Abstract
In the title compound, C14H25NO11·2H2O, the primary hydroxyl group connected to the anomeric C atom of the N-acetyl-β-d-glucopyranose residue exhibits positional disorder, with occupancy factors for the α and β anomers of 0.77 and 0.23, respectively. The two torsion angles (Φ and Ψ) and the bridge angle (τ) that describe conformation of the glycosidic linkage between the galactopyranose and glucopyranose rings are Φ = −81.6 (3)°, Ψ = 118.1 (2)° and τ = 115.2 (2)°. Two water molecules stabilize the molecular packing by forming hydrogen bonds with the saccharide residues.
Related literature
For the synthesis of the title compound, see: Kitaoka et al. (2005 ▶); Nishimoto & Kitaoka (2007a
▶,b
▶). For the conformation of saccharide rings, see: Cremer & Pople (1975 ▶).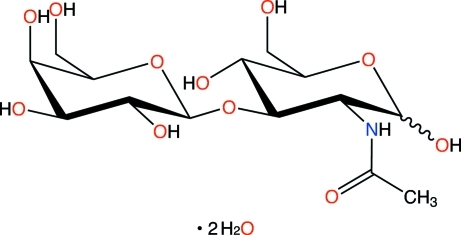
Experimental
Crystal data
C14H25NO11·2H2O
M r = 419.38
Orthorhombic,
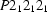
a = 8.284 (1) Å
b = 12.841 (1) Å
c = 17.503 (1) Å
V = 1861.9 (3) Å3
Z = 4
Synchrotron radiation
λ = 0.80000 Å
μ = 0.13 mm−1
T = 95 K
0.10 × 0.10 × 0.10 mm
Data collection
ADSC Quantum 210r diffractometer
Absorption correction: none
25787 measured reflections
2153 independent reflections
2046 reflections with I > 2σ(I)
R int = 0.047
Refinement
R[F 2 > 2σ(F 2)] = 0.042
wR(F 2) = 0.115
S = 1.06
2153 reflections
264 parameters
H-atom parameters constrained
Δρmax = 0.27 e Å−3
Δρmin = −0.31 e Å−3
Data collection: UGUI (Structural Biology Research Center, 2005 ▶); cell refinement: HKL-2000 (Otwinowski & Minor, 1997 ▶); data reduction: HKL-2000; program(s) used to solve structure: SHELXS86 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEPIII (Burnett & Johnson, 1996 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809024775/is2433sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809024775/is2433Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected bond and torsion angles (°).
| C1—O1—C9 | 115.2 (2) |
| O5—C1—O1—C9 | −81.6 (3) |
| C2—C1—O1—C9 | 159.0 (2) |
| C1—O1—C9—C10 | 118.0 (2) |
| C1—O1—C9—C8 | −123.3 (2) |
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1N⋯O2Wi | 0.88 | 2.05 | 2.923 (3) | 169 |
| O2—H2O⋯O12ii | 0.85 | 1.80 | 2.642 (3) | 170 |
| O3—H3O⋯O2W | 0.85 | 1.86 | 2.702 (3) | 169 |
| O4—H4O⋯O13ii | 0.93 | 1.95 | 2.803 (3) | 150 |
| O6—H6O⋯O1Wiii | 0.85 | 1.79 | 2.624 (3) | 168 |
| O10—H10O⋯O6iv | 0.96 | 1.81 | 2.705 (3) | 154 |
| O1W—H11W⋯O10iv | 0.85 | 1.85 | 2.696 (3) | 175 |
| O12—H12O⋯O13v | 0.85 | 1.96 | 2.784 (3) | 162 |
| O1W—H12W⋯O4 | 0.87 | 1.90 | 2.759 (3) | 173 |
| O2W—H21W⋯O11vi | 0.90 | 1.94 | 2.772 (3) | 154 |
| O2W—H22W⋯O6vii | 0.85 | 1.91 | 2.757 (3) | 171 |
| O71—H71O⋯O2i | 0.86 | 1.87 | 2.683 (3) | 159 |
| O72—H72O⋯O1Wv | 0.84 | 2.04 | 2.545 (9) | 119 |
Symmetry codes: (i) 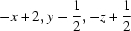 ; (ii)
; (ii) 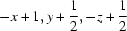 ; (iii)
; (iii) 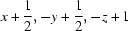 ; (iv)
; (iv) 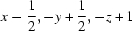 ; (v)
; (v) 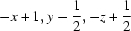 ; (vi)
; (vi) 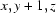 ; (vii)
; (vii) 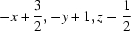 .
