Abstract
The title compound, C10H7F3N2O, is an analogue of pyrazolone derivatives with potential analgesic and anti-inflammatory properties. Its molecular structure consists of phenyl and pyrazol-3(2H)-one units with a dihedral angle between the mean planes of the rings of 33.0 (1)°. The crystal structure is stabilized by an intermolecular hydrogen bond between the N—H group and the carbonyl O atom of the pyrazol-3(2H)-one ring which links the molecules into supramolecular C(5) chains along [001] and by weak π–π stacking interactions between the phenyl rings [centroid-centroid distance = 3.881 (2) Å]. The F atoms are disordered over two positions with refined site occupancies of 0.768(11) and 0.232(11).
Related literature
For the analgesic properties of pyrazolones, see: Mehlisch (1983 ▶); Schnitzer (2003 ▶). For the biological activity of some pyrazolone derivatives, see: Pavlov et al. (1998 ▶). For the pharmacological properties of pyrazolone deriavtives, see: Kees et al. (1996 ▶). For related structures, see: Belmar et al. (2006a
▶,b
▶); Pérez et al. (2005 ▶). For metal complexes, see: Hyun-Shin et al. (2008 ▶); Gallardo et al. (2004 ▶); Meyer et al. (1998 ▶). For the synthesis of pyrazolones, see: Nakagawa et al. (2006 ▶); Belmar et al. (2001 ▶); Bartulín et al. (1994 ▶). For hydrogen-bond motifs, see: Bernstein et al. (1995 ▶).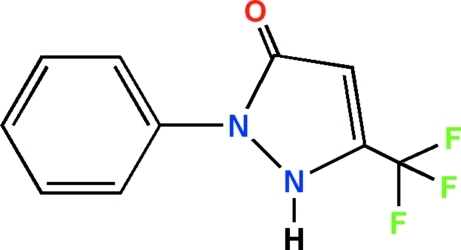
Experimental
Crystal data
C10H7F3N2O
M r = 228.18
Monoclinic,
a = 5.8409 (5) Å
b = 15.2454 (14) Å
c = 11.2291 (17) Å
β = 92.403 (9)°
V = 999.0 (2) Å3
Z = 4
Mo Kα radiation
μ = 0.14 mm−1
T = 293 K
0.46 × 0.40 × 0.20 mm
Data collection
Enraf–Nonius CAD-4 diffractometer
Absorption correction: none
2262 measured reflections
2157 independent reflections
1141 reflections with I > 2σ(I)
R int = 0.024
3 standard reflections every 200 reflections intensity decay: 1%
Refinement
R[F 2 > 2σ(F 2)] = 0.060
wR(F 2) = 0.188
S = 1.03
2157 reflections
173 parameters
81 restraints
H-atom parameters constrained
Δρmax = 0.30 e Å−3
Δρmin = −0.32 e Å−3
Data collection: CAD-4 Software (Enraf–Nonius, 1989 ▶); cell refinement: SET4 in CAD-4 Software; data reduction: HELENA (Spek, 1996 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2009 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809029419/bx2225sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809029419/bx2225Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2⋯O1i | 0.88 | 1.89 | 2.667 (3) | 146 |
Symmetry code: (i) 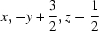 .
.
Acknowledgments
The authors are grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Fundação de Apoio à Pesquisa Científica e Tecnológica do Estado de Santa Catarina (FAPESC), the Instituto Nacional de Ciência e Tecnologia (INCT-cat) and the Financiadora de Estudos e Projetos (FINEP) for financial assistance.
supplementary crystallographic information
Comment
The pyrazolone analgesics (such as phenylbutazone) have effects similar to those of aspirin. They were commonly used to treat rheumatoid arthritis and has been the focus of medicinal chemists for over last 100 years because of the outstanding pharmacological properties shown by several of its derivatives (Kees et al., 1996). The interest in such compounds, pyrazolone derivative, arises from the fact that the incorporation of heteroatoms can result an ancillary ligand to study their photoactive lanthanide complexes. These compounds possess several sites for substitution, allowing for a systematic analysis of their effects on the photo optical properties. Particulary the luminescence properties of the Eu and Tb complexes.
