Abstract
Objective
To report rates of amenorrhea and treatment failure after global endometrial ablation and to estimate the association between patient factors and these outcomes by developing and validating prediction models.
Methods
From January 1998 through December 2005, 816 women underwent global endometrial ablation with either a thermal balloon ablation or radiofrequency ablation device; 455 were included in a population-derived cohort (for model development), and 361 were included in a referral-derived cohort (for model validation). Amenorrhea was defined as cessation of bleeding from immediately after ablation through at least 12 months after the procedure. Treatment failure was defined as hysterectomy or re-ablation for patients with bleeding or pain. Logistic and Cox proportional hazard regression models were used in model development and validation of potential predictors of outcomes.
Results
The amenorrhea rate was 23% (95% confidence interval [CI], 19%–28%) and the 5-year cumulative failure rate was 16% (95% CI, 10%–20%). Predictors of amenorrhea were age 45 years or older (adjusted odds ratio [aOR], 2.6; 95% CI, 1.6–4.3); uterine length less than 9 cm (aOR, 1.8; 95% CI, 1.1–3.1); endometrial thickness less than 4 mm (aOR, 2.7; 95% CI, 1.2–6.3); and use of radiofrequency ablation instead of thermal balloon ablation (aOR, 2.8; 95% CI, 1.7–4.9). Predictors of treatment failure included age younger than 45 years (adjusted hazard ratio [aHR], 2.6; 95% CI, 1.3–5.1); parity of 5 or greater (aHR, 6.0; 95% CI, 2.5–14.8); prior tubal ligation (aHR, 2.2; 95% CI, 1.2–4.0); and history of dysmenorrhea (aHR, 3.7; 95% CI, 1.6–8.5). After global endometrial ablation, 23 women (5.1%; 95% CI, 3.2%–7.5%) had pelvic pain, 3 (0.7%; 95% CI, 0.1%–1.9%) were pregnant, and none (95% CI, 0%–0.8%) had endometrial cancer.
Conclusion
Population-derived rates and predictors of treatment outcomes after global endometrial ablation may help physicians offer optimal preprocedural patient counseling.
Background
Excessive menstrual bleeding is a common problem that adversely affects women’s health and health resources worldwide (1). Endometrial ablation (EA), a less invasive alternative to hysterectomy, aims to treat menorrhagia by selectively destroying the endometrium while preserving the uterus (2). Early EA procedures were hysteroscopy dependent, required surgical dexterity, and were associated with clinically significant complications such as uterine perforation and fluid overload in 3% of patients (3,4). Recently, newer global endometrial ablation (GEA) procedures have been introduced, and because of their ease of use and better safety profiles, they gradually have taken the place of older EA procedures. To date, the US Food and Drug Administration has approved 5 GEA technologies: thermal balloon ablation (TBA), cryoablation, circulated hot fluid ablation, microwave ablation, and bipolar radiofrequency ablation (RFA) (5).
Compared with hysterectomy, GEA initially showed similar efficacy with lower cost and complication rates; however, these favorable outcomes appeared to diminish with time because 30% of patients required hysterectomy within 4 years after ablation (6). By identifying predictors of treatment outcomes after GEA, patient counseling could improve and failure rates could decrease with better patient selection (7). Nevertheless, few studies have examined predictors of treatment failure (8,9), and these studies used inconsistent definitions of treatment failure and were not population derived. They also were limited by low statistical power (from relatively small sample sizes) and thus failed to identify valid independent predictors of treatment outcomes. The objectives of our study were to determine population-derived rates of amenorrhea and treatment failure after GEA and to estimate the association between patient factors and these outcomes by developing and validating predicting models.
Methods
This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. The manuscript was written in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement (10).
Participants
Only women who gave consent to use their medical records in research were included in the analysis. The study established 2 independent patient cohorts. The model development cohort included women who underwent GEA from January 1, 1998, through December 31, 2005, and resided in Olmsted County, Minnesota. Data from this population-derived cohort were used to develop prediction models for amenorrhea and treatment failure after GEA. A model validation cohort was constructed from referred patients who underwent GEA during the same period at Mayo Clinic but resided outside Olmsted County.
The model development cohort was constructed using data from the Rochester Epidemiology Project. The Rochester Epidemiology Project maintains a unique medical records linkage system that encompasses all health care delivered to residents of Olmsted County. Mayo Clinic and the Olmsted Medical Center provide primary care and comprehensive care in virtually every medical specialty, and more than 83% of the county’s population is examined at least once per year in one of these facilities (11). The Rochester Epidemiology Project medical records system was used to identify all women who underwent GEA by searching for the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 68.23 for EA (12). The model validation cohort was identified by the electronic medical records linkage system at Mayo Clinic by searching for the same ICD-9-CM code. Verification of the retrieved cases in both cohorts was conducted by chart review.
Outcomes
The main outcome measures were amenorrhea and treatment failure after GEA. Amenorrhea was defined as cessation of bleeding from immediately after ablation through at least 12 months after the procedure. Treatment failure was defined as bleeding or pain after GEA that required performance of hysterectomy or re-ablation. Time to treatment failure was measured for patients who underwent hysterectomy or re-ablation. For women who did not have this outcome, their duration of follow-up was censored at the time of their last contact or death before the end of the study period (December 31, 2006).
Reliability of the outcome categorization was evaluated by a randomly selected sample of 50 women using the random row selection function of JMP software, version 6.0 (SAS Institute Inc, Cary, North Carolina, United States). Random row selection is a function in JMP that allows random selection of patients from a given file of patients in which each patient is entered on a separate row. Outcomes were assigned by one of the investigators (M.R.H.), who was masked to the outcome determined by the initial chart review. The agreement in evaluation of treatment failure between the masked investigator and the initial chart review was 100% (κ=1.0; 95% confidence interval [CI], 0.9–1.0), and the agreement in measuring amenorrhea was 86% (κ=0.7; 95% CI, 0.5–0.9). This was consistent with the “almost perfect” and “substantial” observer agreement for treatment failure and amenorrhea, respectively, according to Landis and Koch (13).
Measurements
The 2 GEA methods used in Olmsted County during the study period were TBA (ThermaChoice; Gynecare, Somerville, New Jersey) and RFA (NovaSure; Cytyc Surgical Products, Palo Alto, California). Women were offered the procedure if an initial trial of medical therapy for menorrhagia had failed or if medical therapy was contraindicated and they had met the Food and Drug Administration–approved inclusion criteria for GEA. Before GEA, all women had a thorough clinical examination, a Papanicolaou test, endometrial sampling, pelvic ultrasonography, and office hysteroscopy if structural uterine lesions were suspected. Only women with benign polyps or submucous leiomyomas not distorting the endometrial cavity or less than 2 cm in size were offered EA; removal was by dilation and curettage (D&C) or ablation in situ. As is consistent with standard GEA practice, women did not routinely receive hormonal or surgical pretreatment of the endometrium.
