Abstract
In the cation of the title compound [systematic name: 3-(9H-carbazol-4-yloxy)-2-hydroxy-N-[2-(2-methoxyphenoxy)ethyl]propan-1-aminium dihydrogen phosphate hemihydrate], C24H27N2O4 +·H2PO4 −·0.5H2O, the mean planes of the tricyclic ring system and the benzene ring form a dihedral angle of 87.2 (2)°. In the crystal structure, the solvent water molecule is situated on a twofold rotation axis linking two cations via O—H⋯O and N—H⋯O hydrogen bonds. The anions contribute to the formation O—H⋯O and N—H⋯O hydrogen bonds between the anions and cations, which consolidate the crystal packing.
Related literature
For the synthesis of the title compound, claimed as Form I, see: Brook et al. (2005 ▶). For the crystal structures of two polymorphs of the carvedilol free base, see: Chen et al. (1998 ▶); Yathirajan et al. (2007 ▶). For details of the indexing algorithm, see: Visser (1969 ▶). The methodology of bond-restrained Rietveld refinement used in this study was the same as described by Chernyshev et al. (2003 ▶).
Experimental
Crystal data
C24H27N2O4 +·H2PO4 −·0.5H2O
M r = 513.47
Monoclinic,
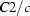
a = 26.600 (2) Å
b = 12.3767 (12) Å
c = 16.5101 (15) Å
β = 106.662 (11)°
V = 5207.2 (8) Å3
Z = 8
Cu Kα1 radiation
μ = 1.38 mm−1
T = 295 K
Specimen shape: flat sheet
15 × 1 × 1 mm
Specimen prepared at 101 kPa
Specimen prepared at 295 K
Particle morphology: no specific habit, light grey
Data collection
Guinier G670 image plate camera
Specimen mounting: thin layer in the specimen holder of the camera
Specimen mounted in transmission mode
Scan method: continuous
2θmin = 5.0, 2θmax = 75.0°
Increment in 2θ = 0.01°
Refinement
R p = 0.026
R wp = 0.035
R exp = 0.014
R B = 0.064
S = 2.43
Wavelength of incident radiation: 1.54059 Å
Excluded region(s): none
Profile function: split-type pseudo-Voigt (Toraya, 1986 ▶)
1346 reflections
157 parameters
125 restraints
H-atom parameters not refined
Preferred orientation correction: March-Dollase (Dollase, 1986 ▶); direction of preferred orientation 100, texture parameter r = 1.038 (5)
Data collection: G670 Imaging Plate Guinier Camera Software (Huber, 2002 ▶); cell refinement: MRIA (Zlokazov & Chernyshev, 1992 ▶); data reduction: G670 Imaging Plate Guinier Camera Software; method used to solve structure: simulated annealing (Zhukov et al., 2001 ▶); program(s) used to refine structure: MRIA; molecular graphics: PLATON (Spek, 2009 ▶); software used to prepare material for publication: MRIA and SHELXL97 (Sheldrick, 2008 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809029353/lh2866sup1.cif
Rietveld powder data: contains datablocks I. DOI: 10.1107/S1600536809029353/lh2866Isup2.rtv
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N19—H19A⋯O32 | 0.90 | 2.06 | 2.93 (2) | 165 |
| N19—H19B⋯O36 | 0.90 | 2.18 | 3.04 (2) | 159 |
| N9—H9⋯O35i | 0.86 | 1.87 | 2.72 (3) | 168 |
| O18—H18⋯O32ii | 0.82 | 2.42 | 3.15 (2) | 148 |
| O18—H18⋯O35ii | 0.82 | 2.46 | 3.02 (2) | 126 |
| O33—H33⋯O35ii | 0.82 | 1.77 | 2.53 (2) | 153 |
| O34—H34⋯O32iii | 0.82 | 1.87 | 2.58 (2) | 144 |
| O36—H36⋯O22 | 0.85 | 2.34 | 2.887 (15) | 122 |
| O36—H36⋯O29 | 0.85 | 2.00 | 2.80 (2) | 155 |
| C21—H21B⋯O34iii | 0.97 | 2.24 | 2.91 (2) | 125 |
Symmetry codes: (i) 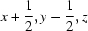 ; (ii)
; (ii) 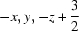 ; (iii)
; (iii) 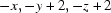 .
.