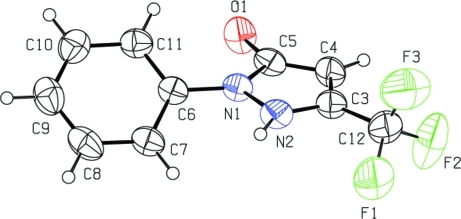
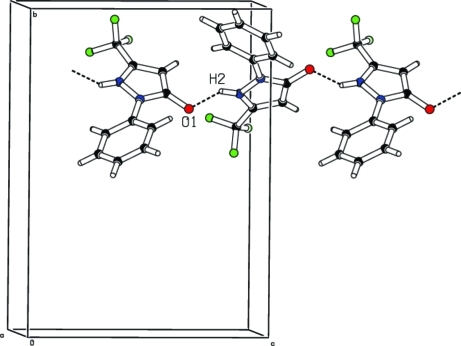
The molecular structure of (I) consists of a phenyl group bonded to 2-N of the dihydropyrazole heterocyclic ring (Fig. 1). These rings are twisted with respect to each other and the dihedral angle between the mean plane is 33.0 (1)°.The molecules are linked into chains by one intermolecular N—H···O hydrogen bond. Atoms N2 in the molecules at (x,y,z) acts as hydrogen bonds donor vía atom H2 to atoms O1 at (-x, 3/2+y, -1-z) so generating by translation one C(5) chains running parallel to [001] direction (Bernstein et al., 1995), (Fig. 2, Table 1) and the crystal structure is reinforced by a weak face-to-face π-π stacking interactions between phenyl rings with the centroid-centroid distance of 3.881 (2) Å.
Refinement
All non-H atoms were refined with anisotropic displacement parameters. HAr atoms were placed at their idealized positions with distances of 0.93 Å and Ueq fixed at 1.2 Uiso of the preceding atom. H atom attached to N atom was located from Fourier difference map and treated with riding model. Fluorine atoms are disordered over two alternative positions with refined site occupancies of 0.768 (11) and 0.232 (11).
Figures
Fig. 1.
The molecular structure of (I) with labeling scheme. Displacement ellipsoids are shown at the 40% probability level.
Fig. 2.
Part of the crystal structure of (I), showing the formation of a C(5) chain pattern. [Symmetry code: (i) x,-y+3/2,z-1/2]
Crystal data
| C10H7F3N2O | F(000) = 464 |
| Mr = 228.18 | Dx = 1.517 Mg m−3 |
| Monoclinic, P21/c | Melting point = 464–465 K |
| Hall symbol: -P 2ybc | Mo Kα radiation, λ = 0.71073 Å |
| a = 5.8409 (5) Å | Cell parameters from 25 reflections |
| b = 15.2454 (14) Å | θ = 3.2–13.8° |
| c = 11.2291 (17) Å | µ = 0.14 mm−1 |
| β = 92.403 (9)° | T = 293 K |
| V = 999.0 (2) Å3 | Irregular, colourless |
| Z = 4 | 0.46 × 0.40 × 0.20 mm |
Data collection
| Enraf–Nonius CAD-4 diffractometer | Rint = 0.024 |
| Radiation source: fine-focus sealed tube | θmax = 27.0°, θmin = 2.3° |
| graphite | h = −7→7 |
| ω–2θ scans | k = −19→0 |
| 2262 measured reflections | l = −14→0 |
| 2157 independent reflections | 3 standard reflections every 200 reflections |