.
Acknowledgments
This study was supported in part by a grant from the Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN) of Japan.
supplementary crystallographic information
Comment
It is widely accepted that oligosaccharides other than lactose in human milk (human milk oligosaccharides, HMOs) play a key role in the growth of Bifidobacteria in the gut. Bifidobacteria, Gram-positive anaerobes, are considered to be beneficial for human health. Recently, a unique metabolic pathway specific for lacto-N-biose I (Gal-β1→3GlcNAc, LNB) was found using Bifidobacteria (Kitaoka et al., 2005; Nishimoto & Kitaoka, 2007a). LNB is one of the basic core disaccharides of HMOs and is suggested to be a bifidus factor.
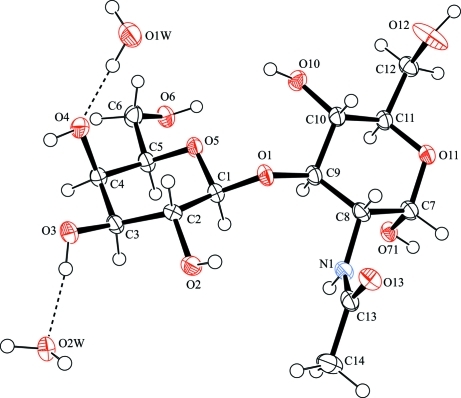
The molecular structure of compound (I) is shown in Fig. 1. There are two water molecules per LNB molecule in the crystal lattice. The primary hydroxyl group connected to the anomeric carbon atom of the GlcNAc residue exhibits disorder, with occupancy factors of O71 (α anomer) and O72 (β anomer) of 0.77 and 0.23, respectively.
The Gal ring is close to the ideal 4C1 chair conformation with ring puckering parameters (Cremer & Pople, 1975) of Q = 0.556 (3) Å, Θ = 7.1 (3)° and Φ = 353 (2)° for the atom sequence O5—C1—C2—C3—C4—C5. The other GlcNAc ring is also close to the ideal chair conformation, with Q = 0.618 (3) Å, Θ = 3.8 (3)°, and Φ = 198 (4)° for the atom sequence O11—C7—C8—C9—C10—C11.
The conformation about the linkage between the Gal and GlcNAc rings is characterized by the torsion angles of Φ (O5—C1—O1—C9) and Ψ (C1—O1—C9—C10), and the bridge angle τ (C1—O1—C9). The values obtained in this study are Φ = -81.6 (3)°, Ψ = 118.1 (2)° and τ = 115.2 (2)° (Table 1).
The conformation of the hydroxymethyl group is defined by two sets of torsion angle: χ and χ'. The values for the Gal ring were χ (O5—C5—C6—O6) = 79.5 (3)° and χ' (C4—C5—C6—O6) = -157.9 (2)°, indicating values close to the gt conformation. The values for the GlcNAc ring are χ (O11—C11—C12—O12) = -64.1 (3)° and χ' (C10—C11—C12—O12) = 57.9 (3)°, indicating the gg conformation.
Both saccharide rings lie approximately parallel to the bc plane and the intermolecular hydrogen bonds were only along the a-axis (Table 2). Two water molecules stabilize the molecular packing by forming hydrogen bonds with sugar molecules in three dimensions.
Experimental
Compound (I) was synthesized from sucrose and GlcNAc by the concurrent action of four enzymes: sucrose phosphorylase, UDP-glucose-hexose-1-phosphate uridylyltransferase, UDP-glucose 4-epimerase, and lacto-N-biose phosphorylase (Nishimoto & Kitaoka, 2007b). Single crystals suitable for X-ray analysis were obtained by slow diffusion of ethanol into an aqueous solution.
Refinement
The anomalous scattering signal of (I) is too weak to predict the accurate absolute structure. Therefore, the merging of Friedel pair data was performed before the final refinement. The hydroxyl H atoms in the saccharides and water molecules, except for H72O, were located in a difference Fourier map. The H72O atom was positioned using the HFIX 83 instruction in the SHELXL97 software package, with O—H = 0.84 Å. These hydroxyl H atoms were subsequently refined as a riding model, with Uiso(H) = 1.2Ueq(O). The methine, methylene, methyl and amide H atoms were positioned using the HFIX 13, HFIX 23, HFIX 137 and HFIX 43 instructions, with C—H = 1.00, 0.99, 0.98 and 0.88 Å, respectively. These C- and N-bound H atoms were also refined as a riding model, with Uiso(H) = 1.2Ueq(C) for the methine, methylene and amide H atoms, and Uiso(H) = 1.5Ueq(C) for methyl H atoms.
