Abstract
Acetaminophen (APAP) overdose is a major cause of acute liver failure. The glutathione (GSH) precursor N-acetylcysteine (NAC) is used to treat patients with APAP overdose for up to 48 h. Although it is well established that early treatment with NAC can improve the scavenging of the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI), protective mechanisms at later times remain unclear. To address this issue, fasted C3Heb/FeJ mice were treated with 300 mg/kg APAP and then received intravenously 0.65 mmol/kg GSH or NAC at 1.5 h after APAP. The animals were sacrificed at 6 h. APAP alone caused severe liver injury with peroxynitrite formation and DNA fragmentation, all of which was attenuated by both treatments. However, GSH (−82%) was more effective than NAC (−46%) in preventing liver injury. Using nuclear magnetic resonance spectroscopy to measure tissue ATP levels and the substrate flux through the mitochondrial Krebs cycle, it was observed that the reduced liver injury correlated with accelerated recovery of mitochondrial GSH content, maintenance of ATP levels and an increased substrate supply for the mitochondrial Krebs cycle compared to APAP alone. NAC treatment was less effective in recovering ATP and mitochondrial GSH levels and showed reduced substrate flux through the Krebs cycle compared to GSH. However, increasing the dose of NAC improved the protective effect similar to GSH suggesting that the amino acids not used for GSH synthesis were used as mitochondrial energy substrates.
Conclusion
Delayed treatment with GSH and NAC protect against APAP overdose by dual mechanisms, i.e. by enhancing hepatic and mitochondrial GSH levels (scavenging of reactive oxygen and peroxynitrite) and by supporting the mitochondrial energy metabolism.
Keywords: Drug-induced liver injury, oncotic necrosis, oxidant stress, acute liver failure, mitochondrial bioenergetics
INTRODUCTION
Acetaminophen (APAP) is a safe analgesic at therapeutic levels. However, an overdose can cause severe liver injury and even acute liver failure. During the last decade, APAP became the most frequent cause of acute liver failure in the US and many other countries.1 Early animal studies established the formation of the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI), which first depletes glutathione and subsequently causes protein binding, as a critical event in the toxicity.2–4 Based on this mechanistic insight, N-acetylcysteine (NAC) was introduced to treat patients with APAP overdose in the 1970s.5 Even today, NAC therapy is still the best therapeutic option for the overdose patient.6 NAC is most effective when given as early as possible after APAP intoxication. The main mechanism of action of NAC is to promote hepatic GSH synthesis,7,8 which supports the detoxification of NAPQI and reduces protein binding.9 However, NAC therapy is clinically effective even when initiated 24 h of APAP overdosing, i.e. at a time when there is no relevant amount of drug left to metabolize.10,11 These observations suggest that there might be other, yet unknown mechanisms of protection. This hypothesis is also supported by the fact that NAC can protect in models without GSH depletion.12
Recent insight into the molecular mechanisms of APAP hepatotoxicity in a mouse model indicated that the early protein binding is an essential initiating event, which requires intracellular propagation and amplification.13,14 Key to this propagation mechanism is mitochondrial dysfunction and oxidant stress.14 Intravenous administration of GSH after APAP treatment resulted in the accelerated recovery of the mitochondrial GSH content.15 The elevated levels of mitochondrial GSH effectively scavenged reactive oxygen and peroxynitrite, which reduced APAP-induced liver injury and promoted regeneration.15–17 Delayed treatment with NAC had a similar protective effect.18 These findings support the hypothesis that the delayed supply of cysteine can be involved in a second mechanism of protection, i.e. the scavenging of reactive oxygen and peroxynitrite.13,14
The dose of GSH we used in our recent experiments15–17 was based on previous data showing its efficacy in supporting the recovery of hepatic GSH levels after starvation.19 This dose provides enough cysteine to re-synthesize twice the entire GSH content of a mouse liver. However, in most studies with NAC generally much higher doses are used, e.g. 300 – 1200 mg/kg of NAC,9,18,20–22 which is sufficient to synthesize the hepatic GSH content between 6 and 23 times. For treatment of acute APAP poisoning in patients, the recommended dosing for NAC is a 150 mg/kg loading dose followed by maintenances doses of 50 mg/kg every 4 h.6 Again, even the loading dose alone would be sufficient cysteine to synthesize several times the entire hepatic GSH content. This raises the question whether GSH is more effective than NAC or if there are additional benefits of high doses of NAC that have not been considered. To address this important question, we compared the efficacy and mechanisms of protection of equimolar doses of GSH and NAC in a murine model of APAP hepatotoxicity.
MATERIALS AND METHODS
Animals
Male C3HeB/FeJ mice (8–10 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME). Animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals”. The experimental protocol was approved by the institutional animal care and use committee of Kansas University Medical Center. The animals were fasted overnight and then received 300 mg/kg APAP (Sigma Chemical Co., St. Louis, MO) dissolved in warm saline (15 mg/ml) (i.p.). Some animals received a single intravenous bolus dose of glutathione (200 mg/kg GSH; 0.65 mmol/kg) dissolved in phosphate-buffered saline (PBS), 106 mg/kg (0.65 mmol/kg) or 318 mg/kg (1.95 mmol/kg) N-acetyl cysteine, a mixture of 3 amino acids [49 mg/kg (0.65 mmol/kg) glycine, 96.4 mg/kg (0.65 mmol/kg) glutamic acid, and 106 mg/kg (0.65 mmol/kg) NAC] or a mixture of 2 amino acids [73.5 mg/kg (0.98 mmol/kg) glycine and 144.6 mg/kg (0.98 mmol/kg) glutamic acid]. All compounds were administered intravenously through the penile vein 1.5 h after APAP injection.15–17 The rationale for the treatment at 1.5 h is based on the assumption that by that time most of the administered APAP has been metabolized and most of the protein binding of NAPQI is completed (see supplemental text for more details).
