Abstract
In the structure of the title compound, 2C7H8NO2 +·2ClO4 −·H2O, the ions are connected via N—H⋯O, N—H⋯(O,O), O—H⋯O, O—H⋯(O,O) and C—H⋯O hydrogen bonds into a three-dimensional network.
Related literature
Hydrogen bonds play a crucial role in supramolecular organization (Jeffrey, 1997 ▶; Nangia & Desiraju, 1998 ▶). Knowledge of hydrogen-bond geometries (Taylor & Kennard, 1984 ▶; Murray-Rust & Glusker, 1984 ▶) and motif formation is vital in the modeling of protein–ligand interactions (Tintelnot & Andrews, 1989 ▶; Böhm & Klebe, 1996 ▶). For hydriogen-bond motifs, see: Bernstein et al. (1995 ▶). For the structures of organic salts of carboxylic acids, see: Bendjeddou et al. (2003 ▶); Cherouana et al. (2003 ▶). For a description of the Cambridge Structural Database, see: Allen (2002 ▶);
Experimental
Crystal data
2C7H8NO2 +·2ClO4 −·H2O
M r = 493.20
Triclinic,
a = 4.9170 (3) Å
b = 12.4030 (2) Å
c = 17.1030 (4) Å
α = 70.520 (2)°
β = 88.697 (3)°
γ = 86.166 (4)°
V = 981.13 (7) Å3
Z = 2
Mo Kα radiation
μ = 0.41 mm−1
T = 120 K
0.3 × 0.03 × 0.02 mm
Data collection
Enraf–Nonius KappaCCD diffractometer
Absorption correction: none
45183 measured reflections
7071 independent reflections
4775 reflections with I > 2σ(I)
R int = 0.049
Refinement
R[F 2 > 2σ(F 2)] = 0.039
wR(F 2) = 0.102
S = 0.99
7071 reflections
292 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.45 e Å−3
Δρmin = −0.57 e Å−3
Data collection: CAD-4 Software (Enraf–Nonius, 1989 ▶); cell refinement: DENZO and SCALEPACK (Otwinowski & Minor, 1997 ▶); data reduction: DENZO and SCALEPACK; program(s) used to solve structure: SIR92 (Altomare et al., 1993 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶), PARST97 (Nardelli, 1995 ▶), Mercury (Macrae et al., 2006 ▶) and POV-RAY (Persistence of Vision Team, 2004 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809025173/bq2150sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809025173/bq2150Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bonding geometry (Å, °) and unitary motifs.
| D—H⋯A | D—A | H⋯A | D⋯A | D—H⋯A | Motifs |
|---|---|---|---|---|---|
| N1—H1A⋯O1Wi | 0.89 | 1.98 | 2.8664 (18) | 171 | D |
| N1—H1B⋯O6ii | 0.89 | 2.11 | 2.9421 (19) | 156 | D |
| N1—H1B⋯O8 | 0.89 | 2.57 | 3.0343 (18) | 114 | D |
| N1—H1C⋯O1Wiii | 0.89 | 1.98 | 2.8636 (18) | 174 | D |
| O1W—H1W⋯O1i | 0.840 (14) | 2.534 (19) | 2.9435 (14) | 111.2 (15) | D |
| O1W—H1W⋯O3iii | 0.840 (14) | 2.258 (14) | 3.0356 (16) | 153.9 (16) | D |
| O1W—H2W⋯O1 | 0.863 (14) | 2.019 (15) | 2.8504 (17) | 161.4 (18) | D |
| N2—H2A⋯O6 | 0.89 | 2.10 | 2.9336 (19) | 155 | D |
| N2—H2A⋯O7 | 0.89 | 2.57 | 2.9584 (18) | 107 | D |
| N2—H2B⋯O4iv | 0.89 | 2.06 | 2.9382 (18) | 168 | D |
| N2—H2C⋯O3v | 0.89 | 2.55 | 3.1176 (19) | 122 | D |
| N2—H2C⋯O4v | 0.89 | 1.98 | 2.8683 (18) | 175 | D |
| O12—H12⋯O11vi | 0.82 | 1.82 | 2.6425 (14) | 178 |
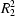 (8) (8) |
| O22—H22⋯O21vii | 0.82 | 1.82 | 2.6428 (17) | 178 |
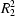 (8) (8) |
| C13—H13⋯O8 | 0.93 | 2.31 | 3.1193 (19) | 145 | D |
| C15—H15⋯O2i | 0.93 | 2.43 | 3.3058 (19) | 156 | D |
| C23—H23⋯O4iv | 0.93 | 2.55 | 3.2529 (19) | 133 | D |
| C25—H25⋯O8viii | 0.93 | 2.49 | 3.384 (2) | 162 | D |
| C27—H27⋯O11vi | 0.93 | 2.60 | 3.136 (2) | 117 | D |
Symmetry codes: (i) 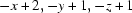 ; (ii)
; (ii) 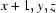 ; (iii)
; (iii) 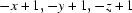 ; (iv)
; (iv) 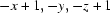 ; (v)
; (v) 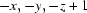 ; (vi)
; (vi) 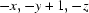 ; (vii)
; (vii) 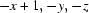 ; (viii)
; (viii) 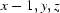 . Motifs: R = ring; D = finite patterns.
