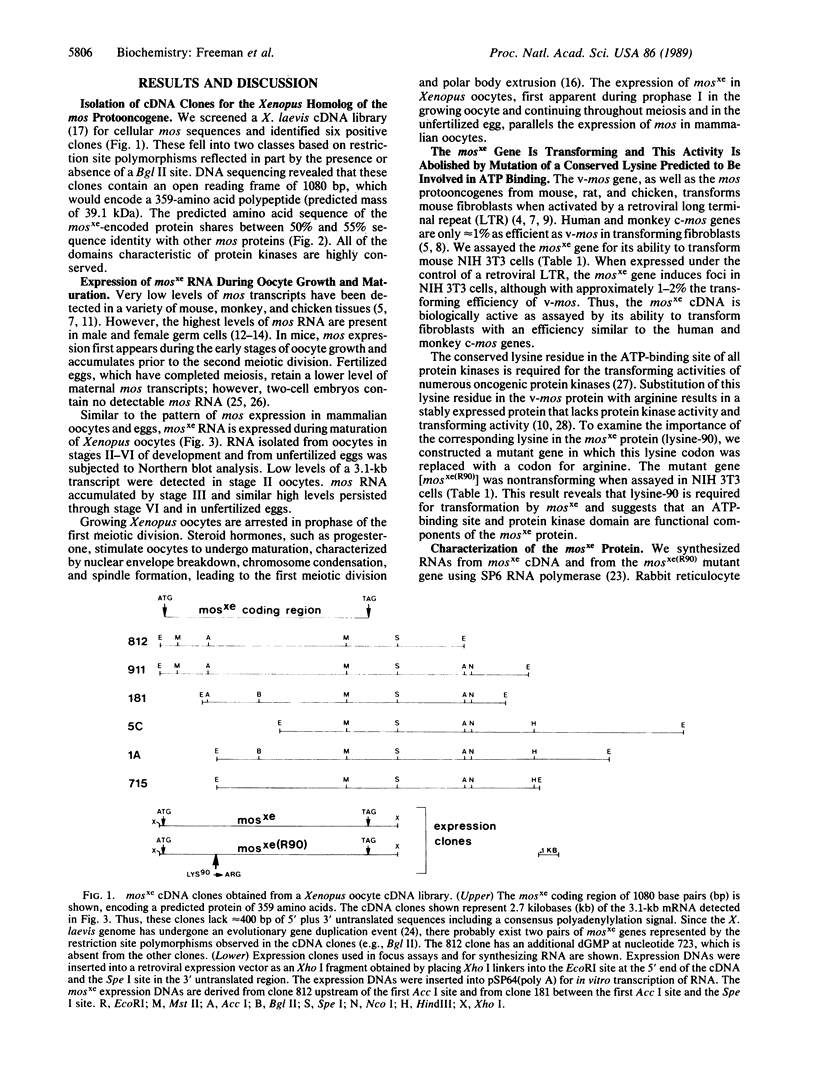
Abstract
The oncogene v-mos transforms mammalian fibroblasts and encodes a serine/threonine protein kinase. Expression of the c-mos protooncogene is most abundant in germ cells, suggesting a normal role for c-mos in meiosis. Here we describe the isolation of cDNA clones containing the complete coding region of the Xenopus laevis homolog of c-mos (mosxe). The mosxe gene is transforming when introduced into murine NIH 3T3 cells, and transformation is abrogated by a lysine-to-arginine mutation in the canonical ATP-binding site. Microinjection of in vitro transcribed mosxe RNA into prophase-arrested Xenopus oocytes causes a resumption of meiosis, leading to germinal vesicle breakdown and oocyte maturation. Oocyte maturation was not observed after microinjection of in vitro transcribed mosxe RNA encoding the lysine-to-arginine mutation. These results demonstrate that the mosxe-encoded protein can induce progression through the cell cycle for both meiotic and mitotic cells and that this property is dependent on the presumptive ATP-binding domain in the protein kinase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allende C. C., Hinrichs M. V., Santos E., Allende J. E. Oncogenic ras protein induces meiotic maturation of amphibian oocytes in the presence of protein synthesis inhibitors. FEBS Lett. 1988 Jul 18;234(2):426–430. doi: 10.1016/0014-5793(88)80130-3. [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Broek D., Wigler M. ras proteins can induce meiosis in Xenopus oocytes. Cell. 1985 Dec;43(3 Pt 2):615–621. doi: 10.1016/0092-8674(85)90233-8. [DOI] [PubMed] [Google Scholar]
- Bisbee C. A., Baker M. A., Wilson A. C., Haji-Azimi I., Fischberg M. Albumin phylogeny for clawed frogs (Xenopus). Science. 1977 Feb 25;195(4280):785–787. doi: 10.1126/science.65013. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Oskarsson M. K., Seth A., Dunn K. J., Dean M., Zweig M., Tainsky M. A., Vande Woude G. F. Analysis of the transforming potential of the human homolog of mos. Cell. 1986 Aug 29;46(5):785–794. doi: 10.1016/0092-8674(86)90354-5. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Oskarsson M., Wood T. G., McClements W. L., Fischinger P. J., Vande Woude G. G. Activation of the transforming potential of a normal cell sequence: a molecular model for oncogenesis. Science. 1981 May 22;212(4497):941–943. doi: 10.1126/science.7233190. [DOI] [PubMed] [Google Scholar]
- Bold R. J., Donoghue D. J. Biologically active mutants with deletions in the v-mos oncogene assayed with retroviral vectors. Mol Cell Biol. 1985 Nov;5(11):3131–3138. doi: 10.1128/mcb.5.11.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande A. K., Kung H. F. Insulin induction of Xenopus laevis oocyte maturation is inhibited by monoclonal antibody against p21 ras proteins. Mol Cell Biol. 1987 Mar;7(3):1285–1288. doi: 10.1128/mcb.7.3.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G., Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988 Jul 1;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Draetta G., Piwnica-Worms H., Morrison D., Druker B., Roberts T., Beach D. Human cdc2 protein kinase is a major cell-cycle regulated tyrosine kinase substrate. Nature. 1988 Dec 22;336(6201):738–744. doi: 10.1038/336738a0. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Brizuela L., Beach D., Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988 Jul 29;54(3):423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Newport J. W. Unraveling of mitotic control mechanisms. Cell. 1988 Dec 23;55(6):925–928. doi: 10.1016/0092-8674(88)90234-6. [DOI] [PubMed] [Google Scholar]