Experimental Protocols
At selected times after APAP treatment, groups of animals were killed by cervical dislocation under isoflurane anesthesia. Blood was drawn from the vena cava into heparinized syringes and centrifuged. The plasma was used for determination of alanine aminotransferase (ALT) activities. Immediately after collecting the blood, the livers were excised and rinsed in saline. A small section from each liver was placed in 10% phosphate buffered formalin. The remaining liver was frozen in liquid nitrogen and stored at −80°C.
Methods
Plasma ALT activities were determined with the kinetic test kit 68-B (Biotron Diagnostics, Inc., Hernet, CA) and expressed as IU/liter. Protein concentrations were assayed using the bicinchoninic acid kit (Pierce, Rockford, IL). Mitochondria were isolated from fresh liver tissue as previously described using a differential centrifugation method.15,17 Purity of the mitochondrial fraction was assessed by cytochrome c content and the absence of lactate dehydrogenase.17 Total soluble GSH and GSSG were measured in the liver homogenate and in isolated mitochondria with a modified method of Tietze as described previously.15,23 Briefly, frozen tissues (or isolated mitochondria) were homogenized at 0°C in 3% sulfosalicylic acid containing 0.1 mM EDTA.23 An aliquot of the homogenate was added to 10 mM N-ethylmaleimide (NEM) in potassium phosphate buffer (KPP), and another aliquot was added to 0.01 N HCl. The NEM-KPP sample was centrifuged, and the supernatant was passed through a C18 cartridge to remove free NEM and NEM-GSH adducts (Sep-Pak; Waters, Milford, MA). The HCl sample was centrifuged, and the supernatant was diluted with KPP. All samples were assayed using dithionitrobenzoic acid. All data are expressed in GSH-equivalents.
Histology and immunohistochemistry
Formalin-fixed tissue samples were embedded in paraffin and 5 μm sections were cut. Replicate sections were stained with hematoxylin and eosin (H&E) for evaluation of necrosis.24 For the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay, sections of liver were stained with the In Situ Cell Death Detection Kit, AP (Roche Diagnostics, Indianapolis, IN) as described in the manufacturer’s instructions.24 Nitrotyrosine protein adducts were detected with standard immunohistochemical methods using an anti-nitrotyrosine antibody (Molecular Probes, Eugene, OR).15
Animals for NMR experiments
For NMR experiments, further groups of mice were treated with APAP and the study substances at t = 1.5 h as mentioned above [GSH, NAC, a mixture of 3 amino acids (glycine, glutamate, cysteine), a mixture of 2 amino acids, or cysteine]. At t = 1.5 h or t = 6 h, all mice received [U-13C]glucose (500 mg/kg; 2.78 mmol/kg) (Cambridge Isotopes, Andover, MA), which was injected intraperitoneally in bolus to study metabolic changes in awake mice. Using 500 mg/kg [U-13C]glucose, plasma glucose was <10 mM in all experiments. The mice were killed by cervical dislocation and the livers freeze-clamped immediately. Blood was taken after severing of the carotid artery and put into heparin tubes. Water-soluble metabolites were extracted from blood or tissue with perchloric acid and analyzed by NMR spectroscopy (see supplemental text for details).12
Statistics
Data are expressed as means ± S.E. Comparison between two groups were performed with Student’s t-test or one-way ANOVA followed by Bonferroni t-test for multiple groups. If the data were not normally distributed, the Mann-Whitney test was applied for comparison of two groups and the Kruskal-Wallis Test (nonparametric ANOVA) followed by Dunn’s Multiple Comparisons Test for multiple groups. P<0.05 was considered significant.
RESULTS
GSH treatment was most effective in preventing APAP-induced liver injury
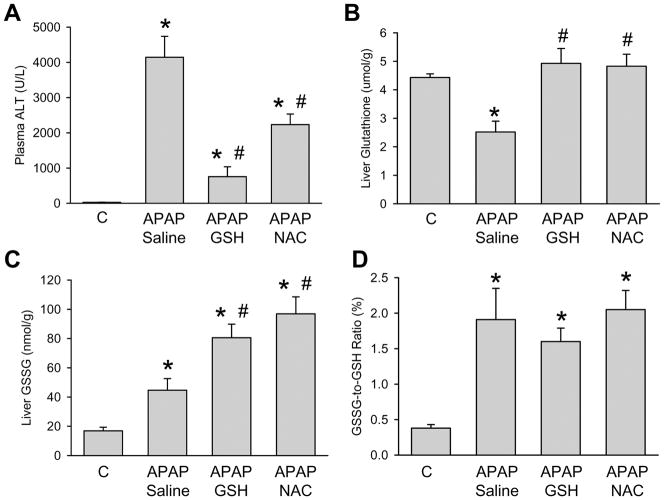
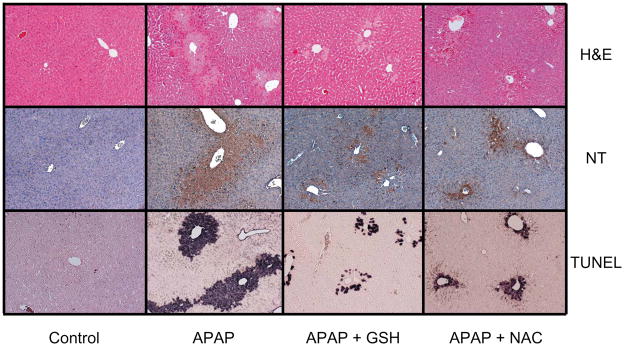
Male C3Heb/FeJ mice treated with an overdose of APAP (300 mg/kg) showed evidence of severe liver injury at 6 h as indicated by the significant increase of plasma ALT values (Figure 1A). In addition, there was only a partial recovery of the hepatic GSH content and evidence of oxidant stress as indicated by the elevated levels of hepatic GSSG and an increased GSSG-to-GSH ratio (Figure 1B–D). Additional treatment of mice with equimolar doses of GSH or NAC (0.65 mmol/kg) at 1.5 h after APAP resulted in a significantly reduced increase of plasma ALT values in both experimental groups (Figure 1). However, GSH treatment was more effective in preventing APAP-induced liver injury as indicated by the 82% lower ALT values compared to the 46% reduction with NAC. On the other hand, both treatments resulted in a complete recovery of hepatic GSH levels (Figure 1). The hepatic GSSG content was further increased compared to APAP alone. However, the GSSG-to-GSH ratio was similar in all APAP-treated groups (Figure 1). These results suggest that