Independent Variables
Preoperative data were obtained from patient records. History of cesarean deliveries and tubal ligation was documented. We also collected data about preoperative characteristics, procedure-related complications, and main outcome measures. To minimize measurement bias when evaluating complications during the chart review, an independent ICD-9 code search for postprocedural endometrial cancer, pregnancy, and pelvic pain was conducted. Variables were categorized for ease of interpretation. Age was categorized by using a threshold value of 45 years (8). Patients were classified as obese or not on the basis of body mass index (obesity was defined as body mass index ≥30 kg/m2) (14). A parity threshold of 5 (the definition of grand multiparity) was used in the treatment failure model, and a parity threshold of 2 (the median parity of the study population) was used in the amenorrhea model. A 4-mm threshold for endometrial thickness was selected (9). The threshold hemoglobin value for anemia was 12 g/dL (15). For duration of bleeding, we used the median duration of 7 days as a threshold value. For uterine length, we used the median of 9 cm as the threshold value.
Statistical Methods
In the amenorrhea model, associations between amenorrhea (binary end point) and preoperative variables were evaluated by fitting logistic regression models and summarized by odds ratios (ORs) and corresponding 95% CIs. In the treatment failure model, the Kaplan-Meier method was used to estimate the cumulative treatment failure rates and corresponding 95% CIs. Cox proportional hazard models were fit to evaluate associations between demographic and preoperative variables and outcomes. Each association was summarized by a hazard ratio (HR) and corresponding 95% CI from the model estimates.
For each outcome, each preoperative variable was initially evaluated in a univariable model. Variables with a P value less than .20 in the univariable analysis were considered in multivariable modeling (16). The multivariable model was derived using backward and stepwise variable selection procedures, and variables with a P value less than .05 in the final model were retained. The type of GEA procedure performed was included in the final multivariable model, regardless of its significance in the univariable model, to adjust for any methodologic differences. Two-way interactions between the different variables in the final model were evaluated. For the variables considered in the treatment failure model, the proportional hazards assumption was assessed separately for each variable using the scaled Schoenfeld residual method (17). This method allows evaluation of whether the covariate effect is constant over the follow-up time by visually assessing a plot of the scaled residuals versus time and by examining their correlation. The discriminative ability of the final models was summarized using a concordance statistic (c index) (18). A value of 1.0 for the c index indicates that the factors in the model perfectly separate patients with different outcomes, while a value of 0.5 indicates that the factors in the model contain predictive information equal to that obtained by chance alone.
All statistical analyses were 2-sided, and P values less than .05 were considered statistically significant. The statistical analysis was performed using SAS software, version 8.2 (SAS Institute Inc).
Results
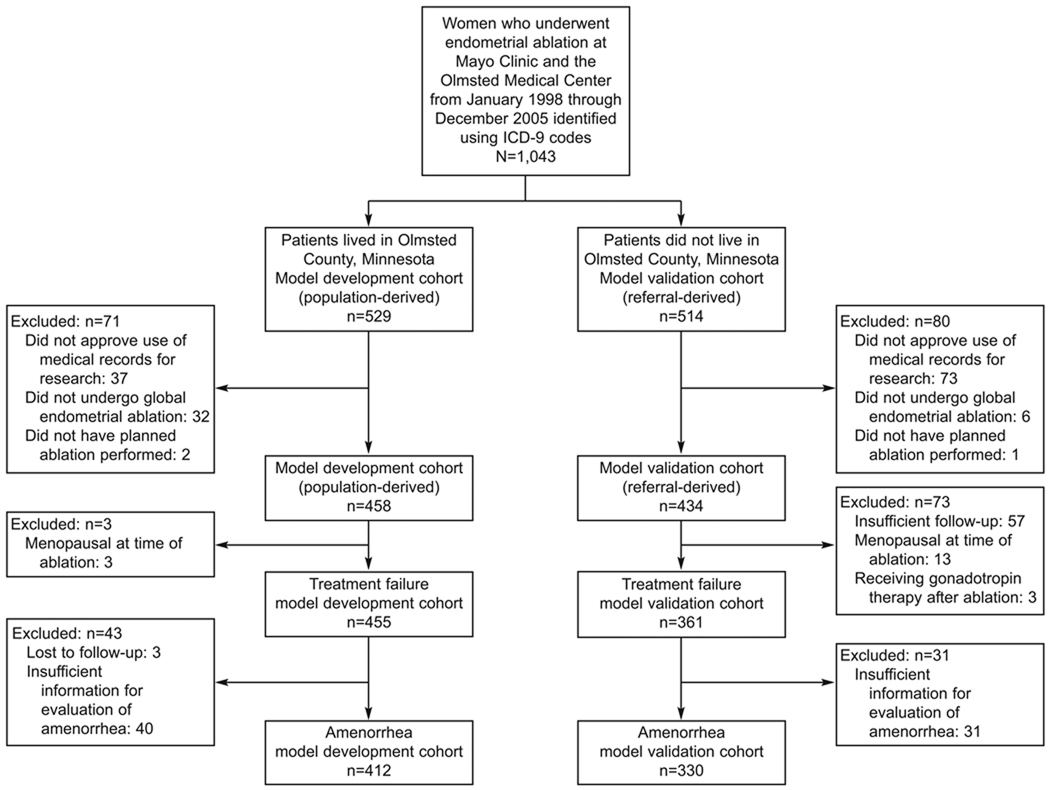
The women included in the study (N=816) underwent GEA from January 1, 1998, through December 31, 2005. Patients were divided into 2 groups: 455 were in the model development cohort and 361 were in the model validation cohort (Figure 1). The baseline characteristics of both cohorts are shown in Table 1.
Figure 1.
Study flow chart shows distribution of study patients in the model development and model validation cohorts.
Table 1.
Baseline Patient Characteristics (N=816)
| Model Development (Population-Derived) Cohort(n=455) |
Model Validation (Referral-Derived)Cohort(n=361) |
||||||
|---|---|---|---|---|---|---|---|
| Patients With Available Data |
Patients With Available Data |
||||||
| Characteristic | Valuea | No. | % | Valuea | No. | % | P Value |
| Age, y | 43.2±5.5 | 455 | 100 | 43.0±5.4 | 361 | 100 | .43 |
| Body mass index, kg/m2 | 29.4±7.5 | 411 | 90 | 29.6±7.8 | 334 | 93 | .69 |
| Parity, No. | 2.0 (2.0–3.0) | 448 | 98 | 2.0 (2.0–3.0) | 359 | 99 | .12 |
| Previous cesarean delivery, No. of patients (%) | 74 (16) | 417 | 92 | 78 (22) | 351 | 97 | .12 |
| Tubal ligation, No. of patients (%) | 148 (33) | 455 | 100 | 176 (49) | 358 | 99 | <.001 |
| Preoperative bleeding, d | 9.8±5.9 | 379 | 83 | NA | NA | NA | NA |
| Preoperative accidents or blood clots, No. of patients (%) | 238 (62) | 455 | 100 | NA | NA | NA | NA |
| Metrorrhagia, No. of patients (%) | 217 (48) | 455 | 100 | NA | NA | NA | NA |
| Preoperative dysmenorrhea, No. of patients (%) | 22 (5) | 455 | 100 | 49 (14) | 360 | 99 | <.001 |
| Uterine length, cm | 9.1±1.3 | 434 | 95 | 9.2±1.1 | 344 | 95 | .05 |
| Retroverted uterus, No. of patients (%) | 27 (6) | 455 | 100 | NA | NA | NA | NA |
| Hemoglobin, g/dL | 12.2±1.6 | 439 | 96 | NA | NA | NA | NA |
| Preprocedure US, No. of patients (%) | 349 (77) | 455 | 100 | 195 (54) | 360 | 99 | <.001 |
| Evidence of intramural fibroids, No. of patients (%) | 98 (28) | 349 | 77 | NA | NA | NA | NA |
| Evidence of adenomyosis, No. of patients (%) | 20 (6) | 349 | 77 | 14 (8) | 165 | 46 | .24 |
| Endometrial thickness, mm | 9.2±5.5 | 291 | 64 | 9.2±5.5 | 171 | 47 | .96 |
| Duration of follow-up, y | 2.5 (1.2–3.6) | 455 | 100 | 2.7 (1.7–4.0) | 361 | 100 | .003 |
Abbreviations: IQR, interquartile range; NA, not available; US, ultrasonography.