Acknowledgments
VVC and YAV acknowledge the International Centre for Diffraction Data (ICDD) for supporting this study (GiA 03–06).
supplementary crystallographic information
Comment
Earlier, the crystal structures of two polymorphs of carvedilol free base have been reported (Chen et al., 1998; Yathirajan et al., 2007). Herein we report the crystal structure of the title compound (I), also known as carvedilol dihydrogen phosphate hemihydrate, Form I (Brook et al., 2005).
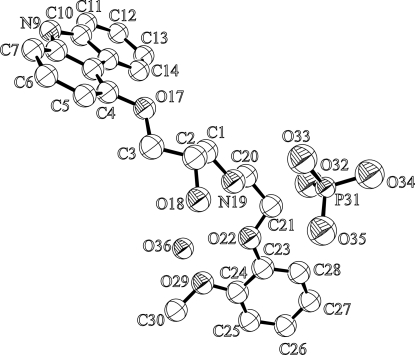
In (I) (Fig. 1), all bond lengths and angles in the cation are comparable with those reported earlier for two monoclinic polymorphs of carvedilol free base (Chen et al., 1998; Yathirajan et al., 2007). The mean planes of tricycle and benzene ring form a dihedral angle of 87.2 (2)°. The crystalline water molecule is situated on a twofold rotational axis linking two cations via O—H···O and N—H···O hydrogen bonds (Table 1). The anions contribute to formation O—H···O and N—H···O hydrogen bonds (Table 1) between the anions and cations giving rise to three-dimensional hydrogen-bonding network.
Experimental
The title compound was synthesized in accordance with the known procedure, invented by Brook et al. (2005) for Form I.
Refinement
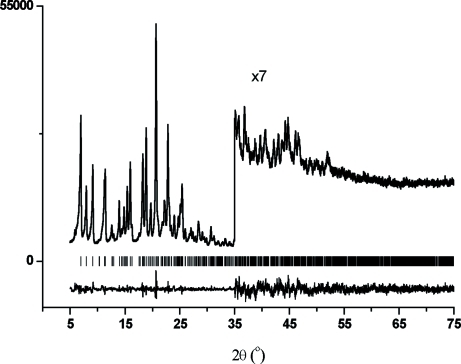
During the exposure, the specimen was spun in its plane to improve particle statistics. The monoclinic unit-cell dimensions were determined with the indexing program ITO (Visser, 1969), M20=35, using the first 30 peak positions. The space group C2/c was chosen on the basis of systematic extinction rules and confirmed later by the crystal structure solution. The structure of (I) was solved by simulated annealing procedure (Zhukov et al., 2001) and refined following the methodology described in details elsewhere (Chernyshev et al., 2003) by the subsequent bond-restrained Rietveld refinement with the program MRIA (Zlokazov & Chernyshev, 1992). All non-H atoms were refined isotropically: two overall Uiso parameters were refined for the cation, and two Uiso parameters were refined for the anion - one for P and one for all O atoms. All H atoms were placed in geometrically calculated positions and not refined. The diffraction profiles and the differences between the measured and calculated profiles are shown in Fig. 2.
Figures
Fig. 1.
The molecular structure of (I) with the atomic numbering and 40% displacement spheres. H atoms are not shown.
Fig. 2.
The Rietveld plot, showing the observed and difference profiles for (I). The reflection positions are shown above the difference profile.