. Motifs: R = ring; D = finite patterns.
Acknowledgments
Technical support (X-ray measurements at SCDRX) from Université Henry Poincaré, Nancy 1, is gratefully acknowledged.
supplementary crystallographic information
Comment
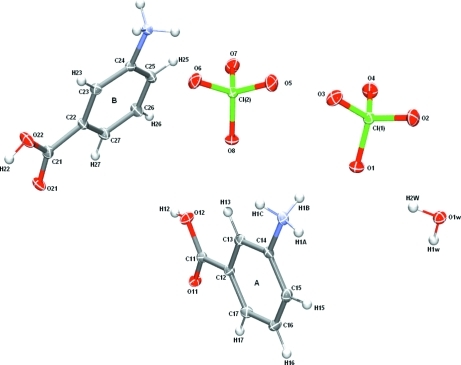
Hydrogen bonds play a crucial role in supramolecular organization (Jeffrey, 1997; Nangia & Desiraju, 1998). Knowledge of hydrogen-bond geometries (Taylor & Kennard, 1984; Murray-Rust & Glusker, 1984) and motif formation is vital in the modeling of protein-ligand interactions (Tintelnot & Andrews, 1989; Böhm & Klebe, 1996). The supramolecular networks become especially interesting when the cation and anion can participate in hydrogen-bonding. In this regard previous studies have been concerned with organic salts of carboxylic acids (Bendjeddou et al., 2003), Cherouana et al., 2003). The asymmetric unit of (I) (Fig. 1) contains two carboxyanilinium cations (A and B), two perchlorate anion and one water molecule. A proton transfer from the perchloric acid to atom N1 and N2 of m-carboxyalinine resulted in the formation of salts.
1- Supramolecular organization:
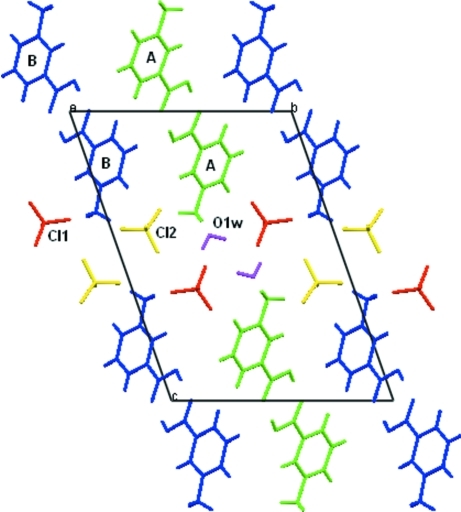
The structure is formed by double anionic and cationic chains that extend along the b axis, giving rise to layers parallel to the plane (b, c). Chains of water molecules are sandwiched between the anionic double chains (Fig2.).
1–1- Overview.
The supramolecular architecture is generated by the nineteen independent interactions of the Table 1. Three types of intermolecular interactions are present in the structure, including O—H···O, N—H···O and C—H···O hydrogen bonds. The presence of water molecule in the structure results in the presence of additional hydrogen bonds. The construction of graphs-set of the twenty hydrogen bonds in this compound has led to a first-level graph set noted: N1 = R22(8)R22(8) (Bernstein et al., 1995) (Table 1.).
1–2- The O—H···O hydrogen-bonded network
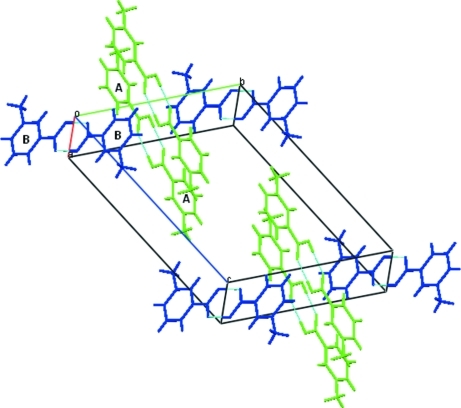
The carboxylic acid groups at the opposite end of the carboxyanilinium cations forms a centrosymmetric hydrogen-bonded dimers with its counterpart in a cation from an adjacent ribbon and are centered at (0 1/2 0) and (1/2 0 0) respectively for cation A and cation B (Fig. 3). These interactions lead to the graph-set motif R22(8), which is a characteristic feature found in most salts of 3- and 4-aminobenzoic acid (Cambridge Structural Database; Allen, 2002).
