Abstract
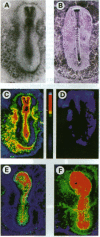
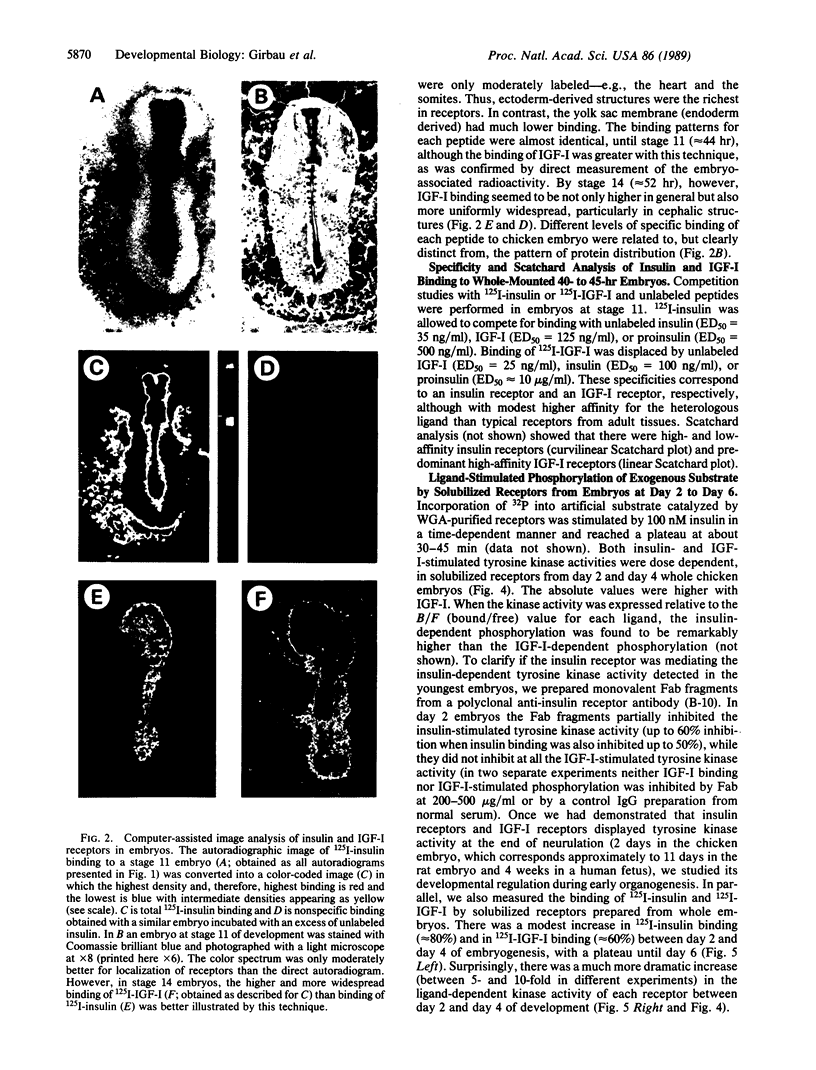
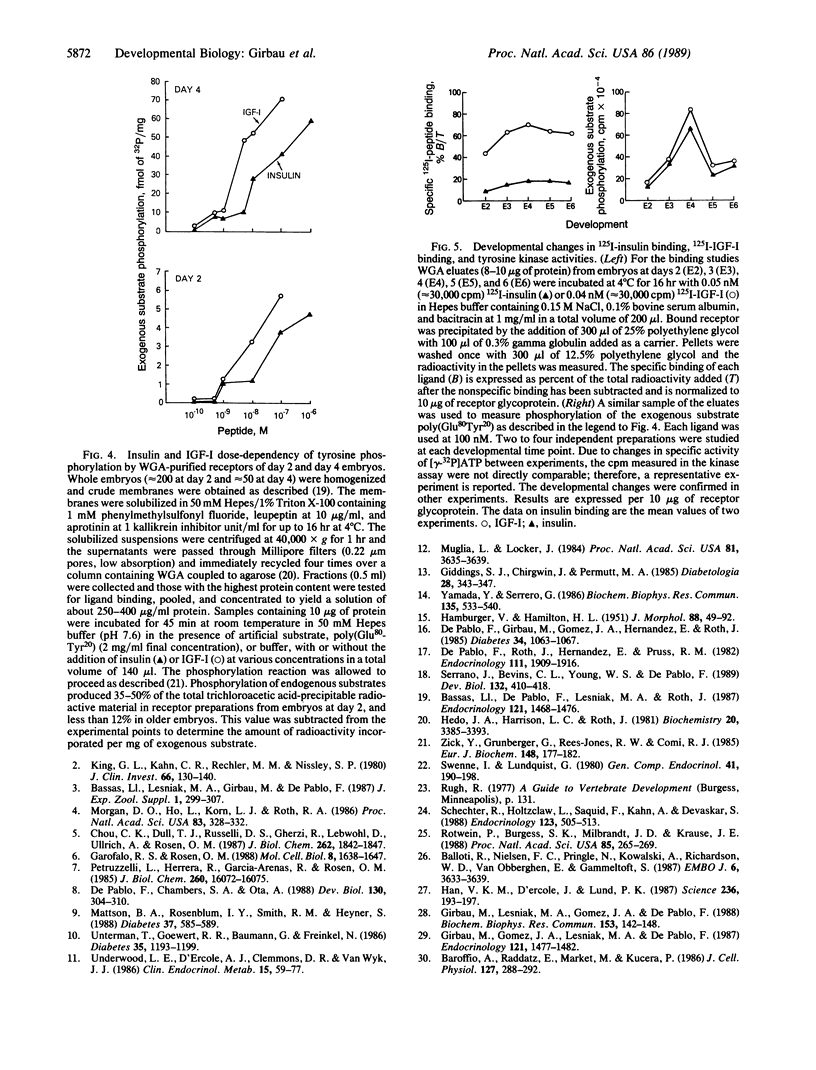
We previously reported specific cross-linking of 125I-labeled insulin and 125I-labeled insulin-like growth factor I (IGF-I) to the alpha subunit of their respective receptors in chicken embryos of 20 somites and older. To achieve adequate sensitivity and localize spatially the receptors in younger embryos, we adapted an autoradiographic technique using whole-mounted chicken blastoderms. Insulin receptors and IGF-I receptors were expressed and could be localized as early as gastrulation, before the first somite is formed. Relative density was analyzed by a computer-assisted image system, revealing overall slightly higher binding of IGF-I than of insulin. Structures rich in both types of receptors were predominantly of ectodermal origin: Hensen's node in gastrulating embryos and neural folds, neural tube and optic vesicles during neurulation. The signal transduction capability of the receptors in early organogenesis was assessed by their ability to phosphorylate the exogenous substrate poly(Glu80Tyr20). Ligand-dependent tyrosine phosphorylation was demonstrable with both insulin and IGF-I in glycoprotein-enriched preparations from embryos at days 2 through 6 of embryogenesis. There was a developmentally regulated change in ligand-dependent tyrosine kinase activity, with a sharp increase from day 2 to day 4, in contrast with a small increase in the ligand binding. Binding of 125I-labeled IGF-I was, with the solubilized receptors, severalfold higher than binding of 125I-labeled insulin. However, the insulin-dependent phosphorylation was as high as the IGF-I-dependent phosphorylation at each developmental stage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballotti R., Nielsen F. C., Pringle N., Kowalski A., Richardson W. D., Van Obberghen E., Gammeltoft S. Insulin-like growth factor I in cultured rat astrocytes: expression of the gene, and receptor tyrosine kinase. EMBO J. 1987 Dec 1;6(12):3633–3639. doi: 10.1002/j.1460-2075.1987.tb02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroffio A., Raddatz E., Markert M., Kucera P. Transient stimulation of glucose metabolism by insulin in the 1-day chick embryo. J Cell Physiol. 1986 May;127(2):288–292. doi: 10.1002/jcp.1041270215. [DOI] [PubMed] [Google Scholar]
- Bassas L., Lesniak M. A., Girbau M., de Pablo F. Insulin-related receptors in the early chick embryo: from tissue patterns to possible function. J Exp Zool Suppl. 1987;1:299–307. [PubMed] [Google Scholar]
- Bassas L., de Pablo F., Lesniak M. A., Roth J. The insulin receptors of chick embryo show tissue-specific structural differences which parallel those of the insulin-like growth factor I receptors. Endocrinology. 1987 Oct;121(4):1468–1476. doi: 10.1210/endo-121-4-1468. [DOI] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- De Pablo F., Roth J., Hernandez E., Pruss R. M. Insulin is present in chicken eggs and early chick embryos. Endocrinology. 1982 Dec;111(6):1909–1916. doi: 10.1210/endo-111-6-1909. [DOI] [PubMed] [Google Scholar]