Crystal data
| C24H27N2O4+·H2PO4−·0.5H2O | F(000) = 2168 |
| Mr = 513.47 | Dx = 1.310 Mg m−3 |
| Monoclinic, C2/c | Cu Kα1 radiation, λ = 1.54059 Å |
| Hall symbol: -C 2yc | µ = 1.38 mm−1 |
| a = 26.600 (2) Å | T = 295 K |
| b = 12.3767 (12) Å | Particle morphology: no specific habit |
| c = 16.5101 (15) Å | light grey |
| β = 106.662 (11)° | flat_sheet, 15 × 1 mm |
| V = 5207.2 (8) Å3 | Specimen preparation: Prepared at 295 K and 101 kPa |
| Z = 8 |
Data collection
| Guinier G670 diffractometer | Data collection mode: transmission |
| Radiation source: line-focus sealed tube | Scan method: continuous |
| Curved Germanium (111) | 2θmin = 5.00°, 2θmax = 75.00°, 2θstep = 0.01° |
| Specimen mounting: thin layer in the specimen holder of the camera |
Refinement
| Refinement on Inet | Profile function: split-type pseudo-Voigt (Toraya, 1986) |
| Least-squares matrix: full with fixed elements per cycle | 157 parameters |
| Rp = 0.026 | 125 restraints |
| Rwp = 0.035 | 27 constraints |
| Rexp = 0.014 | H-atom parameters not refined |
| RBragg = 0.064 | Weighting scheme based on measured s.u.'s |
| χ2 = 5.928 | (Δ/σ)max = 0.004 |
| 7001 data points | Background function: Chebyshev polynomial up to the 5th order |
| Excluded region(s): none | Preferred orientation correction: March-Dollase (Dollase, 1986); direction of preferred orientation 100, texture parameter r = 1.038(5) |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.1090 (10) | 0.7439 (16) | 0.8662 (14) | 0.096 (9)* | |
| H1A | 0.1218 | 0.6723 | 0.8585 | 0.115* | |
| H1B | 0.1339 | 0.7771 | 0.9146 | 0.115* | |
| C2 | 0.1044 (9) | 0.8122 (14) | 0.7871 (12) | 0.096 (9)* | |
| H2 | 0.1110 | 0.8888 | 0.8012 | 0.115* | |
| C3 | 0.1405 (11) | 0.7694 (13) | 0.7371 (13) | 0.096 (9)* | |
| H3A | 0.1289 | 0.6980 | 0.7153 | 0.115* | |
| H3B | 0.1386 | 0.8167 | 0.6895 | 0.115* | |
| C4 | 0.2317 (10) | 0.7415 (15) | 0.7495 (11) | 0.078 (7)* | |
| C5 | 0.2335 (8) | 0.7825 (16) | 0.6713 (14) | 0.078 (7)* | |
| H5 | 0.2069 | 0.8273 | 0.6402 | 0.094* | |
| C6 | 0.2761 (11) | 0.7553 (13) | 0.6398 (12) | 0.078 (7)* | |
| H6 | 0.2745 | 0.7766 | 0.5851 | 0.094* | |
| C7 | 0.3201 (9) | 0.6987 (17) | 0.6859 (15) | 0.078 (7)* | |
| H7 | 0.3502 | 0.6922 | 0.6684 | 0.094* | |
| C8 | 0.3156 (9) | 0.6522 (16) | 0.7609 (13) | 0.078 (7)* | |
| N9 | 0.3515 (8) | 0.5885 (13) | 0.8183 (10) | 0.078 (7)* | |
| H9 | 0.3797 | 0.5622 | 0.8107 | 0.094* | |
| C10 | 0.3351 (9) | 0.5734 (15) | 0.8899 (14) | 0.078 (7)* | |
| C11 | 0.3572 (11) | 0.5123 (16) | 0.9629 (15) | 0.078 (7)* | |
| H11 | 0.3890 | 0.4766 | 0.9703 | 0.094* | |
| C12 | 0.3304 (12) | 0.5062 (17) | 1.0244 (15) | 0.078 (7)* | |
| H12 | 0.3454 | 0.4682 | 1.0741 | 0.094* | |
| C13 | 0.2814 (10) | 0.5561 (14) | 1.0128 (12) | 0.078 (7)* | |
| H13 | 0.2646 | 0.5522 | 1.0550 | 0.094* | |