- Gautier J., Norbury C., Lohka M., Nurse P., Maller J. Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+. Cell. 1988 Jul 29;54(3):433–439. doi: 10.1016/0092-8674(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Gerhart J., Wu M., Kirschner M. Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J Cell Biol. 1984 Apr;98(4):1247–1255. doi: 10.1083/jcb.98.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. S., Kiessling A. A., Millette C. F., Cooper G. M. Expression of c-mos RNA in germ cells of male and female mice. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4509–4513. doi: 10.1073/pnas.84.13.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hannink M., Donoghue D. J. Lysine residue 121 in the proposed ATP-binding site of the v-mos protein is required for transformation. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7894–7898. doi: 10.1073/pnas.82.23.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog N. K., Singh B., Elder J., Lipkin I., Trauger R. J., Millette C. F., Goldman D. S., Wolfes H., Cooper G. M., Arlinghaus R. B. Identification of the protein product of the c-mos proto-oncogene in mouse testes. Oncogene. 1988 Aug;3(2):225–229. [PubMed] [Google Scholar]
- Keshet E., Rosenberg M. P., Mercer J. A., Propst F., Vande Woude G. F., Jenkins N. A., Copeland N. G. Developmental regulation of ovarian-specific Mos expression. Oncogene. 1988 Mar;2(3):235–240. [PubMed] [Google Scholar]
- Korn L. J., Siebel C. W., McCormick F., Roth R. A. Ras p21 as a potential mediator of insulin action in Xenopus oocytes. Science. 1987 May 15;236(4803):840–843. doi: 10.1126/science.3554510. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J. L. Regulation of amphibian oocyte maturation. Cell Differ. 1985 Jun;16(4):211–221. doi: 10.1016/0045-6039(85)90570-6. [DOI] [PubMed] [Google Scholar]
- Maxwell S. A., Arlinghaus R. B. Serine kinase activity associated with Maloney murine sarcoma virus-124-encoded p37mos. Virology. 1985 May;143(1):321–333. doi: 10.1016/0042-6822(85)90119-9. [DOI] [PubMed] [Google Scholar]
- Melton D. A. Translation of messenger RNA in injected frog oocytes. Methods Enzymol. 1987;152:288–296. doi: 10.1016/0076-6879(87)52033-x. [DOI] [PubMed] [Google Scholar]
- Mutter G. L., Grills G. S., Wolgemuth D. J. Evidence for the involvement of the proto-oncogene c-mos in mammalian meiotic maturation and possibly very early embryogenesis. EMBO J. 1988 Mar;7(3):683–689. doi: 10.1002/j.1460-2075.1988.tb02863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutter G. L., Wolgemuth D. J. Distinct developmental patterns of c-mos protooncogene expression in female and male mouse germ cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5301–5305. doi: 10.1073/pnas.84.15.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell. 1982 Oct;30(3):675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Nigg E. A., Hunter T. The transforming protein of Moloney murine sarcoma virus is a soluble cytoplasmic protein. Cell. 1983 May;33(1):161–172. doi: 10.1016/0092-8674(83)90345-8. [DOI] [PubMed] [Google Scholar]