ROS are still being formed after GSH or NAC treamtnet but this oxidant stress is more efficiently detoxified due to the higher GSH levels. The biochemical data could be confirmed by histological findings. H&E staining of liver sections indicated the substantial centrilobular necrosis 6 h after APAP administration (Figure 2). The loss of basophilic staining, vacuolization, cell swelling and karyolysis around the centrilobular veins documents that the cells undergoing oncotic necrosis (Supplemental Figure 1). The extent of necrosis was correlated with massive DNA fragmentation as demonstrated by the TUNEL assay and peroxynitrite formation as indicated by the presence of nitrotyrosine protein adducts in the centrilobular areas (Figure 2). Delayed treatment with GSH markedly reduced the area of necrosis, nitrotyrosine protein adducts as well as the number of TUNEL-positive cells (Figure 2; Supplemental Figure 1). Treatment with NAC also attenuated the areas of necrosis, nitrotyrosine staining and the extent of DNA damage (Figure 2). However, the protective effect of NAC was less pronounced compared to GSH. Together, these results suggest that treatment with GSH 1.5 h after APAP administration was more effective in protecting the liver compared to an equimolar dose of NAC. Since hepatic GSSG levels further increased after GSH or NAC treatment compared to APAP alone, this indicates that ROS are more effectively detoxified but are still being formed. Thus, both delayed treatments did not affect the oxidant stress induced by APAP overdose (Figure 1C, D).
Figure 1.
Plasma alanine aminotransferase (ALT) activities and the hepatic content of glutathione (GSH+GSSG) and glutathione disulfide (GSSG) were measured in control animals or mice treated with 300 mg/kg acetaminophen (APAP) for 6 h. The ratio between GSSG and total glutathione was calculated. Some of the animals received additionally 10 ml/kg saline, 0.65 mmol/kg GSH or 0.65 mmol/kg N-acetylcysteine (NAC) iv 1.5 h after APAP. Data represent means ± SE of n = 5 animals per group. *P<0.05 (compared to controls); #P<0.05 (compared to APAP/saline)
Figure 2.
Histological assessment of liver injury (hematoxilin & eosin, H&E), peroxynitrite formation (nitrotyrosine staining) and DNA fragmentation (TUNEL assay) in control animals or mice treated with 300 mg/kg acetaminophen (APAP) for 6 h. Some of the animals received additionally 10 ml/kg saline, 0.65 mmol/kg GSH or 0.65 mmol/kg N-acetylcysteine (NAC) iv 1.5 h after APAP. The representative pictures show extensive centrilobular necrosis, which correlated with the areas of nitrotyrosine staining and TUNEL-positive cells in APAP-treated animals. Both, GSH and NAC treatment improved all parameters with GSH being more effective than NAC. (×100 for all panels)
GSH and NAC treatment had similar effects on early GSH recovery
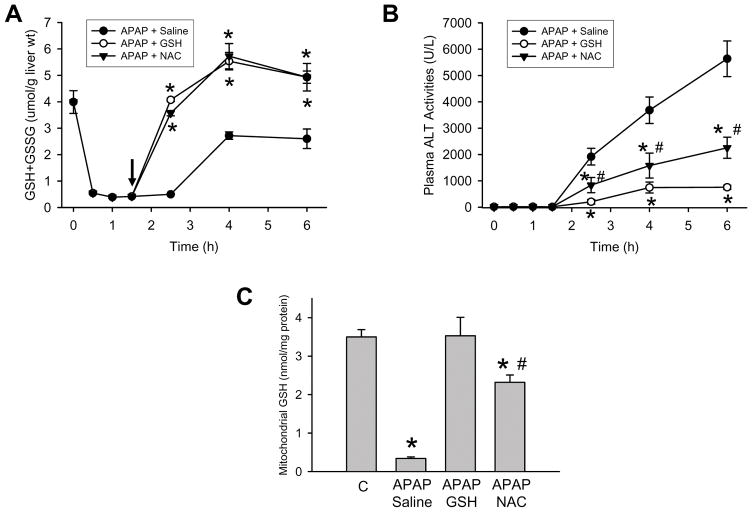
Although there was a clear difference in tissue injury between the GSH- and the NAC-treated groups, based on the GSH and GSSG data of the 6 h time point (Figure 1), it appears that the efficacy in supplying cysteine for recovery of hepatic GSH levels and scavenging reactive oxygen was similar between the 2 treatment groups. To verify that this conclusion is justified, a more detailed time course of the depletion and recovery of hepatic GSH levels was studied (Figure 3A). Treatment with APAP resulted in an 86% depletion of hepatic GSH levels within 30 min and no relevant further decline afterwards (Figure 3A). When saline was injected at 1.5 after APAP, there was no immediate relevant recovery of the GSH content. However, between 2.5 and 4 h, GSH levels spontaneously recovered to about 70% of baseline with no further improvement thereafter (Figure 3A). In contrast, injection of GSH or NAC resulted in a rapid and complete recovery of hepatic GSH levels within 1 h and an additional increase over the next 1.5 h (Figure 3A). Despite the similar recovery of the hepatic GSH content, plasma ALT values indicated improved protection in the GSH-treated animals compared to NAC (Figure 3B). Since our previous studies identified the mitochondria as the main source of oxidant stress and peroxynitrite formation,17,25–27 the mitochondrial GSH content was evaluated 45 min after the injection of GSH or NAC (Figure 3C). Compared to an untreated control, mitochondrial GSH levels are still 90% depleted 2.25 h after APAP exposure. Injection of GSH resulted in a complete recovery of mitochondrial GSH levels (Figure 3C). However, injection of an equimolar dose of NAC caused only a partial recovery of the mitochondrial GSH content to levels 34% below controls or GSH-treated animals (Figure 3C). These results indicate that the supply of cysteine through GSH or NAC is sufficient to re-synthesize GSH in the cytosol but there is a delay of the energy-dependent transport of GSH into the mitochondria with NAC.