Figures
Fig. 1.
Displacement ellipsoid plot and atomic numbering scheme of compound (I). The ellipsoids are drawn at the 50% probability level, and the H atoms are shown as small spheres with arbitrary radii. Broken lines indicate hydrogen bonds. The minor conformer of the disordered part has been omitted for clarity.
Crystal data
| C14H25NO11·2H2O | F(000) = 896 |
| Mr = 419.38 | Dx = 1.496 Mg m−3 |
| Orthorhombic, P212121 | Synchrotron radiation, λ = 0.80000 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 25787 reflections |
| a = 8.284 (1) Å | θ = 2.2–30.0° |
| b = 12.841 (1) Å | µ = 0.13 mm−1 |
| c = 17.503 (1) Å | T = 95 K |
| V = 1861.9 (3) Å3 | Block, colorless |
| Z = 4 | 0.10 × 0.10 × 0.10 mm |
Data collection
| ADSC Quantum 210r diffractometer | 2046 reflections with I > 2σ(I) |
| Radiation source: Photon Facrory NW12A | Rint = 0.047 |
| silicon | θmax = 30.0°, θmin = 2.2° |
| Detector resolution: 9.7466 pixels mm-1 | h = −10→10 |
| ω scans | k = −16→16 |
| 25787 measured reflections | l = −21→21 |
| 2153 independent reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: difmap&geom |
| R[F2 > 2σ(F2)] = 0.042 | H-atom parameters constrained |
| wR(F2) = 0.115 | w = 1/[σ2(Fo2) + (0.0806P)2 + 0.7215P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.06 | (Δ/σ)max = 0.001 |
| 2153 reflections | Δρmax = 0.27 e Å−3 |
| 264 parameters | Δρmin = −0.31 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.084 (6) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| C1 | 0.7021 (3) | 0.32502 (19) | 0.34879 (14) | 0.0205 (5) | |
| H1 | 0.8227 | 0.3214 | 0.3471 | 0.025* | |
| C2 | 0.6435 (3) | 0.4253 (2) | 0.31127 (15) | 0.0211 (5) | |
| H2 | 0.5234 | 0.4232 | 0.3059 | 0.025* | |
| C3 | 0.6921 (3) | 0.52070 (19) | 0.35853 (15) | 0.0215 (5) | |
| H3 | 0.8113 | 0.5310 | 0.3535 | 0.026* | |
| C4 | 0.6518 (3) | 0.5082 (2) | 0.44263 (16) | 0.0226 (6) | |
| H4 | 0.7071 | 0.5646 | 0.4721 | 0.027* | |
| C5 | 0.7119 (4) | 0.4031 (2) | 0.47122 (15) | 0.0235 (6) | |
| H5 | 0.8323 | 0.4017 | 0.4671 | 0.028* | |
| C6 | 0.6658 (4) | 0.3832 (2) | 0.55375 (16) | 0.0279 (6) | |
| H61 | 0.6722 | 0.4494 | 0.5826 | 0.033* | |
| H62 | 0.5526 | 0.3585 | 0.5559 | 0.033* | |
| O1 | 0.6358 (2) | 0.24111 (13) | 0.30922 (11) | 0.0217 (4) | |
| O2 | 0.7150 (2) | 0.43673 (14) | 0.23805 (11) | 0.0241 (4) | |
| H2O | 0.6648 | 0.4055 | 0.2024 | 0.029* | |
| O3 | 0.6135 (3) | 0.61167 (14) | 0.33229 (11) | 0.0261 (5) | |