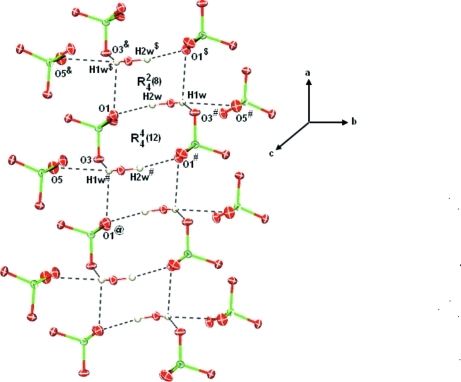
The water molecule, bridges the anionic perchlorate via the O—H···O hydrogen bonds. The centrosymmetric hydrogen-bonded rings formed by two water molecules and two Cl(1)O4- anions can be described by the graph-set R44(12) and R24(8). The aggregation of this two ring motifs results in an overall one-dimensional hydrogen-bonded chain structure along the [100] direction (Fig. 4).
1–3- The N—H···O hydrogen-bonded network
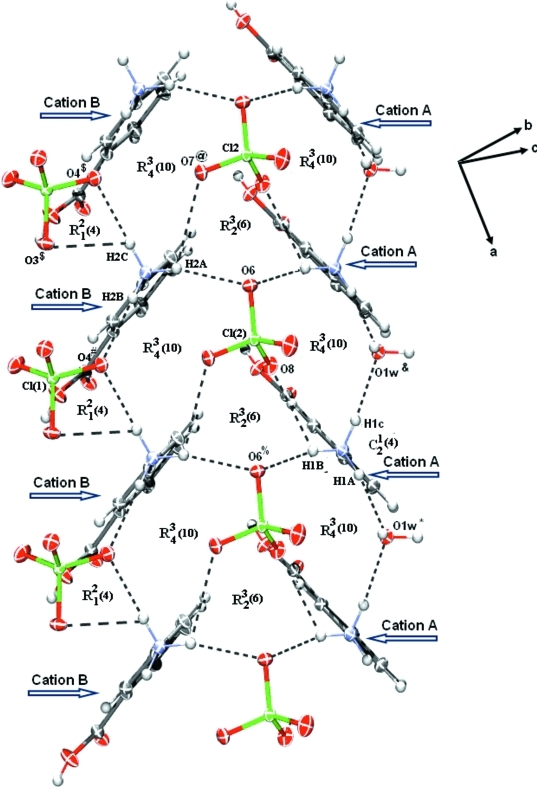
In the first cationic (A) entity, all ammonium H atoms are involved in hydrogen bonds with the perchlorate (Cl(2) O4-) anion and water molecule. Two of these interactions link the anions and cations in an alternating fashion into extended chains along the [100] direction, which can be described by the graph-set C22(4). The two other interactions are in a crosslink from an adjacent chain C22(4). The combination of these two chain motifs generates noncentrosymmetric fused rings which can be described by the graph-set motif R34(10).
In the second cationic (B) entitie, all ammonium H atoms are involved in hydrogen bonds, with the two different perchlorate ion, so forming an alternating noncentrosymmetric rings a long [100] direction which can be described by the graph-set R34(10). Only one H atom (H2C) is involved in bifurcated hydrogen bonds with O(3) and O(4) perchlorate atoms, to form a four-membered hydrogen bonded ring R21(4). The junction betwen this two different cations (A and B) entities are assured by the perchlorate (Cl(2) O4-) anion via N1—H1B···O6, N1—H1B···O8, N2—H2A···O6, N2—H2A···O7 hydrogen bonds, so generete R32(6) rings along [100] direction (Fig. 5)
1–4- The C—H···O hydrogen-bonded network:
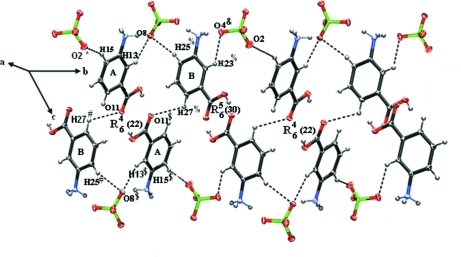
The junction between the cationic entity is consolidated by five weak independent C—H···O hydrogen bonds via the perchlorate anions, forming an alternating of R46(22) and R56(30)centrosymmetric Rings a long b axis (Fig. 6).
Experimental
m-carboxyanilinium acid and perchloric acid were mixed in a 2:2 stoichiometric ratio and dissolved in water. Crystal were obtained by slow evaporation.
Refinement
H atoms were positioned geometrically and refined in the riding-model approximation, with C—H = 0.97 Å, N—H = 0.89 Å, O—H = 0.82 Å, and with Uiso(H) = 1.2Ueq(C, N) or 1.5Ueq(O). The H atoms of the water molecule were located in a difference Fourier map and reined as riding, with O—H = 0.85 Å and Uiso(H) = 1.5Ueq(O).
Figures
Fig. 1.
The asymmetric unit of (I), showing the crystallographic numbering scheme. Displacement ellipsoids are drawn at the 50% probability level and H atoms are shown as spheres of arbitrary radii.
Fig. 2.
A packing diagram for the title compound, viewed along the a axis, showing the mixture layers.
Fig. 3.
Part of the crystal structure, showing the formation of dimers A and B via O—H···O hydrogen bonds.
Fig. 4.