Categorical data are presented as number of patients (percentage of sample). Continuous data are presented as mean±standard deviation for normally distributed data or median (IQR) for skewed data.
Population-Derived Rates of Amenorrhea and Treatment Failure
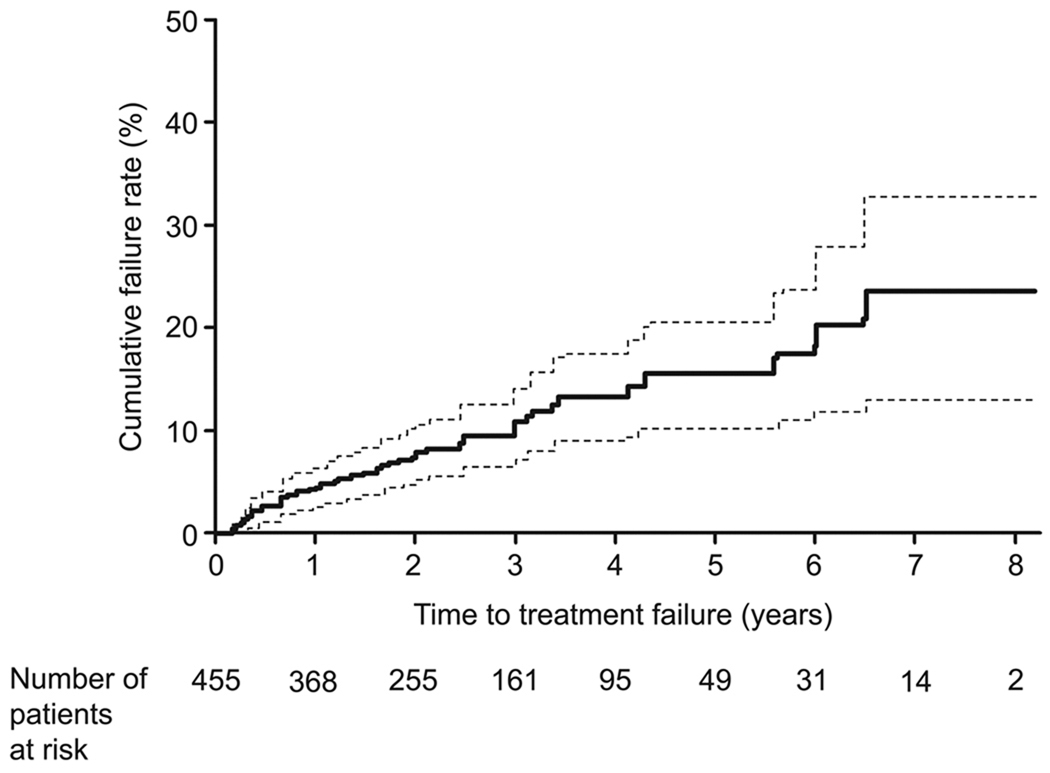
Of the 412 women in the amenorrhea model development cohort, 96 had postablation amenorrhea (23% of patients; 95% CI, 19%–28%). During the 1,201 person-year follow-up, 45 women had hysterectomies and 5 had re-ablations; the 5-year cumulative failure rate was 16% (95% CI, 10%–20%) (Figure 2). All re-ablations were performed to treat persistent bleeding and were considered indications of treatment failure. Forty of 45 hysterectomies met our criteria of treatment failure; 28 were performed for persistent bleeding and 12 for persistent pain. Indications for the remaining 5 hysterectomies were not associated with the GEA procedure (2 benign ovarian masses, 1 uterine prolapse, 1 fibroid uterus, and 1 cervical adenocarcinoma in situ); in the analysis, these data were censored at the time of surgery.
Figure 2.
Cumulative treatment failure rates after global endometrial ablation. Solid line shows the percentage of patients with treatment failure, dotted lines show the boundaries of the 95% confidence interval.
Predictors of Amenorrhea
In the final model, the following preoperative variables were significantly associated with amenorrhea: age 45 years or older (adjusted OR [aOR], 2.6; 95% CI, 1.6–4.3); uterine length less than 9 cm (aOR, 1.8; 95% CI, 1.1–3.1); endometrial thickness less than 4 mm (aOR, 2.7; 95% CI, 1.2–6.3); and use of bipolar RFA (aOR, 2.8; 95% CI, 1.7–4.9) (Table 2). The c index for the final model was 0.706. No significant interactions between variables in the final model were detected. For 67 women in the RFA subgroup, D&C was performed to thin the endometrium before ablation; however, the difference in rates of postablation amenorrhea in those who had D&C and those who did not was not statistically significant (P=.15). Table 3 shows the predicted and observed rates of amenorrhea after applying the final model to the model validation cohort.
Table 2.
Preoperative Predictors of Amenorrhea After Global Endometrial Ablation
| Unadjusted Analyses | Adjusted Analyses (Final Model)a,b | |||||
|---|---|---|---|---|---|---|
| Predictors | Univariable Odds Ratio |
95% CI | P Value | Multivariable Odds Ratio |
95% CI | P Value |
| Age ≥45 y | 2.3 | 1.4–3.6 | <.001 | 2.6 | 1.6–4.3 | <.001 |
| Parity <2 | 1.3 | 0.8–2.4 | .29 | |||
| Body mass index ≥30 kg/m2 | 1.2 | 0.8–2.0 | .38 | |||
| Previous cesarean delivery | 0.8 | 0.4–1.5 | .56 | |||
| Tubal ligation | 1.0 | 0.6–1.7 | .85 | |||
| Preoperative bleeding <7 d | 1.0 | 0.6–1.8 | .90 | |||
| Preoperative menstrual accidents | 1.1 | 0.7–1.8 | .70 | |||
| Regular bleeding | 1.2 | 0.8–1.9 | .39 | |||
| Preoperative dysmenorrhea | 1.3 | 0.5–3.5 | .56 | |||
| Uterine length <9 cm | 1.6 | 1.0–2.5 | .05 | 1.8 | 1.1–3.1 | .02 |
| Retroverted uterus | 0.5 | 0.1–1.6 | .21 | |||
| Hemoglobin ≥11.5 g/dL | 2.1 | 1.1–4.0 | .02 | |||
| Ultrasound suggestive of adenomyosis | 1.7 | 0.6–4.8 | .30 | |||
| Endometrial thickness <4 mm | 2.7 | 1.2–5.9 | .014 | 2.7 | 1.2–6.3 | .02 |
| Uterine cavity lesion (hysteroscopy) | 1.3 | 0.8–2.1 | .32 | |||
| Uterine polyp | 1.5 | 0.9–2.5 | .12 | |||
| Submucous fibroid | 2.3 | 1.1–4.6 | .02 | |||
| Nonsubmucous fibroid | 1.3 | 0.8–2.3 | .29 | |||
| Radiofrequency ablation vs thermal balloon ablation | 2.9 | 1.8–4.9 | <.001 | 2.8 | 1.7–4.9 | <.001 |
Abbreviation: CI, confidence interval.