| 1141 reflections with I > 2σ(I) | intensity decay: 1% |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.060 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.188 | H-atom parameters constrained |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0843P)2 + 0.3445P] where P = (Fo2 + 2Fc2)/3 |
| 2157 reflections | (Δ/σ)max = 0.001 |
| 173 parameters | Δρmax = 0.30 e Å−3 |
| 81 restraints | Δρmin = −0.32 e Å−3 |
Special details
| Experimental. The title compound was synthesized by the condensation of ethyl 4,4,4-trifluoroacetoacetate (5.0?g, 27.2?mmol) in acetic acid (50?ml) with phenylhydrazine (2.9?g, 27.2?mmol) which was added drop wise, with stirring for 3?h. The solvent was removed by evaporation; resulting crude solid was extracted with AcOEt. The organic layer was washed with saturated aqueous NaHCO3 and water, then brine, and evaporation the solvent. The compound, obtained as colorless single crystals, was recrystallized using ethylacetate and n-hexane (2:1) and was suitable for X-ray structure determination. Yield 76% mp: 191–192 °C, lit. 195–196 °C (Nakagawa et al., 2006). 1H-NMR (DMSO, 400?MHz, d, p.p.m.) 12,42 (1H, s), 7,70 (2H, d, J = 8?Hz), 7,49 (2H, t, J = 8?Hz), 7,36 (1H, t, J = 8?Hz), 5,92 (1H, s). 13C-NMR (DMSO, 400?MHz, d, p.p.m.) 153,68 (C5), 140,21 (C3), 13,70 (C6), 129,07 (C10; C8), 127,18 (C9), 122,25 (C11; C7), 119,98 (C12), 85,53 (C4). |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| N1 | 0.2789 (5) | 0.78258 (17) | 0.98039 (19) | 0.0486 (7) | |
| N2 | 0.3999 (5) | 0.73266 (19) | 0.90418 (19) | 0.0534 (7) | |
| H2 | 0.3729 | 0.7405 | 0.8270 | 0.064* | |
| C3 | 0.5425 (6) | 0.6863 (2) | 0.9721 (3) | 0.0535 (8) | |
| C4 | 0.5221 (6) | 0.7045 (2) | 1.0923 (3) | 0.0567 (9) | |
| H4 | 0.6052 | 0.6801 | 1.1566 | 0.068* | |
| C5 | 0.3528 (6) | 0.7661 (2) | 1.0950 (2) | 0.0523 (8) | |
| C6 | 0.1101 (5) | 0.8436 (2) | 0.9370 (2) | 0.0471 (8) | |
| C7 | 0.1383 (6) | 0.8843 (2) | 0.8282 (2) | 0.0582 (9) | |
| H7 | 0.2671 | 0.8730 | 0.7847 | 0.070* | |
| C8 | −0.0280 (7) | 0.9420 (3) | 0.7854 (3) | 0.0730 (11) | |
| H8 | −0.0111 | 0.9694 | 0.7123 | 0.088* | |
| C9 | −0.2193 (7) | 0.9594 (3) | 0.8499 (4) | 0.0745 (11) | |
| H9 | −0.3304 | 0.9983 | 0.8204 | 0.089* | |
| C10 | −0.2439 (6) | 0.9189 (3) | 0.9575 (3) | 0.0691 (10) | |
| H10 | −0.3715 | 0.9309 | 1.0016 | 0.083* | |
| C11 | −0.0805 (6) | 0.8604 (2) | 1.0010 (3) | 0.0583 (9) | |