| H3O | 0.6709 | 0.6420 | 0.2988 | 0.031* | |
| O4 | 0.4818 (3) | 0.51573 (15) | 0.45519 (11) | 0.0268 (5) | |
| H4O | 0.4508 | 0.5788 | 0.4331 | 0.032* | |
| O5 | 0.6475 (2) | 0.31996 (13) | 0.42597 (10) | 0.0219 (4) | |
| O6 | 0.7678 (3) | 0.30802 (14) | 0.58916 (11) | 0.0266 (5) | |
| H6O | 0.7491 | 0.2462 | 0.5742 | 0.032* | |
| C7 | 0.8597 (3) | −0.00264 (19) | 0.25298 (15) | 0.0220 (5) | |
| H71 | 0.8919 | −0.0327 | 0.2025 | 0.026* | 0.77 |
| H72 | 0.9533 | 0.0120 | 0.2874 | 0.026* | 0.23 |
| C8 | 0.7699 (3) | 0.10082 (19) | 0.24040 (15) | 0.0213 (5) | |
| H8 | 0.6707 | 0.0870 | 0.2094 | 0.026* | |
| C9 | 0.7191 (3) | 0.14403 (19) | 0.31837 (15) | 0.0206 (5) | |
| H9 | 0.8171 | 0.1549 | 0.3508 | 0.025* | |
| C10 | 0.6100 (3) | 0.06275 (19) | 0.35568 (15) | 0.0213 (5) | |
| H10 | 0.5151 | 0.0491 | 0.3218 | 0.026* | |
| C11 | 0.7058 (3) | −0.03748 (19) | 0.36589 (15) | 0.0218 (5) | |
| H11 | 0.8047 | −0.0211 | 0.3964 | 0.026* | |
| C12 | 0.6157 (4) | −0.1242 (2) | 0.40603 (16) | 0.0248 (6) | |
| H121 | 0.6855 | −0.1866 | 0.4092 | 0.030* | |
| H122 | 0.5888 | −0.1022 | 0.4587 | 0.030* | |
| O71 | 0.9959 (3) | 0.01943 (18) | 0.29538 (14) | 0.0217 (5) | 0.77 |
| H71O | 1.0752 | −0.0218 | 0.2860 | 0.026* | 0.77 |
| O72 | 0.9226 (12) | −0.0517 (7) | 0.1856 (5) | 0.031 (2) | 0.23 |
| H72O | 0.8861 | −0.0216 | 0.1467 | 0.037* | 0.23 |
| O10 | 0.5548 (3) | 0.09459 (15) | 0.42885 (11) | 0.0251 (5) | |
| H10O | 0.4729 | 0.1468 | 0.4241 | 0.030* | |
| O11 | 0.7561 (2) | −0.07395 (14) | 0.29198 (11) | 0.0229 (4) | |
| O12 | 0.4722 (3) | −0.1495 (2) | 0.36648 (17) | 0.0485 (7) | |
| H12O | 0.4325 | −0.2091 | 0.3760 | 0.058* | |
| N1 | 0.8734 (3) | 0.17181 (16) | 0.19831 (13) | 0.0214 (5) | |
| H1N | 0.9621 | 0.1942 | 0.2205 | 0.026* | |
| C13 | 0.8410 (3) | 0.20474 (19) | 0.12798 (16) | 0.0220 (6) | |
| O13 | 0.7167 (3) | 0.18034 (14) | 0.09211 (11) | 0.0258 (4) | |
| C14 | 0.9628 (4) | 0.2771 (2) | 0.09275 (17) | 0.0312 (7) | |
| H141 | 1.0519 | 0.2884 | 0.1287 | 0.047* | |
| H142 | 0.9112 | 0.3438 | 0.0810 | 0.047* | |
| H143 | 1.0048 | 0.2461 | 0.0456 | 0.047* | |
| O1W | 0.2143 (3) | 0.39008 (16) | 0.43738 (13) | 0.0370 (6) | |
| H11W | 0.1688 | 0.3974 | 0.4806 | 0.044* | |
| H12W | 0.2950 | 0.4330 | 0.4398 | 0.044* | |
| O2W | 0.8087 (2) | 0.72473 (14) | 0.24058 (11) | 0.0247 (4) | |
| H21W | 0.7630 | 0.7867 | 0.2498 | 0.030* | |
