Abstract
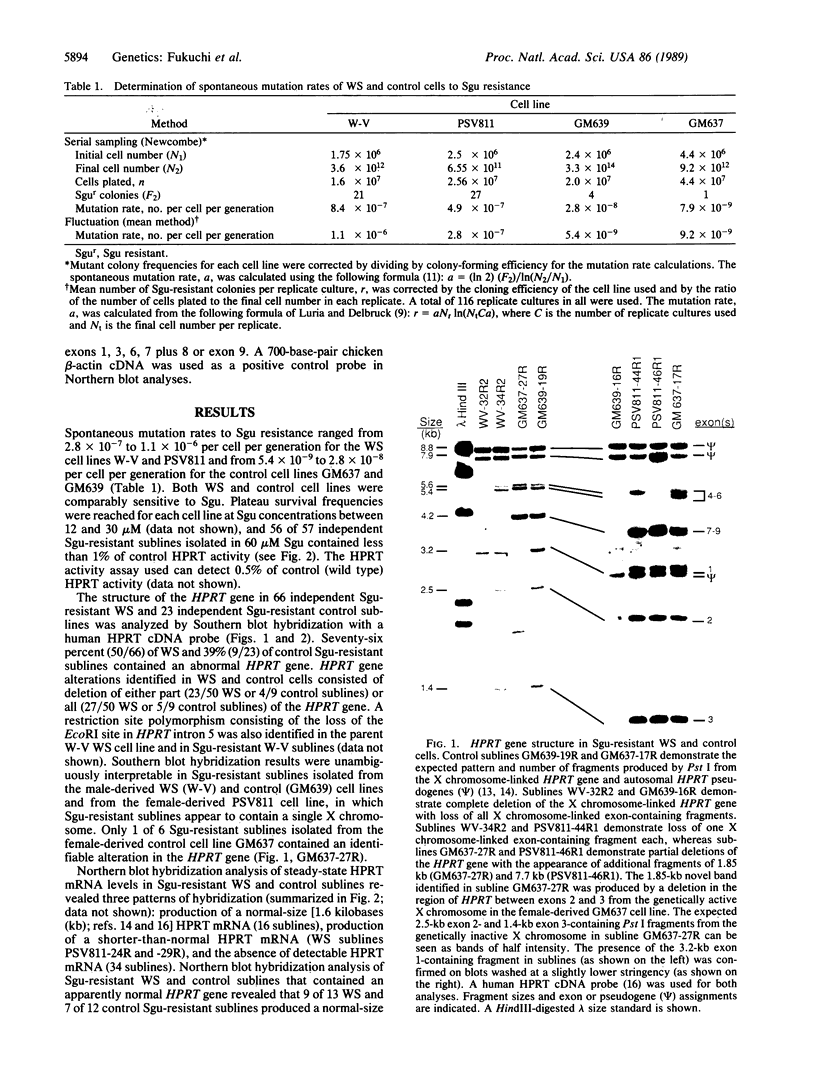
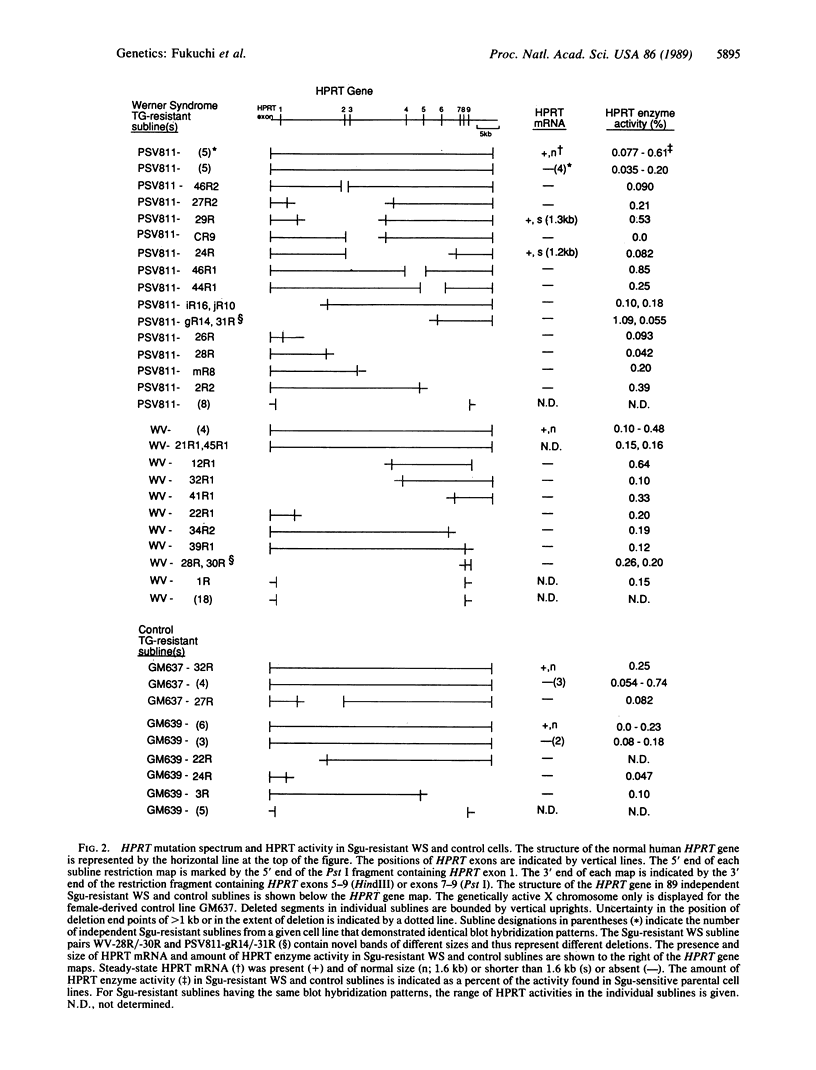
Werner syndrome (WS) is a rare autosomal-recessive disorder characterized by the premature appearance of features of normal aging in young adults. The extensive phenotypic overlap between WS and normal aging suggests they may also share pathogenetic mechanisms. We reported previously that somatic cells from WS patients demonstrate a propensity to develop chromosomal aberrations, including translocations, inversions, and deletions, and that WS cell lines demonstrate a high spontaneous mutation rate to 6-thioguanine resistance. We report here the biochemical and molecular characterization of spontaneous mutations at the X chromosome-linked hypoxanthine phosphoribosyltransferase (HPRT) locus in 6-thioguanine-resistant WS and control cells. Blot hybridization analysis of 89 independent spontaneous HPRT mutations in WS and control mutants lacking HPRT activity revealed an unusually high proportion of HPRT deletions in WS as compared with control cells (76% vs. 39%). Approximately half (58%) of the deletions in WS cells consisted of the loss of greater than 20 kilobases of DNA from the HPRT gene. These results suggest that an elevated somatic mutation rate, and particularly deletions, may play pathogenetically important roles in WS and in several associated age-dependent human disease processes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benditt E. P., Benditt J. M. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer: 1988. Leukemia. 1988 Apr;2(4):199–208. [PubMed] [Google Scholar]
- Breimer L. H. Ionizing radiation-induced mutagenesis. Br J Cancer. 1988 Jan;57(1):6–18. doi: 10.1038/bjc.1988.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breimer L. H., Nalbantoglu J., Meuth M. Structure and sequence of mutations induced by ionizing radiation at selectable loci in Chinese hamster ovary cells. J Mol Biol. 1986 Dec 5;192(3):669–674. doi: 10.1016/0022-2836(86)90284-6. [DOI] [PubMed] [Google Scholar]
- Epstein C. J., Martin G. M., Schultz A. L., Motulsky A. G. Werner's syndrome a review of its symptomatology, natural history, pathologic features, genetics and relationship to the natural aging process. Medicine (Baltimore) 1966 May;45(3):177–221. doi: 10.1097/00005792-196605000-00001. [DOI] [PubMed] [Google Scholar]
- Fukuchi K., Tanaka K., Nakura J., Kumahara Y., Uchida T., Okada Y. Elevated spontaneous mutation rate in SV40-transformed Werner syndrome fibroblast cell lines. Somat Cell Mol Genet. 1985 Jul;11(4):303–308. doi: 10.1007/BF01534688. [DOI] [PubMed] [Google Scholar]