The c index of this model was 0.706.
If the variable had a P value less than .20 in the unadjusted (univariable) analysis, it was considered in the final (multivariable) model.
Table 3.
Predicted and Observed Rates of Amenorrhea After GEA
| Preoperative Risk Factorsa | Observed Rates of Amenorrhea in the Validation Cohort, % (No. at Risk)c |
||||
|---|---|---|---|---|---|
| GEA Procedure |
Age ≥45 y |
Uterine Length <9 cm |
Endometrial Thickness <4 mm |
Predicted Rate of Amenorrhea (95% CI)b |
|
| RFA | Yes | Yes | Yes | 71% (50%–86%) | |
| RFA | Yes | No | Yes | 57% (36%–76%) | |
| RFA | No | Yes | Yes | 49% (29%–68%) | |
| RFA | Yes | Yes | No | 47% (34%–61%) | 58% (12) |
| RFA | No | No | Yes | 34% (18%–54%) | |
| RFA | Yes | No | No | 33% (24%–43%) | 32% (31) |
| RFA | No | Yes | No | 26% (17%–37%) | 56% (18) |
| RFA | No | No | No | 16% (10%–24%) | 39% (38) |
| TBA | Yes | Yes | Yes | 46% (24%–70%) | |
| TBA | Yes | No | Yes | 32% (15%–55%) | |
| TBA | No | Yes | Yes | 25% (12%–45%) | |
| TBA | Yes | Yes | No | 24% (14%–38%) | |
| TBA | No | No | Yes | 15% (7%–32%) | |
| TBA | Yes | No | No | 15% (9%–23%) | 0% (16) |
| TBA | No | Yes | No | 11% (6%–19%) | |
| TBA | No | No | No | 6% (3%–11%) | 7% (14) |