| H22W | 0.7901 | 0.7083 | 0.1942 | 0.030* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0228 (13) | 0.0155 (11) | 0.0234 (12) | −0.0008 (11) | 0.0008 (11) | −0.0003 (9) |
| C2 | 0.0226 (13) | 0.0174 (12) | 0.0234 (12) | −0.0030 (10) | 0.0052 (11) | 0.0017 (10) |
| C3 | 0.0216 (12) | 0.0146 (11) | 0.0284 (13) | −0.0008 (10) | 0.0015 (11) | 0.0010 (10) |
| C4 | 0.0235 (13) | 0.0161 (11) | 0.0282 (13) | 0.0002 (11) | 0.0020 (11) | 0.0000 (10) |
| C5 | 0.0270 (13) | 0.0168 (11) | 0.0266 (13) | −0.0004 (11) | −0.0002 (11) | 0.0000 (10) |
| C6 | 0.0334 (16) | 0.0232 (13) | 0.0271 (14) | 0.0061 (12) | 0.0018 (12) | 0.0011 (11) |
| O1 | 0.0240 (10) | 0.0130 (8) | 0.0280 (9) | 0.0006 (8) | −0.0020 (8) | −0.0001 (7) |
| O2 | 0.0269 (10) | 0.0221 (9) | 0.0233 (9) | −0.0041 (8) | 0.0020 (8) | 0.0008 (7) |
| O3 | 0.0302 (11) | 0.0151 (8) | 0.0329 (10) | 0.0033 (8) | 0.0029 (9) | 0.0043 (7) |
| O4 | 0.0272 (10) | 0.0196 (9) | 0.0336 (10) | 0.0058 (8) | 0.0065 (9) | 0.0026 (8) |
| O5 | 0.0286 (10) | 0.0147 (8) | 0.0225 (9) | 0.0002 (8) | 0.0013 (8) | −0.0005 (7) |
| O6 | 0.0311 (11) | 0.0193 (9) | 0.0293 (10) | 0.0021 (8) | −0.0036 (8) | 0.0022 (8) |
| C7 | 0.0244 (13) | 0.0165 (11) | 0.0251 (12) | −0.0016 (11) | 0.0001 (11) | 0.0006 (10) |
| C8 | 0.0255 (13) | 0.0146 (11) | 0.0237 (12) | −0.0025 (11) | 0.0002 (11) | 0.0029 (10) |
| C9 | 0.0212 (12) | 0.0138 (11) | 0.0268 (12) | 0.0010 (11) | −0.0009 (11) | 0.0000 (10) |
| C10 | 0.0254 (14) | 0.0160 (11) | 0.0225 (12) | 0.0003 (11) | 0.0018 (11) | −0.0005 (10) |
| C11 | 0.0252 (13) | 0.0162 (11) | 0.0241 (12) | 0.0004 (11) | 0.0004 (11) | −0.0004 (10) |
| C12 | 0.0257 (14) | 0.0182 (12) | 0.0304 (13) | 0.0001 (11) | −0.0022 (12) | 0.0039 (11) |
| O71 | 0.0196 (11) | 0.0169 (11) | 0.0284 (12) | 0.0029 (10) | −0.0022 (10) | −0.0015 (9) |
| O72 | 0.039 (5) | 0.024 (4) | 0.028 (4) | 0.008 (4) | 0.011 (4) | 0.001 (4) |
| O10 | 0.0307 (11) | 0.0200 (9) | 0.0246 (9) | 0.0042 (8) | 0.0048 (8) | 0.0018 (8) |
| O11 | 0.0289 (10) | 0.0153 (8) | 0.0246 (9) | −0.0021 (8) | 0.0027 (8) | −0.0012 (7) |
| O12 | 0.0368 (13) | 0.0384 (12) | 0.0703 (17) | −0.0204 (11) | −0.0240 (13) | 0.0300 (12) |
| N1 | 0.0208 (10) | 0.0168 (10) | 0.0268 (11) | −0.0020 (9) | 0.0010 (9) | 0.0007 (9) |
| C13 | 0.0235 (13) | 0.0148 (11) | 0.0278 (12) | −0.0005 (10) | −0.0005 (11) | 0.0002 (10) |
| O13 | 0.0256 (10) | 0.0232 (9) | 0.0286 (10) | −0.0046 (8) | −0.0015 (8) | 0.0015 (8) |
| C14 | 0.0330 (16) | 0.0308 (14) | 0.0298 (14) | −0.0107 (13) | 0.0024 (12) | 0.0058 (12) |