A view of the two-dimensional O—H···O hydrogen-bonded network paralel to the (100) plane of (I), showing the aggregation of R44(12) and R24(8) hydrogen-bonding motifs. Atoms marked with an ampersand (&), an at sign (@), a hash symbol (#) or a dollar sign ($) are at the symmetry positions (1 + x, y, z), (-1 + x, y, z), (1 - x, 1 - y, 1 - z), (2 - x, 1 - y, 1 - z), respectively.
Fig. 5.
Part of the crystal structure, showing the aggregation of C22(4) R34(10) and R32(6) motifs via N—H···O hydrogen bonds. Atoms marked with an ampersand (&), an at sign (@), a hash symbol (#), dollar sign ($), percent sign (%), or a star sign (*) are at the symmetry positions (1 - x, 1 - y, 1 - z), (-1 + x, y, z), (1 - x, y, 1 - z), (-x, -y, 1 - z), (1 + x, y, z), (2 - x, 1 - y, 1 - z), respectively.
Fig. 6.
A view of part of the crystal structure of (I), showing the formation of R46(22) and R56(30) rings. Atoms marked with an ampersand (&), a hash symbol (#), dollar sign ($), percent sign (%), or a star sign (*) are at the symmetry positions (2 - x, -y, 1 - z), (-x, 1 - y, -z), (1 - x, 1 - y, -z), (1 + x, y, z), (2 - x, 1 - y, 1 - z), respectively.
Crystal data
| 2C7H8NO2+·2ClO4−·H2O | Z = 2 |
| Mr = 493.20 | F(000) = 508 |
| Triclinic, P1 | Dx = 1.669 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 4.9170 (3) Å | Cell parameters from 7071 reflections |
| b = 12.4030 (2) Å | θ = 3.3–32.6° |
| c = 17.1030 (4) Å | µ = 0.41 mm−1 |
| α = 70.520 (2)° | T = 120 K |
| β = 88.697 (3)° | Needle, brown |
| γ = 86.166 (4)° | 0.3 × 0.03 × 0.02 mm |
| V = 981.13 (7) Å3 |
Data collection
| Enraf–Nonius KappaCCD diffractometer | 4775 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.049 |
| graphite | θmax = 32.6°, θmin = 3.3° |
| ω scans | h = 0→7 |
| 45183 measured reflections | k = −18→18 |
| 7071 independent reflections | l = −25→25 |
Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.039 | w = 1/[σ2(Fo2) + (0.0526P)2] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.102 | (Δ/σ)max = 0.021 |
| S = 0.99 | Δρmax = 0.45 e Å−3 |
| 7071 reflections | Δρmin = −0.57 e Å−3 |
| 292 parameters |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O11 | 0.2368 (2) | 0.58575 (9) | 0.00162 (6) | 0.0167 (3) | |
| O12 | 0.1451 (2) | 0.42214 (9) | 0.10223 (6) | 0.0176 (3) | |
| N1 | 0.6845 (3) | 0.40582 (11) | 0.35093 (7) | 0.0142 (4) | |
| C11 | 0.2756 (3) | 0.51695 (13) | 0.07149 (9) | 0.0136 (4) | |
| C12 | 0.4736 (3) | 0.53492 (13) | 0.12947 (9) | 0.0131 (4) | |
| C13 | 0.4963 (3) | 0.45953 (13) | 0.21027 (9) | 0.0132 (4) | |
| C14 | 0.6728 (3) | 0.48155 (13) | 0.26416 (9) | 0.0127 (4) | |
| C15 | 0.8297 (3) | 0.57585 (13) | 0.23929 (9) | 0.0154 (4) | |
| C16 | 0.8057 (3) | 0.65062 (13) | 0.15814 (9) | 0.0172 (5) | |
| C17 | 0.6279 (3) | 0.63090 (13) | 0.10338 (9) | 0.0154 (4) | |
| O21 | 0.2424 (2) | 0.08889 (10) | −0.00149 (6) | 0.0195 (3) | |
| O22 | 0.3663 (3) | −0.06863 (10) | 0.10513 (7) | 0.0231 (4) | |
| N2 | −0.2091 (3) | −0.04135 (11) | 0.34838 (7) | 0.0132 (3) | |
