Abstract
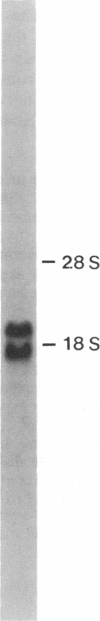
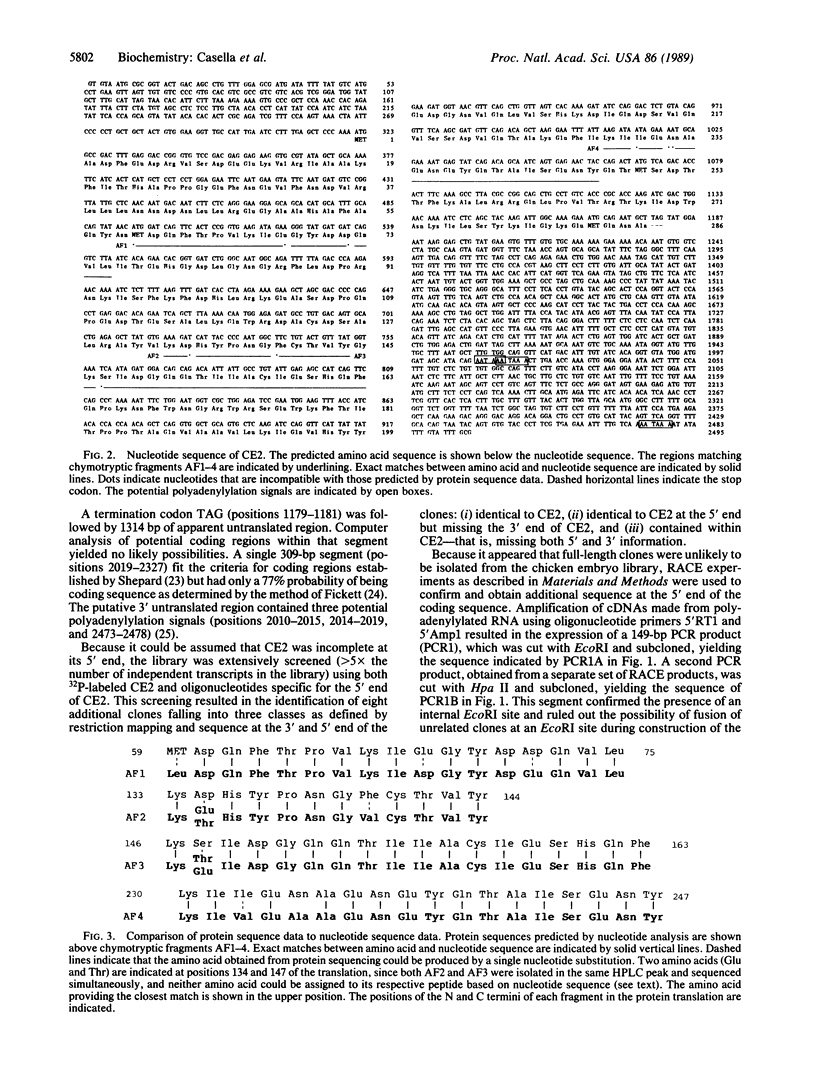
cDNA encoding the alpha chain of Cap Z has been isolated by screening a lambda gt11 library with affinity-purified antibodies. A single cDNA insert (designated CE2) of 2153 base pairs (bp) contains an open reading frame of 836 bp, which is incomplete at its 5' end. The technique of "rapid amplification of cDNA ends" has been used to extend the 5' end of this open reading frame to a potential transcription initiation site that is preceded by 320 bp of an apparently untranslated region. The protein predicted by the resulting nucleotide sequence has a Mr of 32,960 and contains four regions that show close homology with four alpha-chymotryptic digestion fragments of the alpha chain. The amino acid composition of the alpha chain of Cap Z and the predicted protein are also similar. Northern blot analysis of whole chicken embryos shows two mRNA species of 1.9 and 2.4 kilobases, respectively, that hybridize with CE2. Three potential polyadenylylation signals in two regions of CE2 460 bp apart are identified, suggesting that the two messages may result from the use of alternative polyadenylylation sites. Comparison of the sequence data with that of other known actin-capping and severing proteins shows no significant homologies, suggesting that Cap Z may be a member of a unique group of capping, nonsevering proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ampe C., Vandekerckhove J. The F-actin capping proteins of Physarum polycephalum: cap42(a) is very similar, if not identical, to fragmin and is structurally and functionally very homologous to gelsolin; cap42(b) is Physarum actin. EMBO J. 1987 Dec 20;6(13):4149–4157. doi: 10.1002/j.1460-2075.1987.tb02761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André E., Lottspeich F., Schleicher M., Noegel A. Severin, gelsolin, and villin share a homologous sequence in regions presumed to contain F-actin severing domains. J Biol Chem. 1988 Jan 15;263(2):722–727. [PubMed] [Google Scholar]
- Baron M. D., Davison M. D., Jones P., Patel B., Critchley D. R. Isolation and characterization of a cDNA encoding a chick alpha-actinin. J Biol Chem. 1987 Feb 25;262(6):2558–2561. [PubMed] [Google Scholar]
- Bazari W. L., Matsudaira P., Wallek M., Smeal T., Jakes R., Ahmed Y. Villin sequence and peptide map identify six homologous domains. Proc Natl Acad Sci U S A. 1988 Jul;85(14):4986–4990. doi: 10.1073/pnas.85.14.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Casella J. F., Craig S. W., Maack D. J., Brown A. E. Cap Z(36/32), a barbed end actin-capping protein, is a component of the Z-line of skeletal muscle. J Cell Biol. 1987 Jul;105(1):371–379. doi: 10.1083/jcb.105.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella J. F., Maack D. J. Cap Z(36/32) is a contaminant and the major inhibitor of actin network formation in conventional actin preparations. Biochem Biophys Res Commun. 1987 May 29;145(1):625–630. doi: 10.1016/0006-291x(87)91366-0. [DOI] [PubMed] [Google Scholar]
