Abstract
Although the reductionist approach has served science well for 400 years, the accumulation of details can obscure the truth if the original premise is incorrect. One such premise has been that successful organ transplantation and bone marrow engraftment are fundamentally different outcomes involving separate and distinct mechanisms. Some historical clinical observations pointed to a different conclusion almost from the beginning and included clues about how to induce tolerance with the aid of immunosuppression.
Clinical organ transplantation began between 1959 and 1962 with the greater than 1-year survival of six kidney allograft recipients, the first in Boston (1) and the next five in Paris (2,3). The patients had been conditioned before transplantation with sublethal total body irradiation. There had never been a single example before 1960 of long-term survival after kidney transplantation in an animal model with irradiation or any other kind of treatment. Yet, two of the six recipients (both of fraternal twin kidneys) were not treated with immunosuppression after transplantation and had normal renal function until their deaths more than two decades later. The next milestone was the function for 17 months of a nonrelated allograft from the time of its transplantation in April 1962 under azathioprine therapy, without host cytoablation (4). Except for this case, however, the initial clinical results with drug immunosuppression were discouraging.
In 1963, the two features of the adaptive immune response that eventually would make transplantation of all kinds of organs practical were described in the title of a report of kidney recipients treated at the University of Colorado (5). The first observation was that rejection developing under azathioprine was readily reversible by prednisone. The second was “.. the subsequent development of homograft tolerance.” The evolution of partial tolerance was inferred from the rapid decline of the need for immunosuppression that succeeded the successful reversal of rejection. The term “tolerance” was controversial, however, because the patients were still receiving maintenance treatment. Moreover, it already had become dogma that the donor leukocyte chimerism-associated mechanisms of experimental tolerance models (6,7) were not the same as those of organ engraftment.
In fact, tolerance proved to be the bon mot. Nine (19.6%) of the 46 recipients of allografts from live-related donors treated in Colorado between the autumn of 1962 and March 1963 had continuous graft function for most or all of the next four decades. One of the nine with a serum creatinine level less than 1.0 mg/100 mL was recently murdered and had a normal transplanted kidney at coroner’s autopsy; seven of the other eight still have good renal function after 39 to 40.5 years. Importantly, seven of the nine patients became immunosuppression free for periods ranging from 3 to 38 years (Fig. 1). Those who remain bear eight of the nine longest-surviving allografts in the world today, including the four longest (8).
Figure 1.
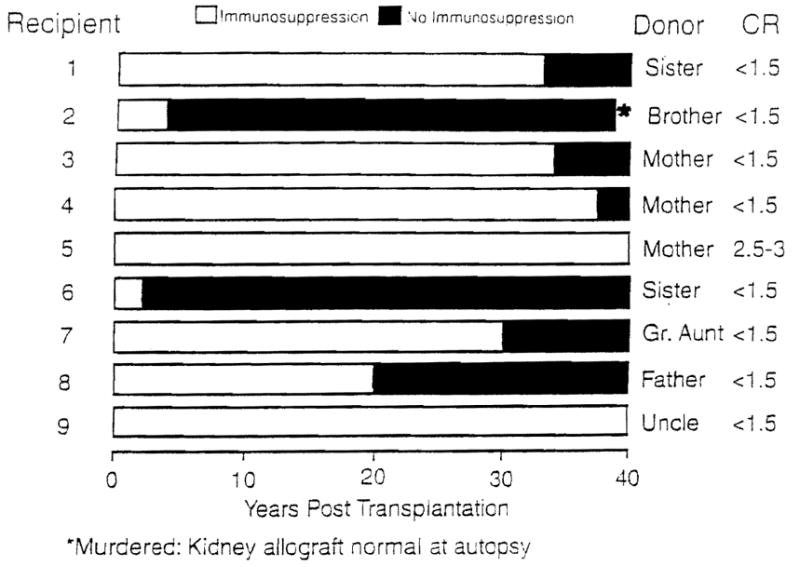
Long-term survival and drug-free tolerance in kidney allograft recipients treated in 1962 to 1963. Follow-up is to May 2003. Cr, serum creatinine. Black boxes, off immunosuppression.
Although a few isolated cases of uneventful prolonged drug discontinuance have been reported since (usually because of noncompliance), no comparable cluster of immunosuppression-free kidney recipients was compiled anywhere during the next 40 years. What was the explanation for the unique Denver experience? It was not a simple matter of accidental good histocompatibility. Although all nine of the allografts were from blood relatives (Fig. 1), only two were HLA matched, and in one of these (case 1), there was a B to A blood group incompatibility.