| C21 | 0.2141 (3) | 0.02587 (13) | 0.07045 (9) | 0.0162 (4) | |
| C22 | 0.0034 (3) | 0.05317 (13) | 0.12572 (9) | 0.0146 (4) | |
| C23 | −0.0102 (3) | −0.01242 (13) | 0.20967 (9) | 0.0137 (4) | |
| C24 | −0.1986 (3) | 0.02235 (13) | 0.25877 (9) | 0.0130 (4) | |
| C25 | −0.3762 (3) | 0.11787 (14) | 0.22733 (9) | 0.0171 (4) | |
| C26 | −0.3649 (3) | 0.18137 (14) | 0.14357 (10) | 0.0193 (5) | |
| C27 | −0.1724 (3) | 0.14958 (14) | 0.09290 (9) | 0.0173 (4) | |
| Cl1 | 0.58901 (7) | 0.29103 (3) | 0.61432 (2) | 0.0133 (1) | |
| O1 | 0.7010 (2) | 0.36508 (9) | 0.53790 (6) | 0.0191 (3) | |
| O2 | 0.6783 (2) | 0.32216 (11) | 0.68248 (7) | 0.0237 (4) | |
| O3 | 0.2956 (2) | 0.30140 (10) | 0.60921 (7) | 0.0208 (3) | |
| O4 | 0.6805 (2) | 0.17339 (9) | 0.62552 (7) | 0.0201 (3) | |
| Cl2 | 0.14254 (7) | 0.15743 (3) | 0.40892 (2) | 0.0128 (1) | |
| O5 | 0.2017 (3) | 0.17301 (10) | 0.48568 (7) | 0.0244 (4) | |
| O6 | −0.1515 (2) | 0.15994 (10) | 0.39893 (7) | 0.0220 (3) | |
| O7 | 0.2591 (2) | 0.04883 (9) | 0.40697 (7) | 0.0207 (3) | |
| O8 | 0.2470 (2) | 0.24934 (9) | 0.34137 (7) | 0.0205 (3) | |
| O1W | 0.8260 (2) | 0.56613 (10) | 0.57195 (7) | 0.0156 (3) | |
| H1A | 0.82677 | 0.42111 | 0.37583 | 0.0212* | |
| H1B | 0.70185 | 0.33315 | 0.35272 | 0.0212* | |
| H1C | 0.53190 | 0.41728 | 0.37684 | 0.0212* | |
| H12 | 0.02648 | 0.42104 | 0.06961 | 0.0263* | |
| H13 | 0.39432 | 0.39521 | 0.22790 | 0.0159* | |
| H15 | 0.94884 | 0.58893 | 0.27615 | 0.0185* | |
| H16 | 0.90976 | 0.71430 | 0.14051 | 0.0206* | |
| H17 | 0.61145 | 0.68159 | 0.04935 | 0.0185* | |
| H2A | −0.22591 | 0.00789 | 0.37622 | 0.0198* | |
| H2B | −0.05642 | −0.08563 | 0.36380 | 0.0198* | |
| H2C | −0.35139 | −0.08481 | 0.35926 | 0.0198* | |
| H22 | 0.48980 | −0.07414 | 0.07338 | 0.0345* | |
| H23 | 0.10478 | −0.07783 | 0.23186 | 0.0165* | |
| H25 | −0.50154 | 0.13923 | 0.26186 | 0.0205* | |
| H26 | −0.48533 | 0.24494 | 0.12131 | 0.0232* | |
| H27 | −0.16175 | 0.19302 | 0.03700 | 0.0208* | |
| H1W | 0.826 (4) | 0.6178 (12) | 0.5253 (8) | 0.037 (6)* | |
| H2W | 0.827 (4) | 0.5046 (10) | 0.5589 (11) | 0.039 (6)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O11 | 0.0196 (6) | 0.0163 (6) | 0.0123 (5) | −0.0034 (4) | −0.0031 (4) | −0.0017 (4) |
| O12 | 0.0198 (6) | 0.0159 (6) | 0.0155 (5) | −0.0059 (5) | −0.0060 (4) | −0.0020 (4) |
| N1 | 0.0141 (6) | 0.0172 (7) | 0.0120 (6) | −0.0003 (5) | −0.0017 (5) | −0.0059 (5) |
| C11 | 0.0135 (7) | 0.0131 (7) | 0.0144 (7) | −0.0001 (6) | 0.0004 (6) | −0.0049 (6) |
| C12 | 0.0130 (7) | 0.0140 (7) | 0.0124 (7) | −0.0006 (6) | −0.0006 (5) | −0.0044 (6) |
| C13 | 0.0129 (7) | 0.0123 (7) | 0.0148 (7) | −0.0008 (6) | 0.0000 (5) | −0.0049 (6) |
| C14 | 0.0131 (7) | 0.0134 (7) | 0.0114 (7) | 0.0002 (6) | 0.0009 (5) | −0.0040 (5) |
| C15 | 0.0144 (7) | 0.0173 (8) | 0.0165 (7) | −0.0013 (6) | −0.0018 (6) | −0.0080 (6) |
| C16 | 0.0173 (8) | 0.0144 (8) | 0.0200 (8) | −0.0043 (6) | 0.0009 (6) | −0.0054 (6) |
| C17 | 0.0161 (8) | 0.0147 (7) | 0.0134 (7) | −0.0008 (6) | −0.0003 (6) | −0.0020 (6) |