| H11 | −0.0994 | 0.8323 | 1.0735 | 0.070* | |
| C12 | 0.7009 (8) | 0.6240 (3) | 0.9172 (3) | 0.0712 (11) | |
| F1 | 0.7641 (13) | 0.6502 (4) | 0.8115 (4) | 0.118 (3) | 0.768 (11) |
| F1' | 0.617 (3) | 0.5831 (17) | 0.826 (2) | 0.129 (8) | 0.232 (11) |
| F2 | 0.8938 (12) | 0.6159 (7) | 0.9809 (5) | 0.129 (3) | 0.768 (11) |
| F2' | 0.884 (4) | 0.6549 (11) | 0.882 (3) | 0.141 (9) | 0.232 (11) |
| F3 | 0.6134 (13) | 0.5470 (4) | 0.9008 (9) | 0.144 (3) | 0.768 (11) |
| F3' | 0.760 (5) | 0.5604 (14) | 0.9906 (13) | 0.104 (6) | 0.232 (11) |
| O1 | 0.2602 (4) | 0.81041 (16) | 1.18269 (16) | 0.0686 (8) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0599 (16) | 0.0617 (17) | 0.0244 (11) | 0.0019 (14) | 0.0042 (10) | −0.0008 (11) |
| N2 | 0.0666 (17) | 0.0714 (18) | 0.0225 (11) | 0.0001 (14) | 0.0044 (11) | −0.0034 (11) |
| C3 | 0.062 (2) | 0.062 (2) | 0.0374 (16) | 0.0015 (17) | 0.0057 (15) | 0.0011 (15) |
| C4 | 0.068 (2) | 0.068 (2) | 0.0336 (15) | 0.0072 (19) | 0.0006 (14) | 0.0062 (15) |
| C5 | 0.070 (2) | 0.063 (2) | 0.0242 (14) | −0.0023 (18) | 0.0033 (13) | 0.0040 (13) |
| C6 | 0.0554 (19) | 0.0511 (18) | 0.0343 (15) | −0.0052 (16) | −0.0026 (13) | −0.0028 (13) |
| C7 | 0.072 (2) | 0.067 (2) | 0.0360 (15) | −0.0042 (19) | 0.0003 (15) | 0.0052 (15) |
| C8 | 0.093 (3) | 0.068 (3) | 0.056 (2) | −0.011 (2) | −0.015 (2) | 0.0164 (18) |
| C9 | 0.075 (3) | 0.063 (2) | 0.083 (3) | 0.001 (2) | −0.022 (2) | 0.005 (2) |
| C10 | 0.059 (2) | 0.073 (2) | 0.075 (2) | 0.001 (2) | −0.0004 (18) | −0.004 (2) |
| C11 | 0.062 (2) | 0.066 (2) | 0.0470 (18) | −0.0056 (19) | 0.0017 (16) | 0.0011 (16) |
| C12 | 0.078 (3) | 0.083 (3) | 0.054 (2) | 0.010 (2) | 0.018 (2) | −0.003 (2) |
| F1 | 0.142 (6) | 0.152 (5) | 0.064 (3) | 0.052 (4) | 0.050 (3) | 0.008 (3) |
| F1' | 0.083 (11) | 0.172 (19) | 0.129 (14) | 0.035 (11) | −0.038 (10) | −0.112 (12) |
| F2 | 0.100 (4) | 0.187 (7) | 0.099 (4) | 0.068 (4) | −0.016 (3) | −0.030 (4) |
| F2' | 0.105 (13) | 0.127 (13) | 0.20 (2) | −0.035 (10) | 0.083 (14) | −0.071 (15) |
| F3 | 0.164 (6) | 0.077 (3) | 0.197 (8) | −0.008 (3) | 0.088 (6) | −0.041 (4) |
| F3' | 0.133 (15) | 0.104 (11) | 0.074 (8) | 0.059 (10) | −0.003 (9) | −0.004 (8) |
| O1 | 0.0983 (19) | 0.0804 (17) | 0.0276 (11) | 0.0248 (15) | 0.0078 (11) | −0.0044 (10) |
Geometric parameters (Å, °)
| N1—C5 | 1.363 (3) | C8—C9 | 1.382 (6) |