- Garofalo R. S., Rosen O. M. Tissue localization of Drosophila melanogaster insulin receptor transcripts during development. Mol Cell Biol. 1988 Apr;8(4):1638–1647. doi: 10.1128/mcb.8.4.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings S. J., Chirgwin J., Permutt M. A. Evaluation of rat insulin messenger RNA in pancreatic and extrapancreatic tissues. Diabetologia. 1985 Jun;28(6):343–347. doi: 10.1007/BF00283141. [DOI] [PubMed] [Google Scholar]
- Girbau M., Gomez J. A., Lesniak M. A., de Pablo F. Insulin and insulin-like growth factor I both stimulate metabolism, growth, and differentiation in the postneurula chick embryo. Endocrinology. 1987 Oct;121(4):1477–1482. doi: 10.1210/endo-121-4-1477. [DOI] [PubMed] [Google Scholar]
- Girbau M., Lesniak M. A., Gomez J. A., De Pablo F. Insulin action in early embryonic life: anti-insulin receptor antibodies retard chicken embryo growth but not muscle differentiation in vivo. Biochem Biophys Res Commun. 1988 May 31;153(1):142–148. doi: 10.1016/s0006-291x(88)81200-2. [DOI] [PubMed] [Google Scholar]
- Han V. K., D'Ercole A. J., Lund P. K. Cellular localization of somatomedin (insulin-like growth factor) messenger RNA in the human fetus. Science. 1987 Apr 10;236(4798):193–197. doi: 10.1126/science.3563497. [DOI] [PubMed] [Google Scholar]
- Hedo J. A., Harrison L. C., Roth J. Binding of insulin receptors to lectins: evidence for common carbohydrate determinants on several membrane receptors. Biochemistry. 1981 Jun 9;20(12):3385–3393. doi: 10.1021/bi00515a013. [DOI] [PubMed] [Google Scholar]
- King G. L., Kahn C. R., Rechler M. M., Nissley S. P. Direct demonstration of separate receptors for growth and metabolic activities of insulin and multiplication-stimulating activity (an insulinlike growth factor) using antibodies to the insulin receptor. J Clin Invest. 1980 Jul;66(1):130–140. doi: 10.1172/JCI109826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Blinderman L. A., Czech M. P. The high affinity insulin receptor mediates growth stimulation in rat hepatoma cells. J Biol Chem. 1982 Dec 10;257(23):13958–13963. [PubMed] [Google Scholar]
- Mattson B. A., Rosenblum I. Y., Smith R. M., Heyner S. Autoradiographic evidence for insulin and insulin-like growth factor binding to early mouse embryos. Diabetes. 1988 May;37(5):585–589. doi: 10.2337/diab.37.5.585. [DOI] [PubMed] [Google Scholar]