- Casella J. F., Maack D. J., Lin S. Purification and initial characterization of a protein from skeletal muscle that caps the barbed ends of actin filaments. J Biol Chem. 1986 Aug 15;261(23):10915–10921. [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Coutu M. D., Craig S. W. cDNA-derived sequence of chicken embryo vinculin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8535–8539. doi: 10.1073/pnas.85.22.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutu M. D., Simon D. J., Brown A. E., Craig S. W. cDNA cloning and characterization of vinculin mRNA. Biochem Biophys Res Commun. 1987 Sep 15;147(2):637–643. doi: 10.1016/0006-291x(87)90978-8. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fickett J. W. Recognition of protein coding regions in DNA sequences. Nucleic Acids Res. 1982 Sep 11;10(17):5303–5318. doi: 10.1093/nar/10.17.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornwald J. A., Kuncio G., Peng I., Ordahl C. P. The complete nucleotide sequence of the chick a-actin gene and its evolutionary relationship to the actin gene family. Nucleic Acids Res. 1982 Jul 10;10(13):3861–3876. doi: 10.1093/nar/10.13.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Aebi U., Pollard T. D. An actin-binding protein from Acanthamoeba regulates actin filament polymerization and interactions. Nature. 1980 Dec 4;288(5790):455–459. doi: 10.1038/288455a0. [DOI] [PubMed] [Google Scholar]
- Kilimann M. W., Isenberg G. Actin filament capping protein from bovine brain. EMBO J. 1982;1(7):889–894. doi: 10.1002/j.1460-2075.1982.tb01265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski D. J., Stossel T. P., Orkin S. H., Mole J. E., Colten H. R., Yin H. L. Plasma and cytoplasmic gelsolins are encoded by a single gene and contain a duplicated actin-binding domain. Nature. 1986 Oct 2;323(6087):455–458. doi: 10.1038/323455a0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Matsudaira P., Janmey P. Pieces in the actin-severing protein puzzle. Cell. 1988 Jul 15;54(2):139–140. doi: 10.1016/0092-8674(88)90542-9. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Nyström L. E., Lindberg U., Kendrick-Jones J., Jakes R. The amino acid sequence of profilin from calf spleen. FEBS Lett. 1979 May 1;101(1):161–165. doi: 10.1016/0014-5793(79)81317-4. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleicher M., Gerisch G., Isenberg G. New actin-binding proteins from Dictyostelium discoideum. EMBO J. 1984 Sep;3(9):2095–2100. doi: 10.1002/j.1460-2075.1984.tb02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi T., Konishi K., Mabuchi I. Amino acid sequence of starfish oocyte depactin. J Biol Chem. 1988 Mar 5;263(7):3097–3102. [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]