Figure 3.
Time course of hepatic glutathione (GSH+GSSG) levels (A) and plasma ALT activities (B) after treatment with 300 mg/kg APAP. Some animals received additionally 10 ml/kg saline, 0.65 mmol/kg GSH or 0.65 mmol/kg N-acetylcysteine (NAC) iv 1.5 h after APAP. Data represent means ± SE of n = 4 animals per time point. *P<0.05 (compared to APAP/saline); #P<0.05 (compared to APAP/GSH)
C. Mitochondrial glutathione content in controls or 2.5 h after injection of APAP alone or in combination with GSH and NAC. Data represent means ± SE of n = 4 animals per group or time point. *P<0.05 (compared to controls, C); #P<0.05 (compared to APAP/GSH)
Hepatic energy status and metabolism
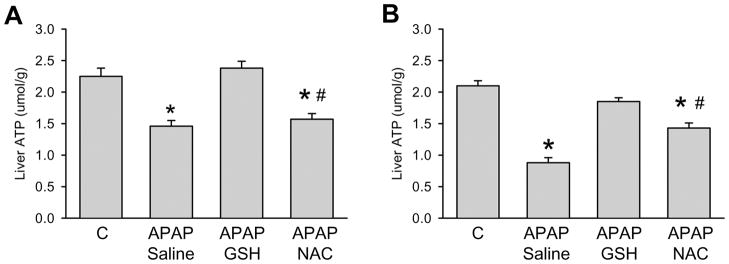
It is well established that APAP hepatotoxicity causes mitochondrial dysfunction with depletion of hepatic ATP levels.26–28. In addition, it was recently shown that high levels of NAC can improve the mitochondrial energy metabolism.12 Therefore, we hypothesized that the difference between GSH and NAC treatment might be caused by differential effects on mitochondrial energy metabolism. Using NMR, we compared changes in ATP levels with alterations in mitochondrial energy metabolism. We chose to evaluate these parameters at two time points, an early time (2.25 h) during the initiation of the injury and a late time (6.75 h) where substantial necrosis was evident. As a measure of the hepatocellular energy state, the concentrations of ATP were determined from 31P-NMR spectra of liver extracts (Figure 4). ATP levels in the livers of mice treated with APAP were reduced to 66% and 42% of saline-treated controls at the early and late time point, respectively. While ATP levels were not significantly different from controls after administration of GSH at both time-points, treatment with NAC did not result in an early ATP recovery and improved the energy status only partially (to 70% of controls) at the late time point.
Figure 4.
