Abstract
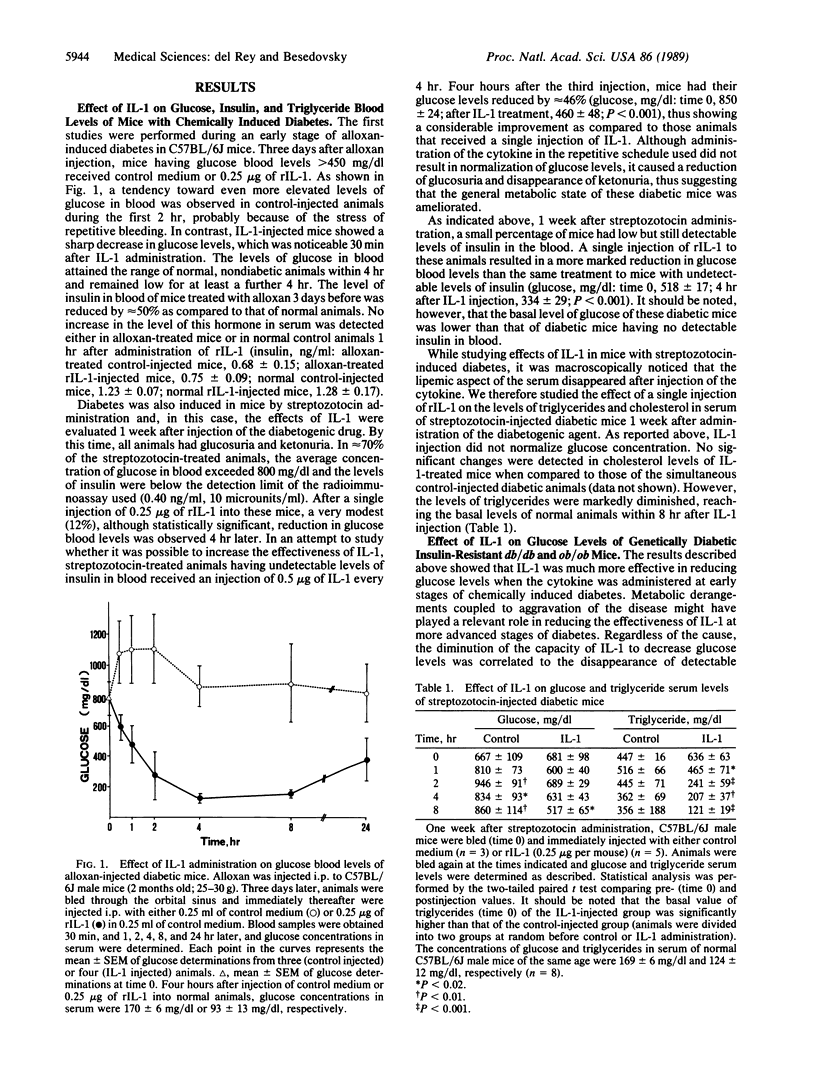
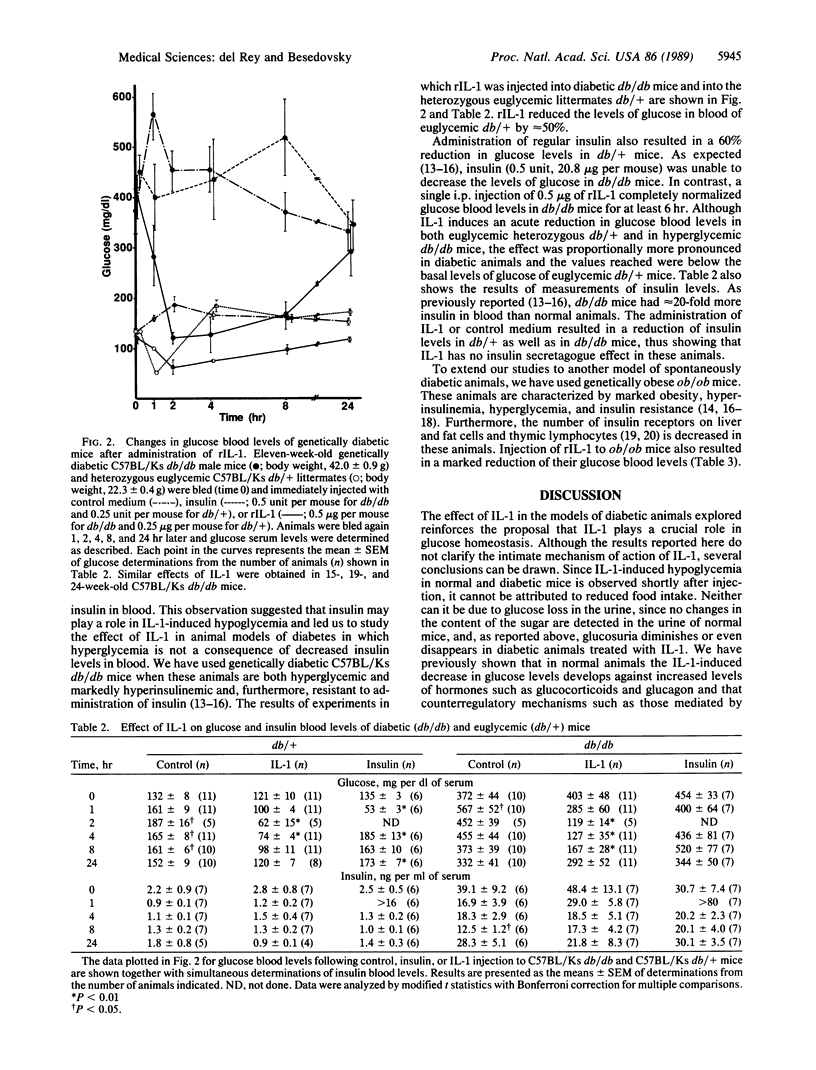
Interleukin 1 (IL-1), a cytokine released mainly by activated macrophages-monocytes, affects glucose homeostasis and may mediate some of the metabolic derangements observed during certain inflammatory and infectious processes. In this report, it is shown that IL-1 acts as a hypoglycemic agent not only in normal animals but also in mice at early stages of alloxan-induced diabetes and in genetically diabetic, insulin-resistant C57BL/Ks db/db mice and C57BL/6J ob/ob mice. In these animal models, a single injection of a low dose of human recombinant IL-1 normalized glucose blood levels for several hours. This effect was not mediated by possible insulin secretagogue actions of the cytokine. Furthermore, IL-1 markedly reduced the levels of triglycerides in blood of streptozotocin-induced diabetic mice at later stages of the disease. Although the final mechanism of action is at present unknown, the results showed that IL-1 is a hormone with powerful antidiabetic properties. Defective production of this cytokine associated with diabetes could contribute to aggravate the course of the disease during infectious and inflammatory processes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abstracts and index of the 19th annual meeting of the European Society for Clinical Investigation, April 24-27, Toulouse, France. Eur J Clin Invest. 1985 Apr;15(2 Pt 2):A1–72. [PubMed] [Google Scholar]
- Attallah A. M., Abdelghaffar H., Fawzy A., Alghraoui F., Alijani M. R., Mahmoud L. A., Ghoneim M. A., Helfrich G. B. Cell-mediated immunity and biological response modifiers in insulin-dependent diabetes mellitus complicated by end-stage renal disease. Int Arch Allergy Appl Immunol. 1987;83(3):278–283. doi: 10.1159/000234308. [DOI] [PubMed] [Google Scholar]
- Beisel W. R. Magnitude of the host nutritional responses to infection. Am J Clin Nutr. 1977 Aug;30(8):1236–1247. doi: 10.1093/ajcn/30.8.1236. [DOI] [PubMed] [Google Scholar]
- Bendtzen K., Mandrup-Poulsen T., Nerup J., Nielsen J. H., Dinarello C. A., Svenson M. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science. 1986 Jun 20;232(4757):1545–1547. doi: 10.1126/science.3086977. [DOI] [PubMed] [Google Scholar]