| O21 | 0.0220 (6) | 0.0200 (6) | 0.0128 (5) | 0.0032 (5) | 0.0050 (4) | −0.0019 (4) |
| O22 | 0.0253 (7) | 0.0218 (6) | 0.0164 (6) | 0.0091 (5) | 0.0070 (5) | −0.0013 (5) |
| N2 | 0.0145 (6) | 0.0139 (6) | 0.0115 (6) | −0.0022 (5) | 0.0010 (5) | −0.0044 (5) |
| C21 | 0.0172 (8) | 0.0159 (8) | 0.0155 (7) | 0.0011 (6) | 0.0004 (6) | −0.0058 (6) |
| C22 | 0.0168 (7) | 0.0145 (7) | 0.0122 (7) | −0.0008 (6) | 0.0022 (6) | −0.0043 (6) |
| C23 | 0.0138 (7) | 0.0128 (7) | 0.0130 (7) | 0.0003 (6) | −0.0009 (5) | −0.0024 (6) |
| C24 | 0.0147 (7) | 0.0125 (7) | 0.0112 (7) | −0.0032 (6) | 0.0006 (5) | −0.0029 (5) |
| C25 | 0.0174 (8) | 0.0177 (8) | 0.0163 (7) | 0.0004 (6) | 0.0044 (6) | −0.0063 (6) |
| C26 | 0.0216 (8) | 0.0160 (8) | 0.0167 (8) | 0.0064 (6) | 0.0009 (6) | −0.0020 (6) |
| C27 | 0.0214 (8) | 0.0157 (8) | 0.0119 (7) | 0.0015 (6) | 0.0021 (6) | −0.0012 (6) |
| Cl1 | 0.0122 (2) | 0.0142 (2) | 0.0143 (2) | −0.0018 (1) | −0.0003 (1) | −0.0057 (1) |
| O1 | 0.0220 (6) | 0.0172 (6) | 0.0156 (5) | −0.0045 (5) | 0.0030 (4) | −0.0018 (4) |
| O2 | 0.0246 (6) | 0.0332 (7) | 0.0196 (6) | −0.0101 (5) | −0.0005 (5) | −0.0156 (5) |
| O3 | 0.0109 (5) | 0.0266 (7) | 0.0263 (6) | 0.0002 (5) | 0.0003 (4) | −0.0109 (5) |
| O4 | 0.0175 (6) | 0.0120 (5) | 0.0289 (6) | 0.0014 (4) | −0.0016 (5) | −0.0048 (5) |
| Cl2 | 0.0145 (2) | 0.0117 (2) | 0.0122 (2) | −0.0018 (1) | 0.0007 (1) | −0.0039 (1) |
| O5 | 0.0355 (7) | 0.0256 (7) | 0.0138 (5) | −0.0047 (5) | −0.0032 (5) | −0.0081 (5) |
| O6 | 0.0127 (5) | 0.0219 (6) | 0.0340 (7) | −0.0021 (5) | 0.0002 (5) | −0.0127 (5) |
| O7 | 0.0203 (6) | 0.0123 (5) | 0.0302 (6) | 0.0016 (4) | 0.0008 (5) | −0.0087 (5) |
| O8 | 0.0284 (7) | 0.0152 (6) | 0.0155 (5) | −0.0072 (5) | 0.0062 (5) | −0.0013 (4) |
| O1W | 0.0175 (6) | 0.0136 (5) | 0.0145 (5) | −0.0022 (4) | −0.0012 (4) | −0.0028 (4) |
Geometric parameters (Å, °)
| Cl1—O2 | 1.4307 (11) | N1—H1C | 0.89 |
| Cl1—O3 | 1.4418 (11) | C22—C27 | 1.389 (2) |
| Cl1—O1 | 1.4454 (11) | C22—C23 | 1.397 (2) |
| Cl1—O4 | 1.4484 (11) | C22—C21 | 1.484 (2) |
| Cl2—O5 | 1.4282 (11) | C24—C23 | 1.379 (2) |
| Cl2—O7 | 1.4386 (11) | C24—C25 | 1.383 (2) |
| Cl2—O8 | 1.4390 (11) | C14—C13 | 1.3833 (19) |
| Cl2—O6 | 1.4568 (11) | C14—C15 | 1.385 (2) |
| O12—C11 | 1.3208 (17) | C12—C13 | 1.388 (2) |
| O12—H12 | 0.82 | C12—C17 | 1.394 (2) |
| O1W—H2W | 0.863 (9) | C12—C11 | 1.484 (2) |
| O1W—H1W | 0.840 (9) | C27—C26 | 1.394 (2) |
| O11—C11 | 1.2252 (17) | C27—H27 | 0.93 |
| O22—C21 | 1.3135 (19) | C16—C17 | 1.386 (2) |
| O22—H22 | 0.82 | C16—C15 | 1.391 (2) |
| O21—C21 | 1.2286 (18) | C16—H16 | 0.93 |
| N2—C24 | 1.4732 (18) | C23—H23 | 0.93 |
| N2—H2A | 0.89 | C15—H15 | 0.93 |
| N2—H2B | 0.89 | C25—C26 | 1.388 (2) |
| N2—H2C | 0.89 | C25—H25 | 0.93 |
| N1—C14 | 1.4679 (18) | C17—H17 | 0.93 |
| N1—H1A | 0.89 | C13—H13 | 0.93 |
| N1—H1B | 0.89 | C26—H26 | 0.93 |
| O2—Cl1—O3 | 110.41 (7) | C15—C14—N1 | 119.21 (13) |
| O2—Cl1—O1 | 109.66 (7) | C13—C12—C17 | 120.21 (13) |