Tissue concentrations of ATP (μmol/g wet weight), as calculated from its resonances in 31P-NMR spectra of liver extracts. The mice were treated with 300 mg/kg APAP and some subsequently received 0.65 mmol/kg GSH or NAC at 1.5 h after APAP. ATP levels were measured 2.25 h (A) or 6.75 h (B) after treatment with APAP. Data represent means ± SE of n = 4 animals per group. *P<0.05 (compared to controls, C); #P<0.05 (compared to APAP/GSH)
GSH causes early and late upregulation of mitochondrial energy metabolism
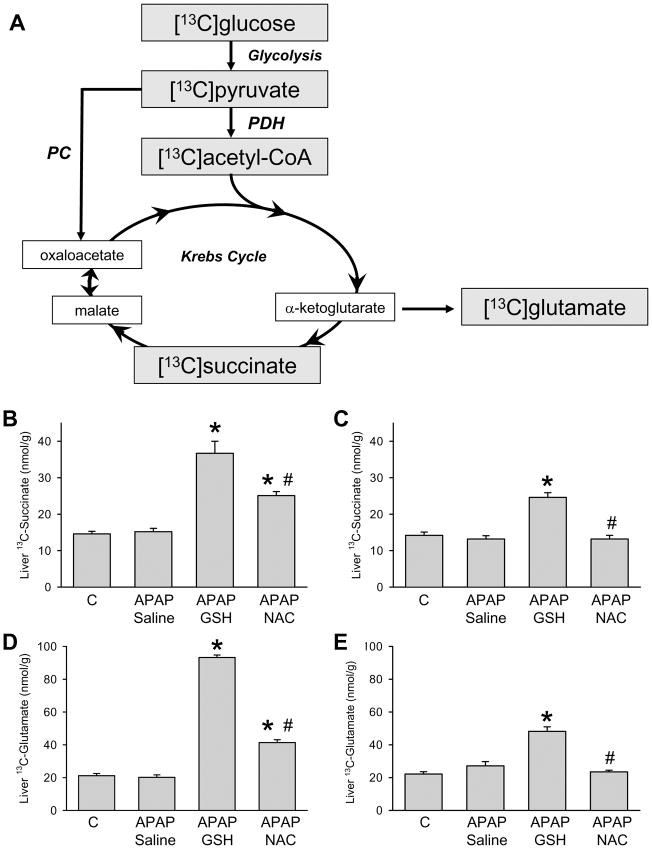
Two pathways mediate the entry of carbon from [U-13C]glucose into the Krebs cycle after conversion to [U-13C]pyruvate (Figure 5A): 1) PDH, which converts pyruvate into acetyl-CoA and is considered the key enzyme for mitochondrial energy metabolism. 2) PC converts pyruvate into the Krebs cycle intermediate oxaloacetate, which needs to combine with acetyl-CoA to allow for synthesis of amino acids such as glutamate. Oxaloacetate can also be replenished after anaplerotic entry of other substrates to the Krebs cycle such as the glutamate residue in GSH. Therefore, we administered GSH or NAC together with [U-13C]glucose 1.5 h after APAP treatment. Additional GSH/NAC-treated animals were injected with [U-13C]glucose at 6 h. Figure 5 shows the amounts of 13C-labelled glutamate (C-4 position), formed through PDH from [U-13C]glucose (Figure 5D, E), as well as of the Krebs cycle intermediate succinate (Figure 5B, C). Despite ATP depletion, treatment of mice with APAP did not result in a diminished flux through PDH with reduced succinate or glutamate formation at both time points (Figure 5B-E). However, administration of GSH to APAP-treated mice caused a significantly augmented formation of both 13C-labelled glutamate and succinate (more than 4-fold and 2-fold elevation at 2.25 h, respectively), which supports early mitochondrial ATP formation (Figure 5B, D). The stimulating effects on glutamate and succinate formation could still be observed 6.75 h after APAP administration (Figure 5C, E). The effects of NAC were much less pronounced compared to GSH at 2.25 h (Figure 5B, D) and disappeared at 6.75 h (Figure 5C, E).
Figure 5.
A. Labeling of glutamate from [U-13C]glucose. [U-13C]glucose is first metabolized to [3-13C]pyruvate, which is converted via pyruvate dehydrogenase (PDH) and pyruvate carboxylase (PC) to [2-13C]acetyl-CoA and [3-13C]oxaloacetate, respectively. The fluxes through PDH and PC lead to a different 13C-labeling pattern in the Krebs cycle intermediate citrate, and are finally measured by the isotopomer pattern of glutamate in 13C-NMR spectra. B-E. Concentrations of 13C-labelled [4,5-13C]glutamate and the Krebs cycle intermediate [2,3-13C]succinate (nmol/g wet weight), as calculated from their resonances in 1H- and 13C-NMR spectra of liver extracts. The mice were treated with 300 mg/kg APAP and some subsequently received 0.65 mmol/kg GSH or NAC at 1.5 h after APAP. Glutamate and succinate levels were measured 45 min after injection of [U-13C]glucose, i.e. at 2.25 h after APAP (B, D) or 45 min after injection of [U-13C]glucose, i.e. 6.75 h after APAP (C, E). Data represent means ± SE of n = 4 animals per group. *P<0.05 (compared to controls, C); #P<0.05 (compared to APAP/GSH)
High doses of NAC effectively protect against APAP hepatotoxicity
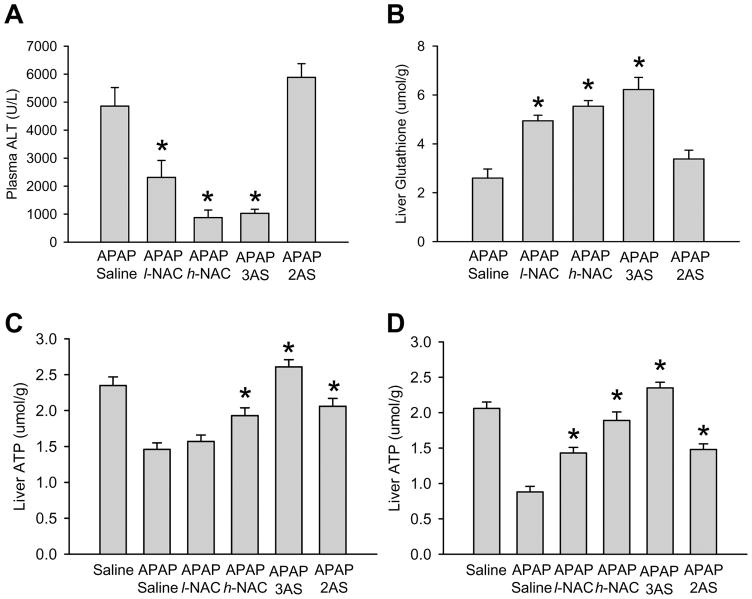
To evaluate if the difference between GSH and NAC in protecting against APAP toxicity is caused by the different amount of amino acids administered, 3 times higher doses of NAC were injected and the efficacy compared to the 3 individual amino acids of GSH (glutamate, glycine and cysteine) and to a similar dose of only 2 amino acids (glutamate, glycine). Both, the high dose of NAC and the 3 amino acid groups were more effective than the low NAC group in protecting against APAP toxicity (Figure 6A) and in maintaining liver ATP levels at early and later time points (Figure 6C, D). All three treatments were equally effective in restoring total hepatic GSH levels (Figure 6B). In contrast, treatment with 2 amino acids (lack of cysteine) did not improve the recovery of hepatic GSH levels and failed to protect despite the partial improvement of hepatic ATP levels (Figure 6A-D). The substrate flux through the Krebs cycle correlated with ATP formation in the different treatment groups (supplemental figure 2).