Abbreviations: CI, confidence interval; GEA, global endometrial ablation; RFA, radiofrequency ablation; TBA, thermal balloon ablation.
Risk factors were significant in the adjusted analysis (final model).
Rates were derived from the final model that was fit using the model development cohort.
Only results for variables with data from 10 or more women in the model validation cohort are presented.
Predictors of Treatment Failure
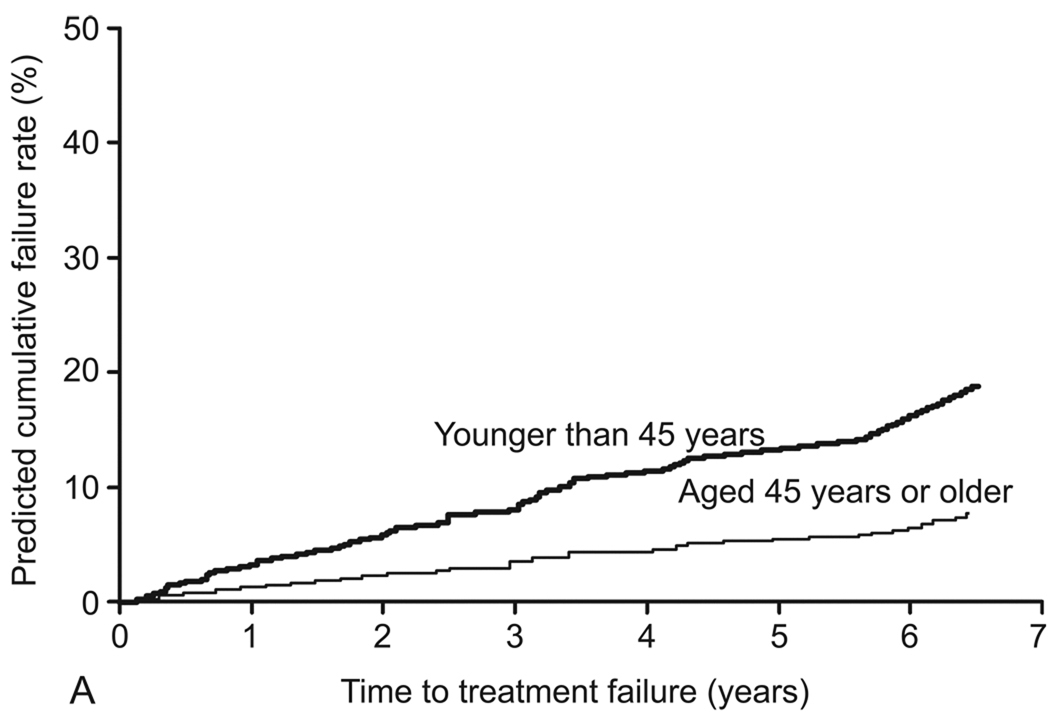
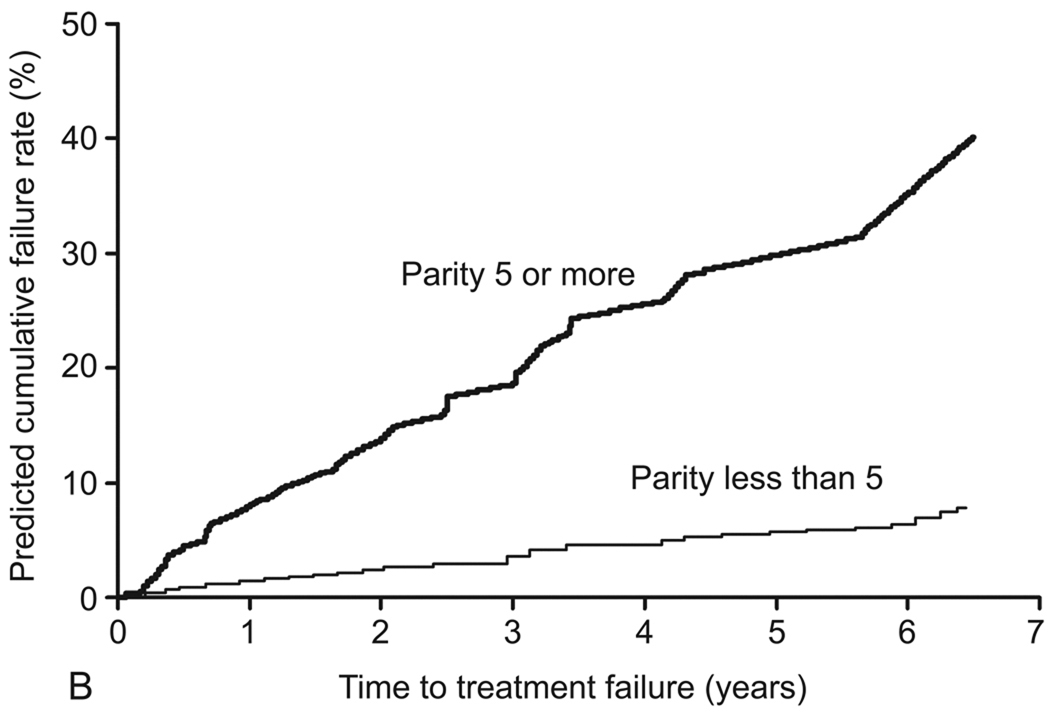
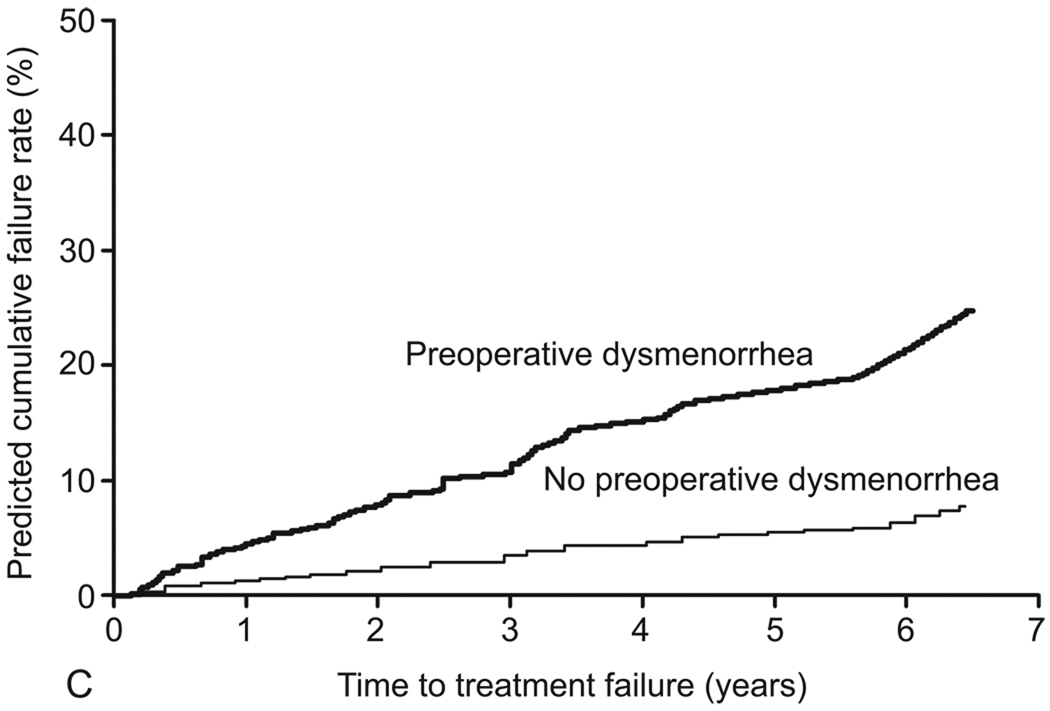
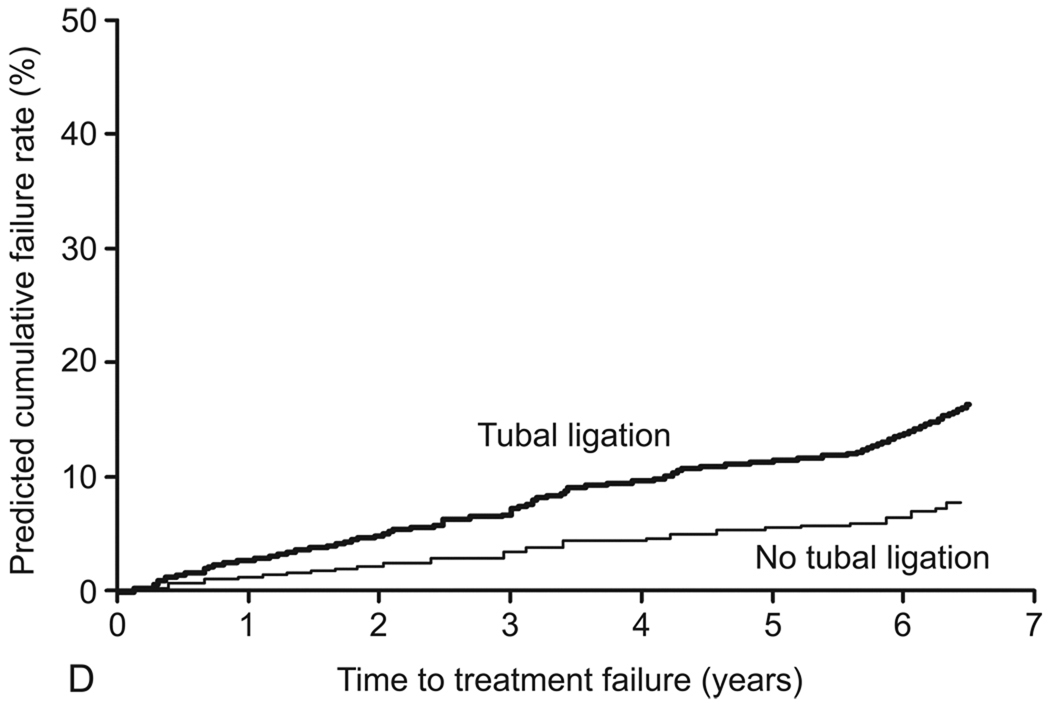
In the final model, the following variables were identified as independent predictors of treatment failure: age younger than 45 years (adjusted HR [aHR], 2.6; 95% CI, 1.3–5.1); parity of 5 or greater (aHR, 6.0; 95% CI, 2.5–14.8); prior tubal ligation (aHR, 2.2; 95% CI, 1.2–4.0); and preoperative dysmenorrhea (aHR, 3.7; 95% CI, 1.6–8.5) (Table 4). The c index of the final model was 0.755. No significant interactions between variables in the final model were detected. Table 5 shows the predicted and observed cumulative treatment failure rates after applying the final model to the model validation cohort. Results were stratified by age, parity, presence of preoperative dysmenorrhea, and tubal ligation status (Figure 3).
Table 4.
