Abstract
The phytochrome holoprotein of plants requires a covalently bound linear tetrapyrrole (bilin) prosthetic group for its photoreceptor function. Here we show that synthetic phytochrome apoprotein prepared by transcription and translation of an Avena phytochrome cDNA construct combines in vitro with phycocyanobilin, an analog of the natural chromophore, to produce a photoactive holoprotein. These results indicate that holoprotein assembly is an “autocatalytic” process.
Keywords: plant photoreceptor, phytochrome biosynthesis, phycocyanobilin attachment, bilin C-S lyase
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. Immunoprecipitation of proteins from cell-free translations. Methods Enzymol. 1983;96:111–120. doi: 10.1016/s0076-6879(83)96012-3. [DOI] [PubMed] [Google Scholar]
- Arciero D. M., Bryant D. A., Glazer A. N. In vitro attachment of bilins to apophycocyanin. I. Specific covalent adduct formation at cysteinyl residues involved in phycocyanobilin binding in C-phycocyanin. J Biol Chem. 1988 Dec 5;263(34):18343–18349. [PubMed] [Google Scholar]
- Arciero D. M., Dallas J. L., Glazer A. N. In vitro attachment of bilins to apophycocyanin. II. Determination of the structures of tryptic bilin peptides derived from the phycocyanobilin adduct. J Biol Chem. 1988 Dec 5;263(34):18350–18357. [PubMed] [Google Scholar]
- Arciero D. M., Dallas J. L., Glazer A. N. In vitro attachment of bilins to apophycocyanin. III. Properties of the phycoerythrobilin adduct. J Biol Chem. 1988 Dec 5;263(34):18358–18363. [PubMed] [Google Scholar]
- Dumont M. E., Ernst J. F., Hampsey D. M., Sherman F. Identification and sequence of the gene encoding cytochrome c heme lyase in the yeast Saccharomyces cerevisiae. EMBO J. 1987 Jan;6(1):235–241. doi: 10.1002/j.1460-2075.1987.tb04744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey H. P., Barker R. F., Idler K. B., Lissemore J. L., Quail P. H. Analysis of cloned cDNA and genomic sequences for phytochrome: complete amino acid sequences for two gene products expressed in etiolated Avena. Nucleic Acids Res. 1985 Dec 9;13(23):8543–8559. doi: 10.1093/nar/13.23.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey H. P., Barker R. F., Idler K. B., Murray M. G., Quail P. H. Nucleotide sequence and characterization of a gene encoding the phytochrome polypeptide from Avena. Gene. 1987;61(3):339–348. doi: 10.1016/0378-1119(87)90197-1. [DOI] [PubMed] [Google Scholar]
- Jagus R. Translation in cell-free systems. Methods Enzymol. 1987;152:267–276. doi: 10.1016/0076-6879(87)52030-4. [DOI] [PubMed] [Google Scholar]
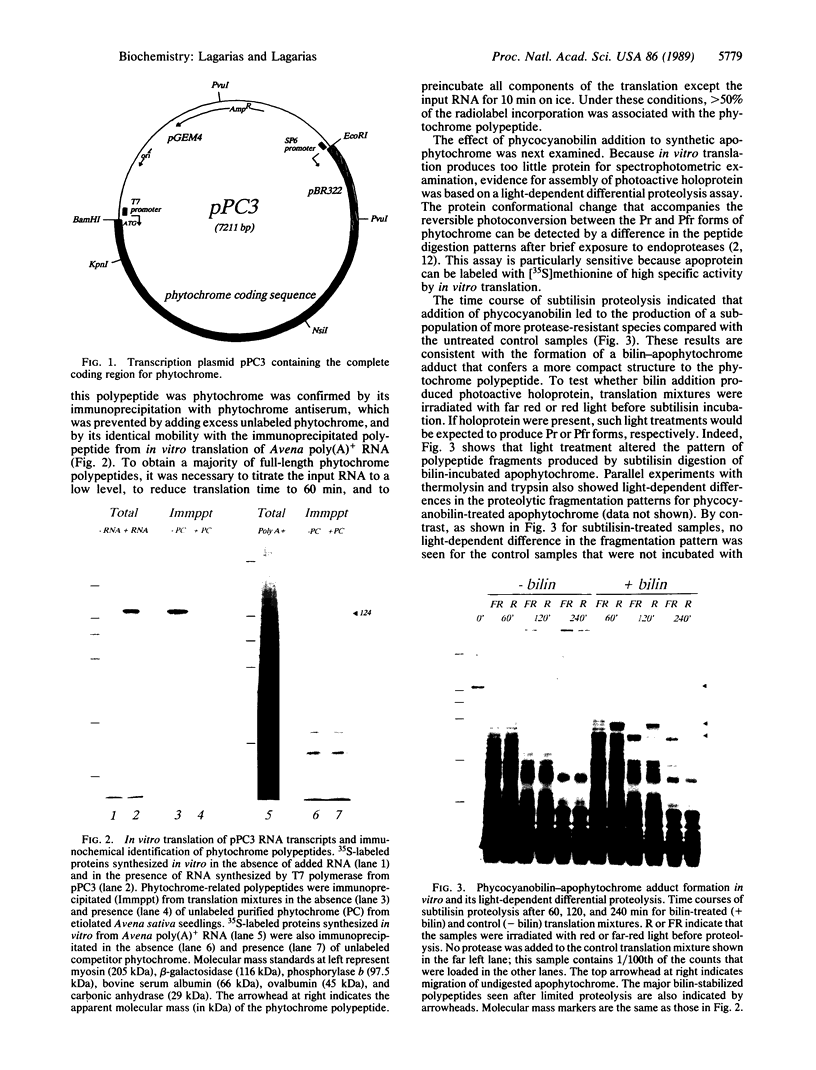
- Lagarias J. C., Mercurio F. M. Structure function studies on phytochrome. Identification of light-induced conformational changes in 124-kDa Avena phytochrome in vitro. J Biol Chem. 1985 Feb 25;260(4):2415–2423. [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Przybyla A. E., Chirgwin J. M. Isolation of RNA using guanidinium salts. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- Nielsen D. A., Shapiro D. J. Preparation of capped RNA transcripts using T7 RNA polymerase. Nucleic Acids Res. 1986 Jul 25;14(14):5936–5936. doi: 10.1093/nar/14.14.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]