Figure 6.
A-B. Plasma alanine aminotransferase (ALT) activities and the hepatic content of glutathione (GSH+GSSG) were measured in mice treated with 300 mg/kg acetaminophen (APAP) for 6 h. Some of the animals received additionally 10 ml/kg saline, 0.65 mmol/kg N-acetylcysteine (l-NAC), 1.95 mmol/kg NAC (h-NAC), a mixture of 3 amino acids (0.65 mmol/kg of glycine, glutamic acid and NAC) (3AS) or a mixture of 2 amino acids (0.98 mmol/kg glycine and glutamic acid) (2AS) iv 1.5 h after APAP. Data represent means ± SE of n = 5 animals per group. *P<0.05 (compared to controls); #P<0.05 (compared to APAP/saline). C-D. Tissue concentrations of ATP (μmol/g wet weight), as calculated from its resonances in 31P-NMR spectra of liver extracts. The mice were treated as under A-B and ATP levels were measured 2.25 h (C) or 6.75 h (D) after treatment with APAP. Data represent means ± SE of n = 4 animals per group. *P<0.05 (compared to APAP/saline)
DISCUSSION
The objectives of this investigation were to evaluate if there is a difference in the efficacy to protect against APAP hepatotoxicity between GSH and clinically relevant doses of NAC and to assess the mechanisms of this protection. Our data indicate that when animals are treated with low equimolar doses of GSH or NAC, GSH is more effective due to the higher amounts of amino acids, which are not only used to re-synthesize hepatic GSH but are also metabolized to serve as energy substrates in the Krebs cycle. This difference in protection between GSH and NAC can be eliminated if higher doses of NAC are used.
Mechanisms of protection I: preventing covalent binding
The best established and most effective mechanism of protection by GSH or NAC is to enhance the scavenging capacity for NAPQI in hepatocytes. This prevents covalent modification of cellular proteins and thereby blocks the initiation of APAP toxicity.7,9 Intravenously administered GSH is rapidly degraded in the kidney with a half-life in plasma of <2 min and the individual amino acids are re-absorbed.19. NAC or the amino acids of GSH are taken up into hepatocytes and are being used for re-synthesis of hepatocellular GSH, which is consumed by conjugation with NAPQI.7–9 Despite the capacity of NAC to directly react with NAPQI, it has been clearly demonstrated that the protection of NAC or GSH against APAP overdose requires the synthesis of GSH.7–9,19 However, in order to be effective through this mechanism, GSH or NAC has to be administered during the metabolism phase of APAP toxicity. The delayed presentation of overdose patients to the emergency room can limit the efficacy of NAC through this mechanism.
Mechanisms of protection II: scavenging of reactive oxygen and peroxynitrite
Covalent binding of NAPQI to mitochondrial proteins is a critical step that links the metabolic activation of APAP to the mitochondrial dysfunction.29 This leads to inhibition of the mitochondrial respiration with enhanced formation of reactive oxygen species and peroxynitrite in mitochondria.17,25–27 The oxidant stress can directly trigger the mitochondrial membrane permeability transition pore opening with collapse of the mitochondrial membrane potential.28,30 In addition, the oxidant stress can activate the c-jun N-terminal kinase, which can translocate to the mitochondria and facilitate the mitochondrial membrane permeability transition pore opening.31 Either mechanism assumes a critical role of mitochondrial reactive oxygen and peroxynitrite formation in the pathophysiology. The toxicity of these reactive oxygen and reactive nitrogen species is potentiated by the fact that mitochondrial GSH levels are severely depleted during APAP metabolism, which leaves these cell organelles highly vulnerable.25 Thus, delayed treatment with GSH or NAC can accelerate the recovery of mitochondrial GSH levels and scavenge reactive oxygen and reactive nitrogen species.15,18 Despite the initial covalent binding, this treatment limits cellular necrosis, improves survival and facilitates repair of the damaged tissue.16 Thus, a critical second mechanism of protection by NAC and GSH is the protection against oxidant stress and peroxynitrite specifically in mitochondria.
Mechanisms of protection III: mitochondrial energy substrates
The higher efficacy of GSH versus an equimolar dose of NAC in protecting against APAP suggests the involvement of a third mechanism. As was shown, both GSH and NAC provide enough cysteine to allow for an effective re-synthesis of the depleted cellular GSH levels. However, two critical differences between GSH- and NAC-treated animals emerged. First, cellular ATP levels were significantly better preserved with GSH treatment than with NAC. Based on the higher substrate supply for the Krebs cycle, this was most likely caused by the use of the excess amino acids not needed for GSH synthesis as energy substrates. The higher ATP levels support energy-requiring cellular functions including the maintenance of ion gradients by Na+/K+-ATPase and Ca2+-ATPase. Dysfunction of these enzymes has been implicated in necrotic cell death.32,33 Although the Km for ATP of transporters and enzymes is lower than the declining tissue ATP levels after APAP treatment, it needs to be kept in mind that these concentrations represent the average in tissue homogenate, which includes healthy as well as dying cells. Thus, one would expect that ATP levels in cells of the centrilobular area are much lower than the average and may well be in the range of the Km for ATP-dependent transporters and enzymes. In addition, the improved energy supply to mitochondria may be responsible for the accelerated uptake of cytosolic GSH into mitochondria as was observed after GSH treatment. GSH is transported into mitochondria by the dicarboxylate carrier and the oxoglutarate carrier, which mediate electroneutral exchange of dicarboxylates for inorganic phosphate and 2-oxoglutarate for other dicarboxylates, respectively.34–36 During the post-metabolism phase of APAP toxicity, mitochondrial GSH is most critical for the effective scavenging of reactive oxygen species and specifically peroxynitrite.15 The higher mitochondrial GSH levels and the reduced areas of nitrotyrosine staining correlated with the reduced areas of necrosis after GSH compared to NAC treatment.