- Berkenbosch F., van Oers J., del Rey A., Tilders F., Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987 Oct 23;238(4826):524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- Bernton E. W., Beach J. E., Holaday J. W., Smallridge R. C., Fein H. G. Release of multiple hormones by a direct action of interleukin-1 on pituitary cells. Science. 1987 Oct 23;238(4826):519–521. doi: 10.1126/science.2821620. [DOI] [PubMed] [Google Scholar]
- Besedovsky H., del Rey A., Sorkin E., Dinarello C. A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986 Aug 8;233(4764):652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- Chandy K. G., Charles A. M., Kershnar A., Buckingham B., Waldeck N., Gupta S. Autologous mixed lymphocyte reaction in man: XV. Cellular and molecular basis of deficient autologous mixed lymphocyte response in insulin-dependent diabetes mellitus. J Clin Immunol. 1984 Nov;4(6):424–428. doi: 10.1007/BF00916571. [DOI] [PubMed] [Google Scholar]
- Coleman D. L., Hummel K. P. Effects of parabiosis of normal with genetically diabetic mice. Am J Physiol. 1969 Nov;217(5):1298–1304. doi: 10.1152/ajplegacy.1969.217.5.1298. [DOI] [PubMed] [Google Scholar]
- Coleman D. L., Hummel K. P. Studies with the mutation, diabetes, in the mouse. Diabetologia. 1967 Apr;3(2):238–248. doi: 10.1007/BF01222201. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. An update on human interleukin-1: from molecular biology to clinical relevance. J Clin Immunol. 1985 Sep;5(5):287–297. doi: 10.1007/BF00918247. [DOI] [PubMed] [Google Scholar]
- Filkins J. P. Endotoxin-enhanced secretion of macrophage insulin-like activity. J Reticuloendothel Soc. 1980 May;27(5):507–511. [PubMed] [Google Scholar]
- Genuth S. M., Przybylski R. J., Rosenberg D. M. Insulin resistance in genetically obese, hyperglycemic mice. Endocrinology. 1971 May;88(5):1230–1238. doi: 10.1210/endo-88-5-1230. [DOI] [PubMed] [Google Scholar]
- Herberg L., Coleman D. L. Laboratory animals exhibiting obesity and diabetes syndromes. Metabolism. 1977 Jan;26(1):59–99. doi: 10.1016/0026-0495(77)90128-7. [DOI] [PubMed] [Google Scholar]
- Hill M. R., Stith R. D., McCallum R. E. Human recombinant IL-1 alters glucocorticoid receptor function in Reuber hepatoma cells. J Immunol. 1988 Sep 1;141(5):1522–1528. [PubMed] [Google Scholar]