Preoperative Predictors of Treatment Failure After Global Endometrial Ablation
| Unadjusted Analyses | Adjusted Analyses (Final Model)a,b | |||||
|---|---|---|---|---|---|---|
| Predictors | Univariable Hazard Ratio |
95% CI | P Value | Multivariable Hazard Ratio |
95% CI | P Value |
| Thermal balloon ablation vs radiofrequency ablation | 1.5 | 0.8–2.9 | .26c | 1.5 | 0.7–2.9 | .27 |
| Age <45 y | 2.4 | 1.2–4.7 | .01 | 2.6 | 1.3–5.1 | .008 |
| Parity ≥5 | 4.8 | 2.0–11.4 | <.001 | 6.0 | 2.5–14.8 | <.001 |
| Body mass index ≥30 kg/m2 | 0.6 | 0.3–1.3 | .20 | |||
| Previous cesarean delivery | 0.7 | 0.3–1.6 | .40c | |||
| Tubal ligation | 2.5 | 1.4–4.5 | .002c | 2.2 | 1.2–4.0 | .01 |
| Preoperative bleeding ≥7 d | 1.1 | 0.5–2.4 | .78 | |||
| Preoperative menstrual accidents or clots | 1.6 | 0.8–3.1 | .16 | |||
| Irregular uterine bleeding (metrorrhagia) | 1.5 | 0.8–2.7 | .18 | |||
| Preoperative dysmenorrhea | 3.9 | 1.7–8.7 | .001c | 3.7 | 1.6–8.5 | .003 |
| Uterine length ≥9 cm | 1.0 | 0.5–1.8 | .94c | |||
| Retroverted uterus | 1.0 | 0.3–3.3 | .97c | |||
| Hemoglobin ≥12 g/dL | 1.8 | 0.9–3.6 | .08 | |||
| Ultrasonogram suggestive of adenomyosis | 1.5 | 0.5–4.9 | .51 | |||
| Endometrial thickness ≥4 mm | 0.8 | 0.3–2.4 | .73c | |||
| Intracavitary lesions (hysteroscopy) | 0.8 | 0.4–1.7 | .58 | |||
| Uterine polyp | 0.6 | 0.3–1.4 | .22c | |||
| Submucous fibroid | 1.0 | 0.3–3.1 | .94c | |||
| Nonsubmucous fibroid | 1.1 | 0.6–2.3 | .75 | |||
Abbreviation: CI, confidence interval.
The c index of this model was 0.755.
Variables with P values less than .20 in the unadjusted (univariable) analysis were considered in the multivariable Cox proportional hazard regression model.
This variable previously was shown in the literature to be associated with treatment failure.
Table 5.
Predicted and Observed Rates of Treatment Failure After GEA
| Risk Variablesa | Observed Rate of Treatment Failure in the Validation Cohort, % (No. at Risk)c |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Predicted Rate of Treatment Failure, % (95% CI)b |
|||||||||
| GEA Procedure |
Age ≥45 y |
Preoperative Dysmenorrhea |
Tubal Ligation |
||||||
| 1y | 3y | 5y | 1y | 3y | 5y | ||||
| RFA | No | No | No | 1 (0–2) | 3 (1–5) | 4 (1–7) | 0 (40) | 0 (16) | 0 (1) |
| RFA | No | No | Yes | 2 (0–4) | 6 (1–10) | 8 (1–15) | 3 (33) | 6 (19) | … (0) |
| RFA | No | Yes | No | 4 (0–8) | 9 (0–18) | 14 (0–27) | |||
| RFA | No | Yes | Yes | 8 (0–16) | 19 (0–34) | 27 (0–48) | |||
| RFA | Yes | No | No | 3 (1–5) | 6 (2–11) | 10 (3–16) | 0 (58) | 0 (26) | … (0) |
| RFA | Yes | No | Yes | 6 (2–10) | 14 (5–22) | 20 (6–32) | 0 (39) | 0 (12) | … (0) |
| RFA | Yes | Yes | No | 10 (0–18) | 22 (0–38) | 31 (0–53) | |||
| RFA | Yes | Yes | Yes | 20 (2–35) | 41 (6–64) | 56 (6–79) | |||
| TBA | No | No | No | 2 (0–3) | 4 (1–7) | 6 (2–10) | 5 (20) | 5 (17) | 11 (5) |
| TBA | No | No | Yes | 4 (1–6) | 8 (2–14) | 12 (3–20) | 10 (19) | 19 (15) | 25 (9) |
| TBA | No | Yes | No | 6 (0–12) | 13 (0–25) | 19 (0–36) | |||
| TBA | No | Yes | Yes | 12 (0–23) | 27 (0–46) | 38 (0–61) | |||
| TBA | Yes | No | No | 4 (1–7) | 9 (4–14) | 14 (7–20) | 0 (16) | 0 (14) | 0 (8) |
| TBA | Yes | No | Yes | 9 (3–14) | 19 (9–29) | 28 (14–40) | 14 (31) | 23 (23) | 26 (12) |
| TBA | Yes | Yes | No | 14 (0–26) | 30 (2–50) | 42 (4–65) | |||
| TBA | Yes | Yes | Yes | 28 (3–47) | 54 (11–77) | 70 (18–89) | |||
Abbreviations: CI, confidence interval; GEA, global endometrial ablation; RFA, radiofrequency ablation; TBA, thermal balloon ablation.
Because few women in our study had 5 or more live births, we reported the predicted rates only for women with parity less than 5.
Rates were derived from the final model that was fit using the model development cohort.
Rates from this cohort were estimated using the Kaplan-Meier method. Rates were calculated only for variables with more than 15 women at risk at baseline.
Figure 3.
Predicted cumulative treatment failure rates for preoperative variables (final model). A, Age (<45 years versus ≥45 years). B, Parity (≥5 versus <5). C, Preoperative dysmenorrhea (presence versus absence). D, Tubal ligation (presence versus absence).
Population-Derived GEA-Related Complications
Complications were generally minor and infrequent. Intraoperative complications were reported in 6 patients (1.3%; 95% CI, 0.5%–2.9%), ie, 5 cervical injuries and 1 uterine perforation. Postprocedural complications were documented in 3 patients (0.7%; 95% CI, 0.1%–1.9%), ie, 2 cases of cystitis and 1 case of endometritis. After GEA, 23 women (5.1%; 95% CI, 3.2%–7.5%) had pelvic pain. In 9, ultrasonograms showed evidence of fluid collection in the uterus (postablation syndrome). Of these 9 patients, 5 had hysterectomy for persistent pain, 3 had evidence of hematometra, 1 had adenomyosis, and 1 had normal findings after a pathologic examination. Postprocedural pregnancy was diagnosed in 3 women (0.7%; 95% CI, 0.1%–1.9%). All pregnancies resulted in spontaneous abortions during the first trimester. In addition, no reports (95% CI, 0%–0.8%) of endometrial cancer or death were made after ablation.
Discussion
We performed a systematic search of the MEDLINE, EMBASE, Web of Science, and Scopus databases from inception to November 2007 by using the terms “endometrial ablation” and “population-derived studies.” According to this search, our study is the first population-derived study of the long-term outcomes and predictors of outcome for women undergoing GEA to treat menorrhagia. The cumulative failure rate of GEA was 16% at 5 years, and most patients with treatment failure required hysterectomy for persistent bleeding or pelvic pain. Predictors of treatment failure included age younger than 45 years, parity of 5 or greater, prior tubal ligation, and history of dysmenorrhea. Predictors of amenorrhea were age 45 years or older, uterine length less than 9 cm, endometrial thickness less than 4 mm, and use of RFA rather than TBA. Predictive models of outcomes were constructed and validated in the population-derived and referral-derived cohorts, respectively.