| O3—Cl1—O1 | 109.24 (7) | C13—C12—C11 | 120.24 (13) |
| O2—Cl1—O4 | 109.82 (7) | C17—C12—C11 | 119.48 (13) |
| O3—Cl1—O4 | 108.57 (7) | O11—C11—O12 | 123.69 (13) |
| O1—Cl1—O4 | 109.11 (7) | O11—C11—C12 | 122.07 (13) |
| O5—Cl2—O7 | 110.85 (7) | O12—C11—C12 | 114.24 (13) |
| O5—Cl2—O8 | 109.49 (7) | O21—C21—O22 | 123.78 (14) |
| O7—Cl2—O8 | 110.20 (7) | O21—C21—C22 | 121.54 (14) |
| O5—Cl2—O6 | 109.62 (7) | O22—C21—C22 | 114.67 (13) |
| O7—Cl2—O6 | 108.29 (7) | C22—C27—C26 | 120.12 (14) |
| O8—Cl2—O6 | 108.34 (7) | C22—C27—H27 | 119.9 |
| C11—O12—H12 | 109.5 | C26—C27—H27 | 119.9 |
| H2W—O1W—H1W | 102.4 (15) | C17—C16—C15 | 120.47 (14) |
| C21—O22—H22 | 109.5 | C17—C16—H16 | 119.8 |
| C24—N2—H2A | 109.5 | C15—C16—H16 | 119.8 |
| C24—N2—H2B | 109.5 | C24—C23—C22 | 118.26 (14) |
| H2A—N2—H2B | 109.5 | C24—C23—H23 | 120.9 |
| C24—N2—H2C | 109.5 | C22—C23—H23 | 120.9 |
| H2A—N2—H2C | 109.5 | C14—C15—C16 | 118.76 (14) |
| H2B—N2—H2C | 109.5 | C14—C15—H15 | 120.6 |
| C14—N1—H1A | 109.5 | C16—C15—H15 | 120.6 |
| C14—N1—H1B | 109.5 | C24—C25—C26 | 119.11 (14) |
| H1A—N1—H1B | 109.5 | C24—C25—H25 | 120.4 |
| C14—N1—H1C | 109.5 | C26—C25—H25 | 120.4 |
| H1A—N1—H1C | 109.5 | C16—C17—C12 | 119.84 (14) |
| H1B—N1—H1C | 109.5 | C16—C17—H17 | 120.1 |
| C27—C22—C23 | 120.41 (14) | C12—C17—H17 | 120.1 |
| C27—C22—C21 | 118.25 (13) | C14—C13—C12 | 118.99 (13) |
| C23—C22—C21 | 121.26 (14) | C14—C13—H13 | 120.5 |
| C23—C24—C25 | 122.28 (14) | C12—C13—H13 | 120.5 |
| C23—C24—N2 | 119.74 (13) | C25—C26—C27 | 119.79 (15) |
| C25—C24—N2 | 117.96 (13) | C25—C26—H26 | 120.1 |
| C13—C14—C15 | 121.73 (14) | C27—C26—H26 | 120.1 |
| C13—C14—N1 | 119.00 (12) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1A···O1Wi | 0.8900 | 1.9800 | 2.8664 (18) | 171.00 |
| N1—H1B···O6ii | 0.8900 | 2.1100 | 2.9421 (19) | 156.00 |
| N1—H1B···O8 | 0.8900 | 2.5700 | 3.0343 (18) | 114.00 |
| N1—H1C···O1Wiii | 0.8900 | 1.9800 | 2.8636 (18) | 174.00 |
| O1W—H1W···O1i | 0.840 (14) | 2.534 (19) | 2.9435 (14) | 111.2 (15) |
| O1W—H1W···O3iii | 0.840 (14) | 2.258 (14) | 3.0356 (16) | 153.9 (16) |
| N2—H2A···O6 | 0.8900 | 2.1000 | 2.9336 (19) | 155.00 |
| N2—H2A···O7 | 0.8900 | 2.5700 | 2.9584 (18) | 107.00 |
| N2—H2B···O4iv | 0.8900 | 2.0600 | 2.9382 (18) | 168.00 |
| N2—H2C···O3v | 0.8900 | 2.5500 | 3.1176 (19) | 122.00 |
| N2—H2C···O4v | 0.8900 | 1.9800 | 2.8683 (18) | 175.00 |
| O1W—H2W···O1 | 0.863 (14) | 2.019 (15) | 2.8504 (17) | 161.4 (18) |
| O12—H12···O11vi | 0.8200 | 1.8200 | 2.6425 (14) | 178.00 |
| O22—H22···O21vii | 0.8200 | 1.8200 | 2.6428 (17) | 178.00 |
| C13—H13···O8 | 0.9300 | 2.3100 | 3.1193 (19) | 145.00 |
| C15—H15···O2i | 0.9300 | 2.4300 | 3.3058 (19) | 156.00 |
| C23—H23···O4iv | 0.9300 | 2.5500 | 3.2529 (19) | 133.00 |
| C25—H25···O8viii | 0.9300 | 2.4900 | 3.384 (2) | 162.00 |
| C27—H27···O11vi | 0.9300 | 2.602 | 3.136 (2) | 117.05 |
Symmetry codes: (i) −x+2, −y+1, −z+1; (ii) x+1, y, z; (iii) −x+1, −y+1, −z+1; (iv) −x+1, −y, −z+1; (v) −x, −y, −z+1; (vi) −x, −y+1, −z; (vii) −x+1, −y, −z; (viii) x−1, y, z.