| O1W | 0.0484 (14) | 0.0261 (10) | 0.0365 (12) | −0.0066 (11) | 0.0090 (11) | −0.0014 (9) |
| O2W | 0.0292 (10) | 0.0169 (8) | 0.0280 (9) | 0.0031 (8) | −0.0015 (9) | −0.0020 (7) |
Geometric parameters (Å, °)
| C1—O1 | 1.394 (3) | C8—N1 | 1.452 (3) |
| C1—O5 | 1.426 (3) | C8—C9 | 1.532 (4) |
| C1—C2 | 1.525 (3) | C8—H8 | 1.0000 |
| C1—H1 | 1.0000 | C9—C10 | 1.528 (4) |
| C2—O2 | 1.419 (3) | C9—H9 | 1.0000 |
| C2—C3 | 1.532 (3) | C10—O10 | 1.420 (3) |
| C2—H2 | 1.0000 | C10—C11 | 1.523 (3) |
| C3—O3 | 1.414 (3) | C10—H10 | 1.0000 |
| C3—C4 | 1.518 (4) | C11—O11 | 1.437 (3) |
| C3—H3 | 1.0000 | C11—C12 | 1.514 (4) |
| C4—O4 | 1.429 (3) | C11—H11 | 1.0000 |
| C4—C5 | 1.523 (3) | C12—O12 | 1.413 (4) |
| C4—H4 | 1.0000 | C12—H121 | 0.9900 |
| C5—O5 | 1.432 (3) | C12—H122 | 0.9900 |
| C5—C6 | 1.516 (4) | O71—H71O | 0.8594 |
| C5—H5 | 1.0000 | O72—H72O | 0.8400 |
| C6—O6 | 1.425 (3) | O10—H10O | 0.9571 |
| C6—H61 | 0.9900 | O12—H12O | 0.8497 |
| C6—H62 | 0.9900 | N1—C13 | 1.329 (4) |
| O1—C9 | 1.434 (3) | N1—H1N | 0.8800 |
| O2—H2O | 0.8500 | C13—O13 | 1.246 (4) |
| O3—H3O | 0.8500 | C13—C14 | 1.504 (4) |
| O4—H4O | 0.9338 | C14—H141 | 0.9800 |
| O6—H6O | 0.8499 | C14—H142 | 0.9800 |
| C7—O71 | 1.380 (4) | C14—H143 | 0.9800 |
| C7—O11 | 1.428 (3) | O1W—H11W | 0.8500 |
| C7—O72 | 1.435 (9) | O1W—H12W | 0.8676 |
| C7—C8 | 1.538 (3) | O2W—H21W | 0.8963 |
| C7—H71 | 1.0000 | O2W—H22W | 0.8523 |
| C7—H72 | 1.0000 | ||
| O1—C1—O5 | 108.1 (2) | O72—C7—H72 | 107.2 |
| O1—C1—C2 | 108.3 (2) | C8—C7—H72 | 107.4 |
| O5—C1—C2 | 110.2 (2) | N1—C8—C9 | 112.7 (2) |
| O1—C1—H1 | 110.1 | N1—C8—C7 | 109.2 (2) |
| O5—C1—H1 | 110.1 | C9—C8—C7 | 108.5 (2) |
| C2—C1—H1 | 110.1 | N1—C8—H8 | 108.8 |
| O2—C2—C1 | 110.1 (2) | C9—C8—H8 | 108.8 |
| O2—C2—C3 | 107.2 (2) | C7—C8—H8 | 108.8 |
| C1—C2—C3 | 111.0 (2) | O1—C9—C10 | 110.9 (2) |
| O2—C2—H2 | 109.5 | O1—C9—C8 | 110.3 (2) |
| C1—C2—H2 | 109.5 | C10—C9—C8 | 107.2 (2) |
| C3—C2—H2 | 109.5 | O1—C9—H9 | 109.5 |
| O3—C3—C4 | 107.5 (2) | C10—C9—H9 | 109.5 |
| O3—C3—C2 | 111.3 (2) | C8—C9—H9 | 109.5 |
| C4—C3—C2 | 112.4 (2) | O10—C10—C11 | 107.8 (2) |
| O3—C3—H3 | 108.5 | O10—C10—C9 | 112.3 (2) |
| C4—C3—H3 | 108.5 | C11—C10—C9 | 108.6 (2) |
| C2—C3—H3 | 108.5 | O10—C10—H10 | 109.4 |
| O4—C4—C3 | 111.0 (2) | C11—C10—H10 | 109.4 |
| O4—C4—C5 | 109.4 (2) | C9—C10—H10 | 109.4 |
| C3—C4—C5 | 109.9 (2) | O11—C11—C12 | 108.7 (2) |
| O4—C4—H4 | 108.8 | O11—C11—C10 | 108.7 (2) |