| N1—N2 | 1.365 (3) | C8—H8 | 0.9300 |
| N1—C6 | 1.426 (4) | C9—C10 | 1.371 (5) |
| N2—C3 | 1.312 (4) | C9—H9 | 0.9300 |
| N2—H2 | 0.8825 | C10—C11 | 1.381 (5) |
| C3—C4 | 1.388 (4) | C10—H10 | 0.9300 |
| C3—C12 | 1.479 (5) | C11—H11 | 0.9300 |
| C4—C5 | 1.366 (5) | C12—F2' | 1.247 (14) |
| C4—H4 | 0.9300 | C12—F1' | 1.279 (13) |
| C5—O1 | 1.327 (4) | C12—F3 | 1.291 (7) |
| C6—C11 | 1.374 (4) | C12—F3' | 1.309 (13) |
| C6—C7 | 1.387 (4) | C12—F2 | 1.315 (6) |
| C7—C8 | 1.381 (5) | C12—F1 | 1.320 (5) |
| C7—H7 | 0.9300 | ||
| C5—N1—N2 | 109.7 (3) | C9—C8—H8 | 119.6 |
| C5—N1—C6 | 129.0 (2) | C10—C9—C8 | 119.5 (4) |
| N2—N1—C6 | 121.2 (2) | C10—C9—H9 | 120.3 |
| C3—N2—N1 | 105.6 (2) | C8—C9—H9 | 120.3 |
| C3—N2—H2 | 136.6 | C9—C10—C11 | 120.4 (4) |
| N1—N2—H2 | 117.7 | C9—C10—H10 | 119.8 |
| N2—C3—C4 | 112.3 (3) | C11—C10—H10 | 119.8 |
| N2—C3—C12 | 119.8 (3) | C6—C11—C10 | 119.9 (3) |
| C4—C3—C12 | 127.9 (3) | C6—C11—H11 | 120.1 |
| C5—C4—C3 | 104.5 (3) | C10—C11—H11 | 120.1 |
| C5—C4—H4 | 127.7 | F2'—C12—F1' | 103.5 (9) |
| C3—C4—H4 | 127.7 | F2'—C12—F3' | 105.9 (9) |
| O1—C5—N1 | 119.0 (3) | F1'—C12—F3' | 103.0 (9) |
| O1—C5—C4 | 133.2 (3) | F3—C12—F2 | 108.5 (5) |
| N1—C5—C4 | 107.9 (3) | F3—C12—F1 | 105.8 (5) |
| C11—C6—C7 | 120.4 (3) | F2—C12—F1 | 104.6 (5) |
| C11—C6—N1 | 120.4 (3) | F2'—C12—C3 | 116.7 (8) |
| C7—C6—N1 | 119.2 (3) | F1'—C12—C3 | 114.9 (7) |
| C8—C7—C6 | 118.9 (3) | F3—C12—C3 | 113.1 (4) |
| C8—C7—H7 | 120.5 | F3'—C12—C3 | 111.6 (7) |
| C6—C7—H7 | 120.5 | F2—C12—C3 | 111.8 (4) |
| C7—C8—C9 | 120.8 (3) | F1—C12—C3 | 112.5 (4) |
| C7—C8—H8 | 119.6 | ||
| C5—N1—N2—C3 | 0.6 (4) | C6—C7—C8—C9 | 0.3 (5) |
| C6—N1—N2—C3 | 178.3 (3) | C7—C8—C9—C10 | 0.0 (6) |
| N1—N2—C3—C4 | −0.6 (4) | C8—C9—C10—C11 | −0.7 (6) |
| N1—N2—C3—C12 | 179.7 (3) | C7—C6—C11—C10 | −0.7 (5) |
| N2—C3—C4—C5 | 0.4 (4) | N1—C6—C11—C10 | −179.4 (3) |
| C12—C3—C4—C5 | 180.0 (4) | C9—C10—C11—C6 | 1.1 (5) |
| N2—N1—C5—O1 | 178.1 (3) | N2—C3—C12—F2' | 84.0 (18) |
| C6—N1—C5—O1 | 0.6 (5) | C4—C3—C12—F2' | −95.6 (18) |
| N2—N1—C5—C4 | −0.4 (4) | N2—C3—C12—F1' | −37.5 (17) |
| C6—N1—C5—C4 | −177.9 (3) | C4—C3—C12—F1' | 143.0 (17) |
| C3—C4—C5—O1 | −178.1 (4) | N2—C3—C12—F3 | −88.1 (7) |
| C3—C4—C5—N1 | 0.0 (4) | C4—C3—C12—F3 | 92.3 (7) |
| C5—N1—C6—C11 | −35.3 (5) | N2—C3—C12—F3' | −154.2 (17) |