- Gennett I. N., Thilly W. G. Mapping large spontaneous deletion endpoints in the human HPRT gene. Mutat Res. 1988 Sep;201(1):149–160. doi: 10.1016/0027-5107(88)90121-2. [DOI] [PubMed] [Google Scholar]
- Green A. R. Recessive mechanisms of malignancy. Br J Cancer. 1988 Aug;58(2):115–121. doi: 10.1038/bjc.1988.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huschtscha L. I., Thompson K. V., Holliday R. The susceptibility of Werner's syndrome and other human skin fibroblasts to SV40-induced transformation and immortalization. Proc R Soc Lond B Biol Sci. 1986 Oct 22;229(1254):1–12. doi: 10.1098/rspb.1986.0070. [DOI] [PubMed] [Google Scholar]
- Jolly D. J., Esty A. C., Bernard H. U., Friedmann T. Isolation of a genomic clone partially encoding human hypoxanthine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5038–5041. doi: 10.1073/pnas.79.16.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly D. J., Okayama H., Berg P., Esty A. C., Filpula D., Bohlen P., Johnson G. G., Shively J. E., Hunkapillar T., Friedmann T. Isolation and characterization of a full-length expressible cDNA for human hypoxanthine phosphoribosyl transferase. Proc Natl Acad Sci U S A. 1983 Jan;80(2):477–481. doi: 10.1073/pnas.80.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendal W. S., Frost P. Pitfalls and practice of Luria-Delbrück fluctuation analysis: a review. Cancer Res. 1988 Mar 1;48(5):1060–1065. [PubMed] [Google Scholar]
- Kim S. H., Moores J. C., David D., Respess J. G., Jolly D. J., Friedmann T. The organization of the human HPRT gene. Nucleic Acids Res. 1986 Apr 11;14(7):3103–3118. doi: 10.1093/nar/14.7.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liber H. L., Call K. M., Little J. B. Molecular and biochemical analyses of spontaneous and X-ray-induced mutants in human lymphoblastoid cells. Mutat Res. 1987 May;178(1):143–153. doi: 10.1016/0027-5107(87)90096-0. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak M. I., Varshaver N. B., Shapiro N. I. Induction of gene mutations and chromosomal aberrations by simian virus 40 in cultured mammalian cells. Mutat Res. 1975 Dec;30(3):383–396. [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A., Epstein C. J. Replicative life-span of cultivated human cells. Effects of donor's age, tissue, and genotype. Lab Invest. 1970 Jul;23(1):86–92. [PubMed] [Google Scholar]
- Monnat R. J., Jr Molecular analysis of spontaneous hypoxanthine phosphoribosyltransferase mutations in thioguanine-resistant HL-60 human leukemia cells. Cancer Res. 1989 Jan 1;49(1):81–87. [PubMed] [Google Scholar]