| C14 | 0.2580 (9) | 0.6116 (13) | 0.9379 (14) | 0.078 (7)* | |
| H14 | 0.2243 | 0.6393 | 0.9280 | 0.094* | |
| C15 | 0.2854 (11) | 0.6256 (15) | 0.8773 (13) | 0.078 (7)* | |
| C16 | 0.2737 (10) | 0.6768 (16) | 0.7952 (12) | 0.078 (7)* | |
| O17 | 0.1930 (7) | 0.7639 (11) | 0.7893 (9) | 0.078 (7)* | |
| O18 | 0.0509 (8) | 0.7940 (12) | 0.7362 (9) | 0.096 (9)* | |
| H18 | 0.0479 | 0.8342 | 0.6957 | 0.144* | |
| N19 | 0.0560 (8) | 0.7353 (13) | 0.8820 (11) | 0.096 (9)* | |
| H19A | 0.0435 | 0.8022 | 0.8851 | 0.115* | |
| H19B | 0.0337 | 0.7015 | 0.8379 | 0.115* | |
| C20 | 0.0576 (10) | 0.6750 (16) | 0.9617 (14) | 0.096 (9)* | |
| H20A | 0.0807 | 0.7124 | 1.0098 | 0.115* | |
| H20B | 0.0718 | 0.6032 | 0.9592 | 0.115* | |
| C21 | 0.0031 (11) | 0.6657 (15) | 0.9737 (12) | 0.096 (9)* | |
| H21A | 0.0051 | 0.6343 | 1.0283 | 0.115* | |
| H21B | −0.0133 | 0.7362 | 0.9701 | 0.115* | |
| O22 | −0.0260 (7) | 0.5974 (11) | 0.9071 (8) | 0.096 (9)* | |
| C23 | −0.0747 (10) | 0.5587 (16) | 0.9079 (13) | 0.096 (9)* | |
| C24 | −0.0925 (12) | 0.4644 (14) | 0.8587 (14) | 0.096 (9)* | |
| C25 | −0.1401 (9) | 0.4201 (15) | 0.8616 (15) | 0.096 (9)* | |
| H25 | −0.1524 | 0.3577 | 0.8309 | 0.115* | |
| C26 | −0.1699 (10) | 0.4678 (16) | 0.9099 (12) | 0.096 (9)* | |
| H26 | −0.2032 | 0.4412 | 0.9062 | 0.115* | |
| C27 | −0.1502 (9) | 0.5542 (17) | 0.9631 (14) | 0.096 (9)* | |
| H27 | −0.1677 | 0.5796 | 1.0006 | 0.115* | |
| C28 | −0.1033 (11) | 0.6023 (14) | 0.9590 (12) | 0.096 (9)* | |
| H28 | −0.0911 | 0.6640 | 0.9907 | 0.115* | |
| O29 | −0.0584 (8) | 0.4261 (12) | 0.8162 (9) | 0.096 (9)* | |
| C30 | −0.0784 (11) | 0.3728 (16) | 0.7357 (14) | 0.096 (9)* | |
| H30A | −0.0497 | 0.3514 | 0.7150 | 0.144* | |
| H30B | −0.1008 | 0.4215 | 0.6961 | 0.144* | |
| H30C | −0.0981 | 0.3101 | 0.7424 | 0.144* | |
| P31 | −0.0045 (5) | 1.0448 (8) | 0.8733 (7) | 0.063 (6)* | |
| O32 | 0.0094 (8) | 0.9380 (12) | 0.9182 (10) | 0.124 (11)* | |
| O33 | 0.0409 (8) | 1.0822 (11) | 0.8416 (11) | 0.124 (11)* | |
| H33 | 0.0373 | 1.0524 | 0.7960 | 0.186* | |
| O34 | −0.0127 (8) | 1.1325 (13) | 0.9331 (10) | 0.124 (11)* | |
| H34 | −0.0244 | 1.1016 | 0.9678 | 0.186* | |
| O35 | −0.0536 (9) | 1.0330 (11) | 0.7999 (9) | 0.124 (11)* | |
| O36 | 0.0000 | 0.5716 (11) | 0.7500 | 0.076 (7)* | |
| H36 | −0.0103 | 0.5310 | 0.7836 | 0.114* |
Geometric parameters (Å, °)
| P31—O35 | 1.51 (2) | C12—C13 | 1.40 (4) |
| P31—O34 | 1.52 (2) | C13—C14 | 1.40 (3) |
| P31—O33 | 1.52 (2) | C14—C15 | 1.41 (3) |
| P31—O32 | 1.509 (18) | C15—C16 | 1.45 (3) |
| O17—C4 | 1.40 (3) | C20—C21 | 1.52 (4) |
| O17—C3 | 1.42 (3) | C23—C28 | 1.40 (3) |
| O18—C2 | 1.45 (3) | C23—C24 | 1.42 (3) |
| O22—C23 | 1.38 (3) | C24—C25 | 1.39 (4) |
| O22—C21 | 1.43 (2) | C25—C26 | 1.41 (3) |
| O29—C24 | 1.38 (3) | C26—C27 | 1.39 (3) |
| O29—C30 | 1.44 (3) | C27—C28 | 1.40 (4) |