Supply of cysteine without providing additional energy substrates leads to sub-optimal protection as was observed with NAC treatment. On the other hand, supply of excess amino acids without cysteine leads to a partial recovery of ATP levels without GSH synthesis but fails to prevent liver cell necrosis. This indicates that just supporting mitochondrial energy metabolism alone is insufficient to prevent cell death. These data extend previous data obtained on the effects of NAC and GSH in non-acetaminophen toxicity in mice.12 In particular, NAC significantly increased the flux through PDH, an effect which has been shown to be uncoupled from GSH synthesis, and was associated with the prevention of liver injury induced by tert-butylhydroperoxide and 3-nitropropionic acid.12 This study also showed that NAC has a limited capacity to increase GSH de novo synthesis, but that the administration of the GSH precursor cysteine alone in equimolar concentrations to NAC does not protect from a disturbed mitochondrial energy metabolism. The present study, on the other hand, provide evidence that injection of GSH itself or of excess amino acids in APAP-treated animals clearly result in a better recovery of ATP levels. It further suggests a dual role of GSH supplementation in prevention of mitochondrial energy failure together with cellular GSH supply. This would also explain the higher efficiency when increasing the NAC doses and therefore of the amino acid cysteine. Thus, based on these observations it can be concluded that a combination of cysteine supply for glutathione synthesis and excess amino acids for metabolism in the Krebs cycle provides the most effective protection against APAP-induced mitochondrial dysfunction and necrotic cell death during the later, post-metabolism phase of the injury. This effect can be achieved by injection of either GSH or NAC in sufficient quantities.
Although our data show that at a dose of 0.65 mmol/kg GSH is more effective than NAC, the effect is clearly caused by the 3-fold higher amount of amino acids injected with GSH compared to an equimolar dose of NAC. In fact, a 3-fold higher dose of NAC shows equally effective protection as with GSH or the mixture of the individual amino acids of GSH. Since the practice guidelines for the use of NAC recommend much higher doses than would be needed for the re-synthesis of hepatic GSH levels alone,6 our data suggest that the reason for the efficacy of high doses of NAC at these later time points is caused by recovery of hepatic and in particular mitochondrial GSH levels and the improved mitochondrial bioenergetics.
In summary, our data indicate that the amino acids supplied with the delayed treatment of GSH or NAC are being used for the re-synthesis of hepatic glutathione levels, which protect against cell injury by scavenging reactive oxygen and peroxynitrite in mitochondria. However, excess amino acids, i.e. amino acids not used for GSH synthesis, serve as energy substrates for the Krebs cycle and support the improved maintenance of hepatic ATP levels. Thus, the optimal protection by delayed GSH or NAC treatment involves the combination of 2 mechanisms, i.e., the accelerated recovery of mitochondrial GSH levels and support of the mitochondrial bioenergetics. These new findings provide the rationale for the clinical use of high doses of NAC well beyond the metabolism of APAP. In addition, these data suggest that more effective substrates for the mitochondrial energy metabolism than excess NAC may further limit cell death and improve regeneration after APAP overdose.
Supplementary Material
Acknowledgments
FINANCIAL SUPPORT: This investigation was supported in part by National Institutes of Health Grants R01 DK070195 and R01 AA12916, and by grants P20 RR016475 and P20 RR 021940 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health.
ABBREVIATIONS
- APAP
acetaminophen
- ALT
alanine aminotransferase
- GSH
glutathione
- GSSG
glutathione disulfide
- NAC
N-acetylcysteine (NAC)
- NAPQI
N-acetyl-p-benzoquinone imine
- NEM
N-ethylmaleimide
- PC
pyruvate carboxylase
- PDH
pyruvate dehydrogenase
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay
Contributor Information
Chieko Saito, Email: csaito@kumc.edu.
Claudia Zwingmann, Email: czwingmann@web.de.
Hartmut Jaeschke, Email: hjaeschke@kumc.edu.