- Newcombe H. B. Delayed Phenotypic Expression of Spontaneous Mutations in Escherichia Coli. Genetics. 1948 Sep;33(5):447–476. doi: 10.1093/genetics/33.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. W., Bradt C. I., Toji L. H., Godley M., Segawa M. Induction of sister chromatid exchanges by transformation with simian virus 40. Cancer Res. 1978 Apr;38(4):960–964. [PubMed] [Google Scholar]
- Patel P. I., Framson P. E., Caskey C. T., Chinault A. C. Fine structure of the human hypoxanthine phosphoribosyltransferase gene. Mol Cell Biol. 1986 Feb;6(2):393–403. doi: 10.1128/mcb.6.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P. I., Nussbaum R. L., gramson P. E., Ledbetter D. H., Caskey C. T., Chinault A. C. Organization of the HPRT gene and related sequences in the human genome. Somat Cell Mol Genet. 1984 Sep;10(5):483–493. doi: 10.1007/BF01534853. [DOI] [PubMed] [Google Scholar]
- Pollard J. W., Harley C. B., Chamberlain J. W., Goldstein S., Stanners C. P. Is transformation associated with an increased error frequency in mammalian cells? J Biol Chem. 1982 Jun 10;257(11):5977–5979. [PubMed] [Google Scholar]
- Salk D., Au K., Hoehn H., Martin G. M. Cytogenetics of Werner's syndrome cultured skin fibroblasts: variegated translocation mosaicism. Cytogenet Cell Genet. 1981;30(2):92–107. doi: 10.1159/000131596. [DOI] [PubMed] [Google Scholar]
- Skulimowski A. W., Turner D. R., Morley A. A., Sanderson B. J., Haliandros M. Molecular basis of X-ray-induced mutation at the HPRT locus in human lymphocytes. Mutat Res. 1986 Aug;162(1):105–112. doi: 10.1016/0027-5107(86)90075-8. [DOI] [PubMed] [Google Scholar]
- Theile M., Strauss M., Luebbe L., Scherneck S., Krause H., Geissler E. SV40-induced somatic mutations: possible relevance to viral transformation. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):377–382. doi: 10.1101/sqb.1980.044.01.042. [DOI] [PubMed] [Google Scholar]
- Vrieling H., Simons J. W., Arwert F., Natarajan A. T., van Zeeland A. A. Mutations induced by X-rays at the HPRT locus in cultured Chinese hamster cells are mostly large deletions. Mutat Res. 1985 Dec;144(4):281–286. doi: 10.1016/0165-7992(85)90065-x. [DOI] [PubMed] [Google Scholar]
- Yang T. P., Patel P. I., Chinault A. C., Stout J. T., Jackson L. G., Hildebrand B. M., Caskey C. T. Molecular evidence for new mutation at the hprt locus in Lesch-Nyhan patients. Nature. 1984 Aug 2;310(5976):412–414. doi: 10.1038/310412a0. [DOI] [PubMed] [Google Scholar]
- van Zeeland A. A., Simons J. U. The use of correction factors in the determination of mutant frequencies in populations of human diploid skin fibroblasts. Mutat Res. 1976 Jan;34(1):149–158. doi: 10.1016/0027-5107(76)90268-2. [DOI] [PubMed] [Google Scholar]