- Kahn C. R., Neville D. M., Jr, Gorden P., Freychet P., Roth J. Insulin receptor defect in insulin resistance: studies in the obese-hyperglycimic mouse. Biochem Biophys Res Commun. 1972 Jul 11;48(1):135–142. doi: 10.1016/0006-291x(72)90354-3. [DOI] [PubMed] [Google Scholar]
- Kazura J. W., Gandola C., Rodman H. R., Mahmoud A. A. Deficient production of the lymphokine eosinophil stimulation promoter in chemically induced and mutation diabetes mellitus in mice. J Immunol. 1979 Nov;123(5):2114–2117. [PubMed] [Google Scholar]
- Kolterman O. G., Olefsky J. M., Kurahara C., Taylor K. A defect in cell-mediated immune function in insulin-resistant diabetic and obese subjects. J Lab Clin Med. 1980 Sep;96(3):535–543. [PubMed] [Google Scholar]
- MAYER J., BATES M. W., DICKIE M. M. Hereditary diabetes in genetically obese mice. Science. 1951 Jun 29;113(2948):746–747. doi: 10.1126/science.113.2948.746. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Rodman H. M., Mandel M. A., Warren K. S. Induced and spontaneous diabetes mellitus and suppression of cell-mediated immunologic responses. Granuloma formation, delayed dermal reactivity and allograft rejection. J Clin Invest. 1976 Feb;57(2):362–367. doi: 10.1172/JCI108287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M. A., Mahmoud A. A. Impairment of cell-mediated immunity in mutation diabetic mice (db/db). J Immunol. 1978 Apr;120(4):1375–1377. [PubMed] [Google Scholar]
- Nachman R. L., Hajjar K. A., Silverstein R. L., Dinarello C. A. Interleukin 1 induces endothelial cell synthesis of plasminogen activator inhibitor. J Exp Med. 1986 Jun 1;163(6):1595–1600. doi: 10.1084/jem.163.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasko K. L., Salvin S. B., Winkelstein A. Mechanisms in the in vivo release of lymphokines. V. Responses in alloxan-treated and genetically diabetic mice. Cell Immunol. 1981 Jul 15;62(1):205–219. doi: 10.1016/0008-8749(81)90314-2. [DOI] [PubMed] [Google Scholar]
- Renold A. E. Spontaneous diabetes and--or obesity in laboratory rodents. Adv Metab Disord. 1968;3:49–84. [PubMed] [Google Scholar]
- Sandler S., Andersson A., Hellerström C. Inhibitory effects of interleukin 1 on insulin secretion, insulin biosynthesis, and oxidative metabolism of isolated rat pancreatic islets. Endocrinology. 1987 Oct;121(4):1424–1431. doi: 10.1210/endo-121-4-1424. [DOI] [PubMed] [Google Scholar]