| C3—C4—H4 | 108.8 | C12—C11—C10 | 114.8 (2) |
| C5—C4—H4 | 108.8 | O11—C11—H11 | 108.2 |
| O5—C5—C6 | 107.9 (2) | C12—C11—H11 | 108.2 |
| O5—C5—C4 | 110.9 (2) | C10—C11—H11 | 108.2 |
| C6—C5—C4 | 112.3 (2) | O12—C12—C11 | 110.9 (2) |
| O5—C5—H5 | 108.5 | O12—C12—H121 | 109.5 |
| C6—C5—H5 | 108.5 | C11—C12—H121 | 109.5 |
| C4—C5—H5 | 108.5 | O12—C12—H122 | 109.5 |
| O6—C6—C5 | 112.3 (2) | C11—C12—H122 | 109.5 |
| O6—C6—H61 | 109.2 | H121—C12—H122 | 108.0 |
| C5—C6—H61 | 109.2 | C7—O71—H71O | 113.3 |
| O6—C6—H62 | 109.2 | C7—O72—H72O | 109.5 |
| C5—C6—H62 | 109.2 | C10—O10—H10O | 110.6 |
| H61—C6—H62 | 107.9 | C7—O11—C11 | 113.28 (19) |
| C1—O1—C9 | 115.2 (2) | C12—O12—H12O | 115.9 |
| C2—O2—H2O | 114.2 | C13—N1—C8 | 123.4 (2) |
| C3—O3—H3O | 110.2 | C13—N1—H1N | 118.3 |
| C4—O4—H4O | 105.5 | C8—N1—H1N | 118.3 |
| C1—O5—C5 | 111.8 (2) | O13—C13—N1 | 123.6 (3) |
| C6—O6—H6O | 113.0 | O13—C13—C14 | 120.2 (2) |
| O71—C7—O11 | 111.5 (2) | N1—C13—C14 | 116.2 (2) |
| O11—C7—O72 | 109.2 (4) | C13—C14—H141 | 109.5 |
| O71—C7—C8 | 107.1 (2) | C13—C14—H142 | 109.5 |
| O11—C7—C8 | 109.4 (2) | H141—C14—H142 | 109.5 |
| O72—C7—C8 | 115.9 (4) | C13—C14—H143 | 109.5 |
| O71—C7—H71 | 109.6 | H141—C14—H143 | 109.5 |
| O11—C7—H71 | 109.6 | H142—C14—H143 | 109.5 |
| C8—C7—H71 | 109.6 | H11W—O1W—H12W | 103.1 |
| O11—C7—H72 | 107.4 | H21W—O2W—H22W | 108.4 |
| O1—C1—C2—O2 | −69.5 (3) | O11—C7—C8—C9 | 58.8 (3) |
| O5—C1—C2—O2 | 172.4 (2) | O72—C7—C8—C9 | −177.2 (5) |
| O1—C1—C2—C3 | 171.9 (2) | C1—O1—C9—C10 | 118.0 (2) |
| O5—C1—C2—C3 | 53.9 (3) | C1—O1—C9—C8 | −123.3 (2) |
| O2—C2—C3—O3 | 70.7 (3) | N1—C8—C9—O1 | 58.5 (3) |
| C1—C2—C3—O3 | −169.0 (2) | C7—C8—C9—O1 | 179.6 (2) |
| O2—C2—C3—C4 | −168.6 (2) | N1—C8—C9—C10 | 179.3 (2) |
| C1—C2—C3—C4 | −48.3 (3) | C7—C8—C9—C10 | −59.5 (3) |
| O3—C3—C4—O4 | 50.3 (3) | O1—C9—C10—O10 | −59.4 (3) |
| C2—C3—C4—O4 | −72.6 (3) | C8—C9—C10—O10 | −179.9 (2) |
| O3—C3—C4—C5 | 171.5 (2) | O1—C9—C10—C11 | −178.5 (2) |
| C2—C3—C4—C5 | 48.6 (3) | C8—C9—C10—C11 | 61.0 (3) |
| O4—C4—C5—O5 | 66.6 (3) | O10—C10—C11—O11 | 177.2 (2) |
| C3—C4—C5—O5 | −55.5 (3) | C9—C10—C11—O11 | −60.9 (3) |
| O4—C4—C5—C6 | −54.2 (3) | O10—C10—C11—C12 | 55.2 (3) |
| C3—C4—C5—C6 | −176.4 (2) | C9—C10—C11—C12 | 177.1 (2) |
| O5—C5—C6—O6 | 79.5 (3) | O11—C11—C12—O12 | −64.1 (3) |
| C4—C5—C6—O6 | −157.9 (2) | C10—C11—C12—O12 | 57.9 (3) |
| O5—C1—O1—C9 | −81.6 (3) | O71—C7—O11—C11 | 57.6 (3) |
| C2—C1—O1—C9 | 159.0 (2) | O72—C7—O11—C11 | 171.4 (5) |