Predicted and Actual Amenorrhea Outcomes
Regardless of a patient’s perception of menstrual pattern change after ablation, amenorrhea is an important clinical outcome. It often is the goal of menorrhagia treatment, and it is the main reason for a high level of patient satisfaction after hysterectomy (19). Conversely, failure to achieve the expected outcome of amenorrhea after EA commonly leads to performance of hysterectomy (20). Although several studies have reported amenorrhea rates of 10% to 60% after EA (5,21–24), this information is of limited value to specific patients. Knowing the patient-specific likelihood of amenorrhea optimizes preoperative counseling. For example, the likelihood of amenorrhea for a 48-year-old woman with a normal-sized uterus (<9 cm) and a thin endometrium (<4 mm) who undergoes RFA is 71%, whereas it is 6% for a 35-year-old woman with a larger uterus (≥9 cm) and thicker endometrium (≥4 mm) who undergoes TBA. This information may improve patient satisfaction and may reduce the need for additional intervention after ablation.
Although the overall amenorrhea rate in this study (23%) was lower than that of previously published reports (21,25), the predicted rate of amenorrhea ranged from 6% to 71% and depended on specific patient characteristics and the type of ablative technology used. Our overall rate was less likely to be confounded by menopause because only patients with amenorrhea that began immediately after the procedure were included in the analysis (21,25). For older women, development of amenorrhea may be facilitated by age-related impairment of endometrial regeneration (8,9). Although we believe the confounding effect of menopause was minimized in the current study by our definition of amenorrhea (cessation of menses immediately after surgery that lasted for at least 12 months), this bias cannot be eliminated completely without performing ovarian function tests.
Another important finding is that women who underwent RFA were more likely to have amenorrhea than those who underwent TBA; our results are consistent with a recent study by Kleijn et al (26), who reported an amenorrhea rate of 48% in RFA patients versus 32% in TBA patients at 5 years of follow-up. The use of impedance-based technology in RFA may optimize the delivery of treatment energy for more complete endometrial destruction (5,26).
Predicted and Actual Treatment Failure Outcomes
During preoperative counseling, patients frequently ask about the probability of hysterectomy after ablation. In 1 study, 60% of women who underwent hysterectomy indicated that they would have chosen EA if the lifetime treatment failure rate was less than 20% (27). A recent clinical trial by the Surgical Treatments Outcomes Project for Dysfunctional Uterine Bleeding (STOP-DUB), which randomly assigned patients to undergo hysterectomy or EA, reported a failure rate of more than 30% after a 4-year follow-up of the GEA subgroup (28). The low failure rate observed in our study may be due to better patient counseling and matching patient expectations with outcomes. For example, patients who prefer complete cessation of menses after EA for menorrhagia are more likely to undergo hysterectomy to treat bleeding symptoms of any severity (20). The randomization in the STOP-DUB study may have contributed to the higher failure rate.
Another important determinant of treatment failure is a good understanding of predictors of failure and application of that knowledge during patient selection and preoperative counseling. The model that we developed predicted clinically useful short- and medium-term rates of treatment failure after GEA. For example, the likelihood of treatment failure for a 35-year-old woman with 3 prior deliveries and a history of dysmenorrhea and tubal ligation at the time of TBA is 70% at 5 years, whereas it would be 6% for a 48-year-old woman with the same parity but no history of dysmenorrhea or tubal ligation. Appropriate patient counseling and selection ultimately may improve treatment outcomes.
Understanding the causal relationship between age, preoperative dysmenorrhea, and tubal ligation status and treatment failure is less clear. Tubal ligation has been identified consistently as a risk factor for treatment failure during rollerball ablation (8), and the threshold for a hysterectomy may be lower in a patient who has had a tubal ligation. Perhaps as important is the association between tubal ligation and post-EA syndrome. This syndrome is characterized by pain from the distention of the proximal end of the fallopian tube that is caused by regeneration of the cornual endometrium, intrauterine adhesions that obstruct the outflow tract, and tubal ligation that prevents emptying into the peritoneal cavity (29). However, no patients in the current study who underwent hysterectomy had a pathologic diagnosis consistent with post-EA syndrome. The association between dysmenorrhea and treatment failure first was suggested by Bongers and colleagues (9), even though their reported association did not reach statistical significance. Undiagnosed adenomyosis may persist after ablation, cause persistent pelvic pain, and require a hysterectomy (5).
Population-Derived Postablation Complications
GEA was generally safe, and minor complications occurred in less than 5% of patients. Our data compare favorably to data from the Manufacturers and Users Facility Device Experience (MAUDE) database, which reported major morbidities after GEA (30). Endometrial cancer after EA was described previously (31), but no such cases were documented in our study population during the relatively short follow-up period. The absence of a cancer diagnosis in the current cohort provides preliminary assurance, but longer follow-up and larger numbers of patients are needed to determine the effect of GEA on the incidence and early diagnosis of endometrial cancer.
Limitations, Strengths, and Generalizability
The main limitation of this study was its retrospective nature, which precluded objective measures of treatment outcomes such as a validated bleeding score. In addition, patient data were incomplete for some variables in the final model. However, only 2 preoperative variables were missing data from more than 20% of patients, and sensitivity analyses (conducted to explore the effect of the missing variables) showed that it did not affect the final model.
This study examined the experience with GEA technologies over a relatively long period. The population-derived nature of the study allowed us to determine treatment outcomes precisely, and the minimal number of patients lost to follow-up provided a fairly complete dataset. The characteristics of our study population were generally similar to those of whites in the United States, but further validation of the model in larger cohorts and in other races would be valuable.
We believe the data presented in this report can be used to optimize preoperative patient counseling. Ultimately, these data may facilitate greater use of GEA technology when treating women with menorrhagia.
Footnotes
This data was presented at the Global Congress of Minimally Invasive Gynecology, 36th AAGL Annual Meeting, Washington, DC, in November 2007.
Editing, proofreading, and reference verification were provided by the Section of Scientific Publications, Mayo Clinic.
Financial Disclosure: The authors have no potential conflicts of interest to disclose.