- Sapolsky R., Rivier C., Yamamoto G., Plotsky P., Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987 Oct 23;238(4826):522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Soli A. H., Kahn C. R., Neville D. M., Jr, Roth J. Insulin receptor deficiency in genetic and acquired obesity. J Clin Invest. 1975 Oct;56(4):769–780. doi: 10.1172/JCI108155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll A. H., Goldfine I. D., Roth J., Kahn C. R. Thymic lymphocytes in obese (ob-ob) mice. A mirror of the insulin receptor defect in liver and fat. J Biol Chem. 1974 Jul 10;249(13):4127–4131. [PubMed] [Google Scholar]
- Spinas G. A., Mandrup-Poulsen T., Mølvig J., Baek L., Bendtzen K., Dinarello C. A., Nerup J. Low concentrations of interleukin-1 stimulate and high concentrations inhibit insulin release from isolated rat islets of Langerhans. Acta Endocrinol (Copenh) 1986 Dec;113(4):551–558. doi: 10.1530/acta.0.1130551. [DOI] [PubMed] [Google Scholar]
- Uehara A., Gottschall P. E., Dahl R. R., Arimura A. Interleukin-1 stimulates ACTH release by an indirect action which requires endogenous corticotropin releasing factor. Endocrinology. 1987 Oct;121(4):1580–1582. doi: 10.1210/endo-121-4-1580. [DOI] [PubMed] [Google Scholar]
- Wingfield P., Payton M., Tavernier J., Barnes M., Shaw A., Rose K., Simona M. G., Demczuk S., Williamson K., Dayer J. M. Purification and characterization of human interleukin-1 beta expressed in recombinant Escherichia coli. Eur J Biochem. 1986 Nov 3;160(3):491–497. doi: 10.1111/j.1432-1033.1986.tb10066.x. [DOI] [PubMed] [Google Scholar]
- Wogensen L. D., Mandrup-Poulsen T., Markholst H., Mølvig J., Lernmark A., Holst J. J., Dinarello C. A., Nerup J. Interleukin-1 potentiates glucose stimulated insulin release in the isolated perfused pancreas. Acta Endocrinol (Copenh) 1988 Mar;117(3):302–306. doi: 10.1530/acta.0.1170302. [DOI] [PubMed] [Google Scholar]
- Zier K. S., Leo M. M., Spielman R. S., Baker L. Decreased synthesis of interleukin-2 (IL-2) in insulin-dependent diabetes mellitus. Diabetes. 1984 Jun;33(6):552–555. doi: 10.2337/diab.33.6.552. [DOI] [PubMed] [Google Scholar]
- del Rey A., Besedovsky H. Interleukin 1 affects glucose homeostasis. Am J Physiol. 1987 Nov;253(5 Pt 2):R794–R798. doi: 10.1152/ajpregu.1987.253.5.R794. [DOI] [PubMed] [Google Scholar]



