C214—C215—C216—C211 | −1.3 (8) |
| O1—S1—C1—F2 | 61.4 (5) | C212—C211—C216—C215 | 1.9 (7) |
| O3—S1—C1—F1 | 62.3 (5) | P2—C211—C216—C215 | 178.6 (4) |
| O2—S1—C1—F1 | −176.5 (4) | C211—P2—C221—C226 | −70.3 (5) |
| O1—S1—C1—F1 | −57.6 (5) | C12—P2—C221—C226 | 43.1 (5) |
| P1—Au1—Au2—P4 | 130.95 (5) | Au2—P2—C221—C226 | 165.1 (4) |
| P3—Au1—Au2—P4 | −46.89 (5) | C211—P2—C221—C222 | 109.6 (4) |
| P1—Au1—Au2—P2 | −47.11 (5) | C12—P2—C221—C222 | −136.9 (4) |
| P3—Au1—Au2—P2 | 135.05 (5) | Au2—P2—C221—C222 | −15.0 (5) |
| Au1—Au2—P2—C221 | −46.58 (18) | C226—C221—C222—C223 | −1.1 (8) |
| Au1—Au2—P2—C211 | −167.81 (18) | P2—C221—C222—C223 | 179.0 (4) |
| Au1—Au2—P2—C12 | 73.24 (19) | C221—C222—C223—C224 | 0.3 (8) |
| O6—S2—C2—F6 | −60.5 (5) | C222—C223—C224—C225 | 0.2 (8) |
| O5—S2—C2—F6 | 61.5 (5) | C223—C224—C225—C226 | 0.1 (8) |
| O4—S2—C2—F6 | −179.1 (4) | C224—C225—C226—C221 | −0.8 (8) |
| O6—S2—C2—F4 | 60.9 (5) | C222—C221—C226—C225 | 1.4 (8) |
| O5—S2—C2—F4 | −177.0 (4) | P2—C221—C226—C225 | −178.7 (4) |
| O4—S2—C2—F4 | −57.7 (5) | C321—P3—C311—C312 | −102.9 (5) |
| O6—S2—C2—F5 | −179.8 (4) | C21—P3—C311—C312 | 11.5 (5) |
| O5—S2—C2—F5 | −57.7 (5) | Au1—P3—C311—C312 | 136.3 (4) |
| O4—S2—C2—F5 | 61.6 (5) | C321—P3—C311—C316 | 77.4 (5) |
| Au2—Au1—P3—C321 | 119.99 (19) | C21—P3—C311—C316 | −168.2 (4) |
| Au2—Au1—P3—C311 | −121.21 (18) | Au1—P3—C311—C316 | −43.4 (5) |
| Au2—Au1—P3—C21 | −3.91 (19) | C316—C311—C312—C313 | −2.1 (8) |
| Au1—Au2—P4—C421 | −48.35 (18) | P3—C311—C312—C313 | 178.2 (4) |
| Au1—Au2—P4—C411 | −172.49 (19) | C311—C312—C313—C314 | 0.8 (8) |
| Au1—Au2—P4—C22 | 73.23 (19) | C312—C313—C314—C315 | 0.6 (9) |
| C121—P1—C11—C12 | −55.4 (4) | C313—C314—C315—C316 | −0.6 (9) |
| C111—P1—C11—C12 | −168.2 (4) | C314—C315—C316—C311 | −0.7 (9) |
| Au1—P1—C11—C12 | 73.1 (4) | C312—C311—C316—C315 | 2.1 (8) |
| P1—C11—C12—P2 | −56.9 (5) | P3—C311—C316—C315 | −178.2 (4) |
| C221—P2—C12—C11 | 94.4 (4) | C311—P3—C321—C326 | −89.9 (5) |
| C211—P2—C12—C11 | −151.8 (4) | C21—P3—C321—C326 | 158.0 (4) |
| Au2—P2—C12—C11 | −29.0 (4) | Au1—P3—C321—C326 | 29.3 (5) |
| C321—P3—C21—C22 | −58.8 (4) | C311—P3—C321—C322 | 91.9 (5) |
| C311—P3—C21—C22 | −173.5 (4) | C21—P3—C321—C322 | −20.2 (5) |
| Au1—P3—C21—C22 | 66.8 (4) | Au1—P3—C321—C322 | −149.0 (4) |
| P3—C21—C22—P4 | −51.2 (5) | C326—C321—C322—C323 | −0.1 (8) |
| C421—P4—C22—C21 | 91.8 (4) | P3—C321—C322—C323 | 178.2 (4) |
| C411—P4—C22—C21 | −153.6 (4) | C321—C322—C323—C324 | 1.5 (8) |
| Au2—P4—C22—C21 | −33.0 (4) | C322—C323—C324—C325 | −1.5 (9) |
| C121—P1—C111—C112 | −112.8 (5) | C323—C324—C325—C326 | 0.0 (9) |
| C11—P1—C111—C112 | −0.3 (5) | C322—C321—C326—C325 | −1.4 (8) |
| Au1—P1—C111—C112 | 124.0 (4) | P3—C321—C326—C325 | −179.7 (4) |
| C121—P1—C111—C116 | 69.1 (5) | C324—C325—C326—C321 | 1.4 (9) |
| C11—P1—C111—C116 | −178.5 (4) | C421—P4—C411—C412 | −163.5 (4) |