| O18—H18 | 0.82 | C1—H1A | 0.97 |
| O33—H33 | 0.82 | C1—H1B | 0.97 |
| O34—H34 | 0.82 | C2—H2 | 0.98 |
| O36—H36 | 0.85 | C3—H3A | 0.97 |
| O36—H36i | 0.85 | C3—H3B | 0.97 |
| N9—C10 | 1.39 (3) | C5—H5 | 0.93 |
| N9—C8 | 1.38 (3) | C6—H6 | 0.93 |
| N19—C1 | 1.51 (3) | C7—H7 | 0.93 |
| N19—C20 | 1.50 (3) | C11—H11 | 0.93 |
| N9—H9 | 0.86 | C12—H12 | 0.93 |
| N19—H19A | 0.90 | C13—H13 | 0.93 |
| N19—H19B | 0.90 | C14—H14 | 0.93 |
| C1—C2 | 1.53 (3) | C20—H20B | 0.97 |
| C2—C3 | 1.53 (3) | C20—H20A | 0.97 |
| C4—C16 | 1.41 (3) | C21—H21B | 0.97 |
| C4—C5 | 1.40 (3) | C21—H21A | 0.97 |
| C5—C6 | 1.42 (4) | C25—H25 | 0.93 |
| C6—C7 | 1.39 (3) | C26—H26 | 0.93 |
| C7—C8 | 1.40 (3) | C27—H27 | 0.93 |
| C8—C16 | 1.42 (4) | C28—H28 | 0.93 |
| C10—C15 | 1.43 (4) | C30—H30A | 0.96 |
| C10—C11 | 1.40 (3) | C30—H30B | 0.96 |
| C11—C12 | 1.40 (4) | C30—H30C | 0.96 |
| O34—P31—O35 | 109.7 (13) | O29—C24—C25 | 128.2 (18) |
| O32—P31—O35 | 110.0 (11) | C24—C25—C26 | 121.3 (19) |
| O32—P31—O33 | 109.2 (13) | C25—C26—C27 | 121 (2) |
| O32—P31—O34 | 111.5 (11) | C26—C27—C28 | 118 (2) |
| O33—P31—O34 | 106.4 (12) | C23—C28—C27 | 120.8 (19) |
| O33—P31—O35 | 110.0 (12) | N19—C1—H1B | 109.77 |
| C3—O17—C4 | 117.0 (16) | N19—C1—H1A | 109.78 |
| C21—O22—C23 | 120.0 (18) | H1A—C1—H1B | 108.18 |
| C24—O29—C30 | 120 (2) | C2—C1—H1A | 109.73 |
| C2—O18—H18 | 103.23 | C2—C1—H1B | 109.71 |
| P31—O33—H33 | 106.53 | C1—C2—H2 | 111.50 |
| P31—O34—H34 | 105.88 | C3—C2—H2 | 111.46 |
| H36—O36—H36i | 107.52 | O18—C2—H2 | 111.57 |
| C8—N9—C10 | 110 (2) | O17—C3—H3A | 109.57 |
| C1—N19—C20 | 113.1 (18) | C2—C3—H3A | 109.48 |
| C10—N9—H9 | 125.12 | C2—C3—H3B | 109.48 |
| C8—N9—H9 | 125.17 | O17—C3—H3B | 109.59 |
| C1—N19—H19B | 108.95 | H3A—C3—H3B | 108.18 |
| H19A—N19—H19B | 107.79 | C4—C5—H5 | 120.24 |
| C20—N19—H19B | 108.92 | C6—C5—H5 | 120.11 |
| C1—N19—H19A | 108.90 | C7—C6—H6 | 118.16 |
| C20—N19—H19A | 109.00 | C5—C6—H6 | 118.07 |
| N19—C1—C2 | 109.6 (19) | C6—C7—H7 | 122.62 |
| C1—C2—C3 | 111.1 (17) | C8—C7—H7 | 122.59 |
| O18—C2—C1 | 103.5 (18) | C10—C11—H11 | 120.69 |
| O18—C2—C3 | 107.3 (16) | C12—C11—H11 | 120.78 |
| O17—C3—C2 | 110.5 (16) | C11—C12—H12 | 119.21 |
| O17—C4—C16 | 116.1 (17) | C13—C12—H12 | 119.26 |
| C5—C4—C16 | 118 (2) | C12—C13—H13 | 120.07 |
| O17—C4—C5 | 125.8 (19) | C14—C13—H13 | 120.07 |
| C4—C5—C6 | 120 (2) | C13—C14—H14 | 120.01 |
| C5—C6—C7 | 123.8 (19) | C15—C14—H14 | 119.97 |
| C6—C7—C8 | 115 (2) | C21—C20—H20A | 109.42 |
| N9—C8—C16 | 108.4 (18) | C21—C20—H20B | 109.44 |
| C7—C8—C16 | 123 (2) | H20A—C20—H20B | 107.96 |
| N9—C8—C7 | 128 (2) | N19—C20—H20A | 109.38 |
| N9—C10—C11 | 131 (2) | N19—C20—H20B | 109.33 |
| N9—C10—C15 | 108.6 (18) | O22—C21—H21A | 110.63 |
| C11—C10—C15 | 121 (2) | C20—C21—H21B | 110.63 |
| C10—C11—C12 | 119 (2) | O22—C21—H21B | 110.54 |