References
- 1.Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health. 2007 May–Jun;10(3):183–194. doi: 10.1111/j.1524-4733.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 2.Lethaby A, Hickey M, Garry R. Endometrial destruction techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005 Oct 19;4:CD001501. doi: 10.1002/14651858.CD001501.pub2. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor H, Broadbent JA, Magos AL, McPherson K. Medical Research Council randomised trial of endometrial resection versus hysterectomy in management of menorrhagia. Lancet. 1997 Mar 29;349(9056):897–901. doi: 10.1016/S0140-6736(96)07285-6. [DOI] [PubMed] [Google Scholar]
- 4.McGurgan P, O’Donovan P. Endometrial ablation. Curr Opin Obstet Gynecol. 2003 Aug;15(4):327–332. doi: 10.1097/01.gco.0000084244.09900.94. [DOI] [PubMed] [Google Scholar]
- 5.Sharp HT. Assessment of new technology in the treatment of idiopathic menorrhagia and uterine leiomyomata. Obstet Gynecol. 2006 Oct;108(4):990–1003. doi: 10.1097/01.AOG.0000232618.26261.75. [DOI] [PubMed] [Google Scholar]
- 6.Dickersin K, Munro MG, Clark M, Langenberg P, Scherer R, Frick K, et al. Surgical Treatments Outcomes Project for Dysfunctional Uterine Bleeding (STOP-DUB) Research Group. Hysterectomy compared with endometrial ablation for dysfunctional uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2007 Dec;110(6):1279–1289. doi: 10.1097/01.AOG.0000292083.97478.38. [DOI] [PubMed] [Google Scholar]
- 7.Parkin DE. Prognostic factors for success of endometrial ablation and resection. Lancet. 1998 Apr 18;351(9110):1147–1148. doi: 10.1016/S0140-6736(05)79116-9. [DOI] [PubMed] [Google Scholar]
- 8.Dutton C, Ackerson L, Phelps-Sandall B. Outcomes after rollerball endometrial ablation for menorrhagia. Obstet Gynecol. 2001 Jul;98(1):35–39. doi: 10.1016/s0029-7844(01)01380-1. [DOI] [PubMed] [Google Scholar]
- 9.Bongers MY, Mol BW, Brolmann HA. Prognostic factors for the success of thermal balloon ablation in the treatment of menorrhagia. Obstet Gynecol. 2002 Jun;99(6):1060–1066. doi: 10.1016/s0029-7844(02)02011-2. [DOI] [PubMed] [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP STROBE Initiative. The STrengthening the Reporting of OBservational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007 Oct 20;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 11.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996 Mar;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 12.H-ICDA, hospital adaptation of ICDA. 2nd ed. Ann Arbor (MI): Commission on Professional and Hospital Activities; c1973. Commission on Professional and Hospital Activities. [Google Scholar]
- 13.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–174. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. About BMI for Adults. [updated 2007 May 22; cited 2007 Feb 7];Atlanta (GA): Centers for Disease Control and Prevention; BMI—Body Mass Index [Internet] Available from: http://www.cdc.gov/nccdphp/dnpa/bmi/about_adult_BMI.htm.
- 15.Johnson-Spear MA, Yip R. Hemoglobin difference between black and white women with comparable iron status: justification for race-specific anemia criteria. Am J Clin Nutr. 1994 Jul;60(1):117–121. doi: 10.1093/ajcn/60.1.117. [DOI] [PubMed] [Google Scholar]
- 16.Robins JM, Greenland S. The role of model selection in causal inference from nonexperimental data. Am J Epidemiol. 1986 Mar;123(3):392–402. doi: 10.1093/oxfordjournals.aje.a114254. [DOI] [PubMed] [Google Scholar]
- 17.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 18.Harrell FE, Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984 Apr–Jun;3(2):143–152. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 19.Weber AM, Munro MG. Endometrial ablation versus hysterectomy: STOP-DUB. Medscape Womens Health. 1998 May;3(3):3. [PubMed] [Google Scholar]
- 20.Aberdeen Endometrial Ablation Trials Group. A randomised trial of endometrial ablation versus hysterectomy for the treatment of dysfunctional uterine bleeding: outcome at four years. Br J Obstet Gynaecol. 1999 Apr;106(4):360–366. doi: 10.1111/j.1471-0528.1999.tb08275.x. Erratum in: Br J Obstet Gynaecol. 1999 Aug;106(8):876. [DOI] [PubMed] [Google Scholar]
- 21.Abbott J, Hawe J, Hunter D, Garry R. A double-blind randomized trial comparing the Cavaterm and the NovaSure endometrial ablation systems for the treatment of dysfunctional uterine bleeding. Fertil Steril. 2003 Jul;80(1):203–208. doi: 10.1016/s0015-0282(03)00549-1. [DOI] [PubMed] [Google Scholar]
- 22.Bongers MY, Bourdrez P, Heintz AP, Brolmann HA, Mol BW. Bioplar radio frequency endometrial ablation compared with balloon endometrial ablation in dysfunctional uterine bleeding: impact on patients’ health-related quality of life. Fertil Steril. 2005 Mar;83(3):724–734. doi: 10.1016/j.fertnstert.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Gallinat A. NovaSure impedance controlled system for endometrial ablation: three-year follow-up on 107 patients. Am J Obstet Gynecol. 2004 Nov;191(5):1585–1589. doi: 10.1016/j.ajog.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Loffer FD, Grainger D. Five-year follow-up of patients participating in a randomized trial of uterine balloon therapy versus rollerball ablation for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002 Nov;9(4):429–435. doi: 10.1016/s1074-3804(05)60514-2. [DOI] [PubMed] [Google Scholar]
- 25.Bongers MY, Bourdrez P, Mol BW, Heintz AP, Brolmann HA. Randomised controlled trial of bipolar radio-frequency endometrial ablation and balloon endometrial ablation. BJOG. 2004 Oct;111(10):1095–1102. doi: 10.1111/j.1471-0528.2004.00253.x. [DOI] [PubMed] [Google Scholar]
- 26.Kleijn JH, Engels R, Bourdrez P, Mol BW, Bongers MY. Five-year follow up of a randomised controlled trial comparing NovaSure and ThermaChoice endometrial ablation. BJOG. 2008 Jan;115(2):193–198. doi: 10.1111/j.1471-0528.2007.01427.x. Epub 2007 Jul 6. [DOI] [PubMed] [Google Scholar]
- 27.Bourdrez P, Bongers MY, Mol BW. Treatment of dysfunctional uterine bleeding: patient preferences for endometrial ablation, a levonorgestrel-releasing intrauterine device, or hysterectomy. Fertil Steril. 2004 Jul;82(1):160–166. doi: 10.1016/j.fertnstert.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 28.Dickersin K, Munro M, Langenberg P, Scherer R, Frick KD, Weber AM, et al. Surgical Treatments Outcomes Project for Dysfunctional Uterine Bleeding Research Group. Surgical Treatments Outcomes Project for Dysfunctional Uterine Bleeding (STOP-DUB): design and methods. Control Clin Trials. 2003 Oct;24(5):591–609. doi: 10.1016/s0197-2456(03)00023-0. [DOI] [PubMed] [Google Scholar]
- 29.Townsend DE, McCausland V, McCausland A, Fields G, Kauffman K. Post-ablation-tubal sterilization syndrome. Obstet Gynecol. 1993 Sep;82(3):422–424. [PubMed] [Google Scholar]
- 30.Gurtcheff SE, Sharp HT. Complications associated with global endometrial ablation: the utility of the MAUDE database. Obstet Gynecol. 2003 Dec;102(6):1278–1282. doi: 10.1016/j.obstetgynecol.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Areia A, Branco M, Frutuoso C, de Oliveira CF. Endometrial adenocarcinoma after endometrial ablation: a case report. Eur J Gynaecol Oncol. 2006;27(4):432–433. [PubMed] [Google Scholar]