Table 1 Hydrogen-bonding geometry (Å, °) and unitary motifs
| D—H···A | D—A | H···A | D···A | D—H···A | Motifs |
| N1—H1A···O1Wi | 0.89 | 1.98 | 2.8664 (18) | 171 | D |
| N1—H1B···O6ii | 0.89 | 2.11 | 2.9421 (19) | 156 | D |
| N1—H1B···O8 | 0.89 | 2.57 | 3.0343 (18) | 114 | D |
| N1—H1C···O1Wiii | 0.89 | 1.98 | 2.8636 (18) | 174 | D |
| O1W—H1W···O1i | 0.840 (14) | 2.534 (19) | 2.9435 (14) | 111.2 (15) | D |
| O1W—H1W···O3iii | 0.840 (14) | 2.258 (14) | 3.0356 (16) | 153.9 (16) | D |
| N2—H2A···O6 | 0.89 | 2.10 | 2.9336 (19) | 155 | D |
| N2—H2A···O7 | 0.89 | 2.57 | 2.9584 (18) | 107 | D |
| N2—H2B···O4iv | 0.89 | 2.06 | 2.9382 (18) | 168 | D |
| N2—H2C···O3v | 0.89 | 2.55 | 3.1176 (19) | 122 | D |
| N2—H2C···O4v | 0.89 | 1.98 | 2.8683 (18) | 175 | D |
| O1W—H2W···O1 | 0.863 (14) | 2.019 (15) | 2.8504 (17) | 161.4 (18) | D |
| O12—H12···O11vi | 0.82 | 1.82 | 2.6425 (14) | 178 | R22(8) |
| O22—H22···O21vii | 0.82 | 1.82 | 2.6428 (17) | 178 | R22(8) |
| C13—H13···O8 | 0.93 | 2.31 | 3.1193 (19) | 145 | D |
| C15—H15···O2i | 0.93 | 2.43 | 3.3058 (19) | 156 | D |
| C23—H23···O4iv | 0.93 | 2.55 | 3.2529 (19) | 133 | D |
| C25—H25···O8viii | 0.93 | 2.49 | 3.384 (2) | 162 | D |
| C27—H27···O11vi | 0.93 | 2.60 | 3.136 (2) | 117 | D |
Symmetry codes: (i) -x+2, -y+1, -z+1; (ii) x+1, y, z; (iii) -x+1, -y+1, -z+1; (iv) -x+1, -y, -z+1; (v) -x, -y, -z+1; (vi) -x, -y+1, -z; (vii) -x+1, -y, -z; (viii) x-1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BQ2150).
References
- Allen, F. H. (2002). Acta Cryst. B58, 380–388. [DOI] [PubMed]
- Altomare, A., Cascarano, G., Giacovazzo, C. & Guagliardi, A. (1993). J. Appl. Cryst.26, 343–350.
- Bendjeddou, L., Cherouana, A., Berrah, F. & Benali-Cherif, N. (2003). Acta Cryst. E59, o574–o576.
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N. L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
- Böhm, H.-J. & Klebe, G. (1996). Angew. Chem. Int. Ed. Engl.35, 2588–2614.
- Cherouana, A., Bendjeddou, L. & Benali-Cherif, N. (2003). Acta Cryst. E59, o1790–o1792.
- Enraf–Nonius (1989). CAD-4 Software Enraf–Nonius, Delft, The Netherlands.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Jeffrey, G. A. (1997). An Introduction to Hydrogen Bonding New York: Oxford University Press Inc.
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst.39, 453–457.
- Murray-Rust, P. & Glusker, J. P. (1984). J. Am. Chem. Soc.106, 1018–1025.
- Nangia, A. & Desiraju, G. R. (1998). Acta Cryst. A54, 934–944.
- Nardelli, M. (1995). J. Appl. Cryst.28, 659.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Persistence of Vision Team (2004). POV-RAY Persistence of Vision Raytracer Pty Ltd, Victoria, Australia. URL: http://www.povray.org/.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Taylor, R. & Kennard, O. (1984). Acc. Chem. Res.17, 320–326.
- Tintelnot, M. & Andrews, P. (1989). J. Comput. Aid. Mol. Des.3, 67–84. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809025173/bq2150sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809025173/bq2150Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report