- Morgan D. O., Ho L., Korn L. J., Roth R. A. Insulin action is blocked by a monoclonal antibody that inhibits the insulin receptor kinase. Proc Natl Acad Sci U S A. 1986 Jan;83(2):328–332. doi: 10.1073/pnas.83.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muglia L., Locker J. Extrapancreatic insulin gene expression in the fetal rat. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3635–3639. doi: 10.1073/pnas.81.12.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli L., Herrera R., Garcia-Arenas R., Rosen O. M. Acquisition of insulin-dependent protein tyrosine kinase activity during Drosophila embryogenesis. J Biol Chem. 1985 Dec 25;260(30):16072–16075. [PubMed] [Google Scholar]
- Rotwein P., Burgess S. K., Milbrandt J. D., Krause J. E. Differential expression of insulin-like growth factor genes in rat central nervous system. Proc Natl Acad Sci U S A. 1988 Jan;85(1):265–269. doi: 10.1073/pnas.85.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter R., Holtzclaw L., Sadiq F., Kahn A., Devaskar S. Insulin synthesis by isolated rabbit neurons. Endocrinology. 1988 Jul;123(1):505–513. doi: 10.1210/endo-123-1-505. [DOI] [PubMed] [Google Scholar]
- Serrano J., Bevins C. L., Young S. W., de Pablo F. Insulin gene expression in chicken ontogeny: pancreatic, extrapancreatic, and prepancreatic. Dev Biol. 1989 Apr;132(2):410–418. doi: 10.1016/0012-1606(89)90237-6. [DOI] [PubMed] [Google Scholar]
- Swenne I., Lundqvist G. Islet structure and pancreatic hormone content of the developing chick embryo. Gen Comp Endocrinol. 1980 Jun;41(2):190–198. doi: 10.1016/0016-6480(80)90143-4. [DOI] [PubMed] [Google Scholar]
- Underwood L. E., D'Ercole A. J., Clemmons D. R., Van Wyk J. J. Paracrine functions of somatomedins. Clin Endocrinol Metab. 1986 Feb;15(1):59–77. doi: 10.1016/s0300-595x(86)80042-1. [DOI] [PubMed] [Google Scholar]
- Unterman T., Goewert R. R., Baumann G., Freinkel N. Insulin receptors in embryo and extraembryonic membranes of early somite rat conceptus. Diabetes. 1986 Nov;35(11):1193–1199. doi: 10.2337/diab.35.11.1193. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Serrero G. Characterization of an insulin-related factor secreted by a teratoma cell line. Biochem Biophys Res Commun. 1986 Mar 13;135(2):533–540. doi: 10.1016/0006-291x(86)90027-6. [DOI] [PubMed] [Google Scholar]
- Zick Y., Grunberger G., Rees-Jones R. W., Comi R. J. Use of tyrosine-containing polymers to characterize the substrate specificity of insulin and other hormone-stimulated tyrosine kinases. Eur J Biochem. 1985 Apr 1;148(1):177–182. doi: 10.1111/j.1432-1033.1985.tb08822.x. [DOI] [PubMed] [Google Scholar]
- de Pablo F., Chambers S. A., Ota A. Insulin-related molecules and insulin effects in the sea urchin embryo. Dev Biol. 1988 Nov;130(1):304–310. doi: 10.1016/0012-1606(88)90436-8. [DOI] [PubMed] [Google Scholar]
- de Pablo F., Girbau M., Gomez J. A., Hernandez E., Roth J. Insulin antibodies retard and insulin accelerates growth and differentiation in early embryos. Diabetes. 1985 Oct;34(10):1063–1067. doi: 10.2337/diab.34.10.1063. [DOI] [PubMed] [Google Scholar]