References
- 1.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. HEPATOLOGY. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187:211–217. [PubMed] [Google Scholar]
- 3.Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973;187:195–202. [PubMed] [Google Scholar]
- 4.Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973;187:185–194. [PubMed] [Google Scholar]
- 5.Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;2:432–434. doi: 10.1016/s0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- 6.Polson J, Lee WM. American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. HEPATOLOGY. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 7.Lauterburg BH, Corcoran GB, Mitchell JR. Mechanism of action of N-acetylcysteine in the protection against the hepatotoxicity of acetaminophen in rats in vivo. J Clin Invest. 1983;71:980–991. doi: 10.1172/JCI110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corcoran GB, Wong BK. Role of glutathione in prevention of acetaminophen-induced hepatotoxicity by N-acetyl-L-cysteine in vivo: studies with N-acetyl-D-cysteine in mice. J Pharmacol Exp Ther. 1986;238:54–61. [PubMed] [Google Scholar]
- 9.Corcoran GB, Racz WJ, Smith CV, Mitchell JR. Effects of N-acetylcysteine on acetaminophen covalent binding and hepatic necrosis in mice. J Pharmacol Exp Ther. 1985;232:864–872. [PubMed] [Google Scholar]
- 10.Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. N Engl J Med. 1988;319:1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 11.Harrison PM, Keays R, Bray GP, Alexander GJM, Williams R. Improved outcome of paracetamol-induced fulminant hepatic failure by late administration of acetylcysteine. Lancet. 1990;335:1572–1573. doi: 10.1016/0140-6736(90)91388-q. [DOI] [PubMed] [Google Scholar]
- 12.Zwingmann C, Bilodeau M. Metabolic insights into the hepatoprotective role of N-acetylcysteine in mouse liver. HEPATOLOGY. 2006;43:454–463. doi: 10.1002/hep.21075. [DOI] [PubMed] [Google Scholar]
- 13.Jaeschke H, Knight TR, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett. 2003;144:279–288. doi: 10.1016/s0378-4274(03)00239-x. [DOI] [PubMed] [Google Scholar]
- 14.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 15.Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exper Ther. 2002;303:468–475. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- 16.Bajt ML, Knight TR, Farhood A, Jaeschke H. Scavenging peroxynitrite with glutathione promotes regeneration and enhances survival during acetaminophen-induced liver injury in mice. J Pharmacol Exper Therap. 2003;307:67–73. doi: 10.1124/jpet.103.052506. [DOI] [PubMed] [Google Scholar]
- 17.Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, et al. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exper Therap. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- 18.James LP, McCullough SS, Lamps LW, Hinson JA. Effect of N-acetylcysteine on acetaminophen toxicity in mice: relationship to reactive nitrogen and cytokine formation. Toxicol Sci. 2003;75:458–467. doi: 10.1093/toxsci/kfg181. [DOI] [PubMed] [Google Scholar]
- 19.Wendel A, Jaeschke H. Drug-induced lipid peroxidation in mice--III. Glutathione content of liver, kidney and spleen after intravenous administration of free and liposomally entrapped glutathione. Biochem Pharmacol. 1982;31:3607–3611. doi: 10.1016/0006-2952(82)90583-4. [DOI] [PubMed] [Google Scholar]
- 20.Salminen WF, Jr, Voellmy R, Roberts SM. Effect of N-acetylcysteine on heat shock protein induction by acetaminophen in mouse liver. J Pharmacol Exp Ther. 1998;286:519–524. [PubMed] [Google Scholar]
- 21.McConnachie LA, Mohar I, Hudson FN, Ware CB, Ladiges WC, Fernandez C, et al. Glutamate cysteine ligase modifier subunit deficiency and gender as determinants of acetaminophen-induced hepatotoxicity in mice. Toxicol Sci. 2007;99:628–636. doi: 10.1093/toxsci/kfm165. [DOI] [PubMed] [Google Scholar]
- 22.Whitehouse LW, Wong LT, Paul CJ, Pakuts A, Solomonraj G. Postabsorption antidotal effects of N-acetylcysteine on acetaminophen-induced hepatotoxicity in the mouse. Can J Physiol Pharmacol. 1985;63:431–437. doi: 10.1139/y85-075. [DOI] [PubMed] [Google Scholar]
- 23.Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752–759. doi: 10.1016/0076-6879(90)86175-u. [DOI] [PubMed] [Google Scholar]
- 24.Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- 25.Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen-induced liver injury: role of mitochondrial oxidant stress. Toxicol Sci. 2001;62:212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- 26.Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990;255:935–941. [PubMed] [Google Scholar]
- 27.Tirmenstein MA, Nelson SD. Acetaminophen-induced oxidation of protein thiols. Contribution of impaired thiol-metabolizing enzymes and the breakdown of adenine nucleotides. J Biol Chem. 1990;265:3059–3065. [PubMed] [Google Scholar]
- 28.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrotic and apoptotic cell death to cultured mouse hepatocytes. HEPATOLOGY. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 29.Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- 30.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Increase of cytosolic ferrous iron induces the mitochondrial permeability transition in acetaminophen-induced toxicity to mouse hepatocytes [ABSTRACT] HEPATOLOGY. 2004;40(Suppl):647A. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 31.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsokos-Kuhn JO, Hughes H, Smith CV, Mitchell JR. Alkylation of the liver plasma membrane and inhibition of the Ca2+ ATPase by acetaminophen. Biochem Pharmacol. 1988;37:2125–2131. doi: 10.1016/0006-2952(88)90570-9. [DOI] [PubMed] [Google Scholar]
- 33.Carini R, Bellomo G, Benedetti A, Fulceri R, Gamberucci A, Parola M, et al. Alteration of Na+ homeostasis as a critical step in the development of irreversible hepatocyte injury after adenosine triphosphate depletion. HEPATOLOGY. 1995;21:1089–1098. [PubMed] [Google Scholar]
- 34.Lash LH. Mitochondrial glutathione transport: physiological, pathological and toxicological implications. Chem Biol Interact. 2006;163:54–67. doi: 10.1016/j.cbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong Q, Putt DA, Xu F, Lash LH. Hepatic mitochondrial transport of glutathione: studies in isolated rat liver mitochondria and H4IIE rat hepatoma cells. Arch Biochem Biophys. 2008;474:119–127. doi: 10.1016/j.abb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez-Checa JC, Kaplowitz N. Hepatic mitochondrial glutathione: transport and role in disease and toxicity. Toxicol Appl Pharmacol. 2005;204:263–273. doi: 10.1016/j.taap.2004.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.