| N2—N1—C6—C11 | 147.5 (3) | C4—C3—C12—F3' | 26.3 (18) |
| C5—N1—C6—C7 | 146.0 (3) | N2—C3—C12—F2 | 149.0 (7) |
| N2—N1—C6—C7 | −31.2 (4) | C4—C3—C12—F2 | −30.5 (9) |
| C11—C6—C7—C8 | 0.0 (5) | N2—C3—C12—F1 | 31.7 (7) |
| N1—C6—C7—C8 | 178.7 (3) | C4—C3—C12—F1 | −147.9 (5) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2···O1i | 0.88 | 1.89 | 2.667 (3) | 146 |
Symmetry codes: (i) x, −y+3/2, z−1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BX2225).
References
- Bartulín, J., Belmar, J., Gallardo, H. & León, G. (1994). J. Heterocycl. Chem.31, 561–563.
- Belmar, J., Alderete, J., Zuñiga, C., Jimenez, C., Jimenez, V., Núñez, H., Grandy, R. & Yori, A. (2001). Bol. Soc. Chil. Quim.46, 459–470.
- Belmar, J., Jiménez, C., Ortiz, L., Garland, M. T. & Baggio, R. (2006a). Acta Cryst. C62, o76–o78. [DOI] [PubMed]
- Belmar, J., Jiménez, C., Ruiz-Pérez, C., Delgado, F. S. & Baggio, R. (2006b). Acta Cryst. C62, o599–o601. [DOI] [PubMed]
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
- Enraf–Nonius (1989). CAD-4 Software Enraf–Nonius, Delft, The Netherlands.
- Gallardo, H., Meyer, E., Bortoluzzi, A. J., Molin, F. & Mangrich, A. S. (2004). Inorg. Chim. Acta, 357, 505–512.
- Hyun-Shin, L., Ji-Hyun, S., Moon, K. C., Young-Kwan, K. & Yunkyoung, H. (2008). J. Phys. Chem. Solids, 69, 1305–1309.
- Kees, K. L., Fitzgerald, J. J. Jr, Steiner, K. E., Mattes, J. F., Mihan, B., Tosi, T., Mondoro, D. & McCalebr, M. L. (1996). J. Med. Chem.39, 3920–3928. [DOI] [PubMed]
- Mehlisch, D. R. (1983). Am. J. Med.75, 47–52. [DOI] [PubMed]
- Meyer, E., Zucco, C. & Gallardo, H. (1998). J. Mater. Chem.8, 1351–1354.
- Nakagawa, H., Ohyama, R., Kimata, A., Suzuki, T. & Miyata, N. (2006). Bioorg. Med. Chem. Lett.16, 5939–5942. [DOI] [PubMed]
- Pavlov, P. T., Goleneva, A. F., Lesnov, A. E. & Prokhorova, T. S. (1998). Pharm. Chem. J.32, 370–372.
- Pérez, F. R., Belmar, J., Jiménez, C., Moreno, Y., Hermosilla, P. & Baggio, R. (2005). Acta Cryst. C61, m318–m320. [DOI] [PubMed]
- Schnitzer, T. (2003). Eur. J. Anaesthesiol. Suppl.28, 13–7. [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (1996). HELENA University of Utrecht, The Netherlands.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809029419/bx2225sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809029419/bx2225Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report