- Propst F., Rosenberg M. P., Iyer A., Kaul K., Vande Woude G. F. c-mos proto-oncogene RNA transcripts in mouse tissues: structural features, developmental regulation, and localization in specific cell types. Mol Cell Biol. 1987 May;7(5):1629–1637. doi: 10.1128/mcb.7.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propst F., Vande Woude G. F. Expression of c-mos proto-oncogene transcripts in mouse tissues. Nature. 1985 Jun 6;315(6019):516–518. doi: 10.1038/315516a0. [DOI] [PubMed] [Google Scholar]
- Rebagliati M. R., Weeks D. L., Harvey R. P., Melton D. A. Identification and cloning of localized maternal RNAs from Xenopus eggs. Cell. 1985 Oct;42(3):769–777. doi: 10.1016/0092-8674(85)90273-9. [DOI] [PubMed] [Google Scholar]
- Rijsewijk F., Schuermann M., Wagenaar E., Parren P., Weigel D., Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987 Aug 14;50(4):649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- Russell P., Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987 May 22;49(4):559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Russell P., Nurse P. The mitotic inducer nim1+ functions in a regulatory network of protein kinase homologs controlling the initiation of mitosis. Cell. 1987 May 22;49(4):569–576. doi: 10.1016/0092-8674(87)90459-4. [DOI] [PubMed] [Google Scholar]
- Sagata N., Oskarsson M., Copeland T., Brumbaugh J., Vande Woude G. F. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988 Oct 6;335(6190):519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Oskarsson M. K., Dunn J. K., Blair D. G., Hughes S., Propst F., Vande Woude G. F. Chicken homolog of the mos proto-oncogene. Mol Cell Biol. 1988 Feb;8(2):923–929. doi: 10.1128/mcb.8.2.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanis V., Nurse P. The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell. 1986 Apr 25;45(2):261–268. doi: 10.1016/0092-8674(86)90390-9. [DOI] [PubMed] [Google Scholar]
- Singh B., Hannink M., Donoghue D. J., Arlinghaus R. B. p37mos-associated serine/threonine protein kinase activity correlates with the cellular transformation function of v-mos. J Virol. 1986 Dec;60(3):1148–1152. doi: 10.1128/jvi.60.3.1148-1152.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Wasserman W. J., Richter J. D., Smith L. D. Protein synthesis during maturation promoting factor- and progesterone-induced maturation in Xenopus oocytes. Dev Biol. 1982 Jan;89(1):152–158. doi: 10.1016/0012-1606(82)90303-7. [DOI] [PubMed] [Google Scholar]
- Watson R., Oskarsson M., Vande Woude G. F. Human DNA sequence homologous to the transforming gene (mos) of Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4078–4082. doi: 10.1073/pnas.79.13.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- van der Hoorn F. A., Hulsebos E., Berns A. J., Bloemers H. P. Molecularly cloned c-mos(rat) is biologically active. EMBO J. 1982;1(11):1313–1317. doi: 10.1002/j.1460-2075.1982.tb01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]