| O1—C1—O5—C5 | 179.3 (2) | C8—C7—O11—C11 | −60.7 (3) |
| C2—C1—O5—C5 | −62.6 (3) | C12—C11—O11—C7 | −172.7 (2) |
| C6—C5—O5—C1 | −172.6 (2) | C10—C11—O11—C7 | 61.8 (3) |
| C4—C5—O5—C1 | 64.0 (3) | C9—C8—N1—C13 | −125.0 (3) |
| O71—C7—C8—N1 | 61.1 (3) | C7—C8—N1—C13 | 114.2 (3) |
| O11—C7—C8—N1 | −178.0 (2) | C8—N1—C13—O13 | 2.1 (4) |
| O72—C7—C8—N1 | −53.9 (5) | C8—N1—C13—C14 | −179.2 (2) |
| O71—C7—C8—C9 | −62.2 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1N···O2Wi | 0.88 | 2.05 | 2.923 (3) | 169 |
| O2—H2O···O12ii | 0.85 | 1.80 | 2.642 (3) | 170 |
| O3—H3O···O2W | 0.85 | 1.86 | 2.702 (3) | 169 |
| O4—H4O···O13ii | 0.93 | 1.95 | 2.803 (3) | 150 |
| O6—H6O···O1Wiii | 0.85 | 1.79 | 2.624 (3) | 168 |
| O10—H10O···O6iv | 0.96 | 1.81 | 2.705 (3) | 154 |
| O1W—H11W···O10iv | 0.85 | 1.85 | 2.696 (3) | 175 |
| O12—H12O···O13v | 0.85 | 1.96 | 2.784 (3) | 162 |
| O1W—H12W···O4 | 0.87 | 1.90 | 2.759 (3) | 173 |
| O2W—H21W···O11vi | 0.90 | 1.94 | 2.772 (3) | 154 |
| O2W—H22W···O6vii | 0.85 | 1.91 | 2.757 (3) | 171 |
| O71—H71O···O2i | 0.86 | 1.87 | 2.683 (3) | 159 |
| O72—H72O···O1Wv | 0.84 | 2.04 | 2.545 (9) | 119 |
Symmetry codes: (i) −x+2, y−1/2, −z+1/2; (ii) −x+1, y+1/2, −z+1/2; (iii) x+1/2, −y+1/2, −z+1; (iv) x−1/2, −y+1/2, −z+1; (v) −x+1, y−1/2, −z+1/2; (vi) x, y+1, z; (vii) −x+3/2, −y+1, z−1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IS2433).
References
- Burnett, M. N. & Johnson, C. K. (1996). ORTEPIII Report ORNL-6895. Oak Ridge National Laboratory. Tennessee, USA.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc.97, 1354–1358.
- Kitaoka, M., Tian, J. & Nishimoto, M. (2005). Appl. Environ. Microbiol.71, 3158–3162. [DOI] [PMC free article] [PubMed]
- Nishimoto, M. & Kitaoka, M. (2007a). Appl. Environ. Microbiol.73, 6444–6449. [DOI] [PMC free article] [PubMed]
- Nishimoto, M. & Kitaoka, M. (2007b). Biosci. Biotechnol. Biochem.71, 2101–2104. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp.307–326. New York: Academic Press.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Structural Biology Research Center (2005). Unified Graphical User Interface (UGUI) Structural Biology Research Center, Photon Factory, High Energy Accelerator Research Organization, Tsukuba, Ibaraki, Japan.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809024775/is2433sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809024775/is2433Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report