| C11—C12—C13 | 122 (2) | C20—C21—H21A | 110.55 |
| C12—C13—C14 | 120 (2) | H21A—C21—H21B | 108.76 |
| C13—C14—C15 | 120 (2) | C26—C25—H25 | 119.37 |
| C10—C15—C16 | 106 (2) | C24—C25—H25 | 119.29 |
| C14—C15—C16 | 135 (2) | C25—C26—H26 | 119.69 |
| C10—C15—C14 | 119.1 (19) | C27—C26—H26 | 119.59 |
| C4—C16—C8 | 120.1 (18) | C26—C27—H27 | 120.79 |
| C4—C16—C15 | 133 (2) | C28—C27—H27 | 120.75 |
| C8—C16—C15 | 107 (2) | C27—C28—H28 | 119.57 |
| N19—C20—C21 | 111.3 (19) | C23—C28—H28 | 119.65 |
| O22—C21—C20 | 105.7 (18) | O29—C30—H30B | 109.47 |
| O22—C23—C28 | 123.0 (19) | O29—C30—H30C | 109.44 |
| C24—C23—C28 | 121 (2) | H30A—C30—H30C | 109.53 |
| O22—C23—C24 | 116 (2) | H30B—C30—H30C | 109.45 |
| C23—C24—C25 | 117 (2) | H30A—C30—H30B | 109.43 |
| O29—C24—C23 | 114 (2) | O29—C30—H30A | 109.50 |
Symmetry codes: (i) −x, y, −z+3/2.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N19—H19A···O32 | 0.90 | 2.06 | 2.93 (2) | 165 |
| N19—H19B···O36 | 0.90 | 2.18 | 3.04 (2) | 159 |
| N9—H9···O35ii | 0.86 | 1.87 | 2.72 (3) | 168 |
| O18—H18···O32i | 0.82 | 2.42 | 3.15 (2) | 148 |
| O18—H18···O35i | 0.82 | 2.46 | 3.02 (2) | 126 |
| O33—H33···O35i | 0.82 | 1.77 | 2.53 (2) | 153 |
| O34—H34···O32iii | 0.82 | 1.87 | 2.58 (2) | 144 |
| O36—H36···O22 | 0.85 | 2.34 | 2.887 (15) | 122 |
| O36—H36···O29 | 0.85 | 2.00 | 2.80 (2) | 155 |
| C21—H21B···O34iii | 0.97 | 2.24 | 2.91 (2) | 125 |
Symmetry codes: (ii) x+1/2, y−1/2, z; (i) −x, y, −z+3/2; (iii) −x, −y+2, −z+2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LH2866).
References
- Brook, C. S., Chen, W., Dell’Orco, P. C., Katrincic, L. M., Lovet, A. M., Oh, C. K., Spoors, P. G. & Werner, C. (2005). US Patent No. 20050240027A1.
- Chen, W.-M., Zeng, L.-M., Yu, K.-B. & Xu, J.-H. (1998). Jiegou Huaxue, 17, 325–328.
- Chernyshev, V. V., Machon, D., Fitch, A. N., Zaitsev, S. A., Yatsenko, A. V., Shmakov, A. N. & Weber, H.-P. (2003). Acta Cryst. B59, 787–793. [DOI] [PubMed]
- Dollase, W. A. (1986). J. Appl. Cryst.19, 267–272.
- Huber (2002). G670 Imaging Plate Guinier Camera Software Huber Diffraktionstechnik GmbH, Rimsting, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Toraya, H. (1986). J. Appl. Cryst.19, 440–447.
- Visser, J. W. (1969). J. Appl. Cryst.2, 89–95.
- Yathirajan, H. S., Bindya, S., Sreevidya, T. V., Narayana, B. & Bolte, M. (2007). Acta Cryst. E63, o542–o544.
- Zhukov, S. G., Chernyshev, V. V., Babaev, E. V., Sonneveld, E. J. & Schenk, H. (2001). Z. Kristallogr. 216, 5–9.
- Zlokazov, V. B. & Chernyshev, V. V. (1992). J. Appl. Cryst.25, 447–451.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809029353/lh2866sup1.cif
Rietveld powder data: contains datablocks I. DOI: 10.1107/S1600536809029353/lh2866Isup2.rtv
Additional supplementary materials: crystallographic information; 3D view; checkCIF report