| Au1—P1—C111—C116 | −54.2 (4) | C22—P4—C411—C412 | 83.2 (5) |
| C116—C111—C112—C113 | −0.3 (8) | Au2—P4—C411—C412 | −37.0 (5) |
| P1—C111—C112—C113 | −178.4 (4) | C421—P4—C411—C416 | 18.7 (5) |
| C111—C112—C113—C114 | 1.3 (8) | C22—P4—C411—C416 | −94.7 (5) |
| C112—C113—C114—C115 | −2.6 (9) | Au2—P4—C411—C416 | 145.1 (4) |
| C113—C114—C115—C116 | 2.8 (9) | C416—C411—C412—C413 | 0.3 (8) |
| C114—C115—C116—C111 | −1.8 (8) | P4—C411—C412—C413 | −177.6 (4) |
| C112—C111—C116—C115 | 0.5 (8) | C411—C412—C413—C414 | −0.8 (9) |
| P1—C111—C116—C115 | 178.7 (4) | C412—C413—C414—C415 | 1.0 (9) |
| C111—P1—C121—C122 | −106.2 (4) | C413—C414—C415—C416 | −0.6 (9) |
| C11—P1—C121—C122 | 142.1 (4) | C412—C411—C416—C415 | 0.0 (8) |
| Au1—P1—C121—C122 | 12.6 (5) | P4—C411—C416—C415 | 177.8 (4) |
| C111—P1—C121—C126 | 74.7 (5) | C414—C415—C416—C411 | 0.1 (9) |
| C11—P1—C121—C126 | −37.1 (5) | C411—P4—C421—C422 | 107.3 (4) |
| Au1—P1—C121—C126 | −166.5 (4) | C22—P4—C421—C422 | −142.9 (4) |
| C126—C121—C122—C123 | 0.8 (8) | Au2—P4—C421—C422 | −18.8 (5) |
| P1—C121—C122—C123 | −178.3 (4) | C411—P4—C421—C426 | −75.3 (5) |
| C121—C122—C123—C124 | −0.2 (9) | C22—P4—C421—C426 | 34.5 (5) |
| C122—C123—C124—C125 | −0.4 (9) | Au2—P4—C421—C426 | 158.6 (4) |
| C123—C124—C125—C126 | 0.3 (9) | C426—C421—C422—C423 | −0.3 (8) |
| C124—C125—C126—C121 | 0.3 (8) | P4—C421—C422—C423 | 177.1 (4) |
| C122—C121—C126—C125 | −0.9 (8) | C421—C422—C423—C424 | −1.4 (8) |
| P1—C121—C126—C125 | 178.3 (4) | C422—C423—C424—C425 | 2.8 (9) |
| C221—P2—C211—C216 | 108.1 (4) | C423—C424—C425—C426 | −2.5 (9) |
| C12—P2—C211—C216 | −5.8 (5) | C424—C425—C426—C421 | 0.7 (8) |
| Au2—P2—C211—C216 | −127.2 (4) | C422—C421—C426—C425 | 0.7 (8) |
| C221—P2—C211—C212 | −75.2 (4) | P4—C421—C426—C425 | −176.7 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C212—H212···O1i | 0.95 | 2.45 | 3.387 (7) | 171 |
| C21—H21B···O1i | 0.99 | 2.34 | 3.268 (7) | 155 |
| C11—H11B···O4ii | 0.99 | 2.36 | 3.301 (7) | 158 |
Symmetry codes: (i) x, −y+1/2, z+1/2; (ii) x, −y+1/2, z−1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IM2111).
References
- Al-Baker, S., Hill, W. E. & McAuliffe, C. A. (1985). J. Chem. Soc. Dalton Trans. pp. 2655–2659.
- Atwood, J. L. & Barbour, L. J. (2003). Cryst. Growth Des.3, 3–8.
- Barbour, L. J. (2001). J. Supramol. Chem.1, 189–191.
- Bruker (2002). SADABS and SMART Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2003). SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Jongh, L.-A. de, Strasser, C. E., Cronje, S. & Raubenheimer, H. G. (2007). Acta Cryst. E63, m2137–m2138.
- Schuh, W., Kopacka, H., Wurst, K. & Peringer, P. (2001). Chem. Commun. pp. 2186–2187. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809025513/im2111sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809025513/im2111Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