A clue was a revision in the use of immunosuppression made in December 1963. Based on the results in preclinical studies in dogs (9), the first 45 patients had been pretreated with daily doses of azathioprine for 1 to 2 weeks (5). Azathioprine was continued after transplantation, adding prednisone only when there was deterioration of initially good postoperative renal function. In subsequent cases, the pre-treatment was de-emphasized because of immunosuppression-related infectious complications encountered in the pre-operative period. A second more formal modification was instituted in December 1963 (10). This policy change was prompted by the early losses of several kidney allografts to nonreversible acute rejection. From this time onward, prophylactic high doses of prednisone were begun at the time of operation, rather than as needed.
Although better control of acute rejection was described, it was specifically noted that the rate of late rejection, which had been 5% in the original patients, rose to 30% in the patients treated prophylactically with steroids (10). The warning notation was generally unheeded, including by us. However, in Belfast, where the nephrologist Mary McGeown persisted in using azathioprine with minimum steroid intervention unless specifically indicated, the best precyclosporine results in the world were obtained (11). Although the Belfast approach was heralded by Peter Morris (Oxford), the consensus movement to heavy prophylactic immunosuppression was inexorable.
Better drugs have resulted in a reduced use of steroids, but the concept of heavy early immunosuppression with multiple agents (often called “induction”) has dominated the practice of transplantation to the present day. In an important exception, Calne et al. (12) recently treated cadaver kidney recipients with a few perioperative doses of a broadly reacting humanized monoclonal antibody (alemtuzumab [campathR)), followed by low daily doses of cyclosporine. As in the earlier Colorado experience, they recognized that a significant degree of tolerance had been achieved (called “prope tolerance”).
Liver Transplantation
Between 1965 and 1967, drug-free survival of canine liver recipients was reported after a short course of azathioprine (13) or a few perioperative doses of antilymphocyte serum or antilymphocyte globulin (ALG) (14). Liver transplantation with lifetime survival subsequently was demonstrated without treatment in some outbred pigs (15) and in all experiments with selective strain combinations of inbred rats and mice. Most such liver recipients accept skin or organ grafts from donors of the same strain but not from third-party donors. Importantly, similar tolerance can be induced spontaneously or under a brief course of immunosuppression by the heart and kidney but in a much more restricted number of strain combinations (reviewed in 16).
In view of these experimental observations, it was not surprising that drug-free tolerance was observed more frequently in humans after transplantation of the liver than of any other organ. However, significant numbers of such cases were compiled only in four periods. The first was the mortality-blighted decade of the 1970s when immunosuppression was with azathioprine (or cyclophosphamide), combined with a short course of pre- and postoperative ALG and the sparing use of prednisone (17). In 1995, 12 of our 42 patients still surviving from this era already had been off all immunosuppression for 1 to 17 years (18). These patients currently remain healthy for as long 33 years after transplantation, and many of the remaining 30 have since been weaned from drugs under supervision (19).
When the exorbitant mortality and the high rate of rejection after liver transplantation declined with the advent of the calcineurin-inhibiting drugs, the frequency of tolerant liver and other organ recipients was expected to increase. This was seen, however, only in liver recipients treated in 1980 to 1981 and in 1989 to 1990 during periods when cyclosporine and tacrolimus were given as monotherapy and high doses of steroids were added only to treat rejection (19). After the consensus move to multiagent prophylactic immunosuppression from the time of transplantation, complete drug weaning became rare. Liver-induced tolerance emerged for the fourth time in the late 1990s in Kyoto, Japan (20), where pediatric recipients of parental livers were successfully weaned from steroid-sparing tacrolimus-based immunosuppression similar to that originally used in 1989 to 1990.
Back to the Future
An explanation for the diverse observations began to emerge with the discovery in 1992 of low-level donor leukocyte chimerism in surviving human kidney, liver, and other kinds of organ recipients from the earlier transplant eras (17,21). With these observations, it was proposed that organ and bone marrow cell engraftment were variations on the same theme. In this paradigm, the two kinds of alloengraftment are mirror image products of a double immune reaction, host-versus-graft (HVG) and graft-versus-host, in which “.. responses of coexisting donor and recipient cells, each to the other, cause reciprocal clonal exhaustion followed by peripheral clonal deletion..” (17,21).
The typically dominant HVG response of organ transplantation is induced by the preferential migration of the graft’s passenger leukocytes (hematolymphopoietic cells of bone marrow origin) to host lymphoid organs, where they induce a donor-specific (clonal) T-cell response. The response proceeds to rejection in untreated humans and most animals. In the historical experimental models of spontaneous tolerance, however, the HVG reaction is too weak to eliminate the migratory leukocytes, most commonly when the allograft is the leukocyte-rich liver. Therefore, the response is exhausted and deleted (Fig. 2A). Because the exhaustion deletion is never complete, maintenance of the level achieved acutely (i.e., in the first 30–60 days) depends on the persistence of peripheralized donor leukocytes. The manner in which a small number of these cells (microchimerism) perpetuate tolerance by the combined mechanisms of clonal exhaustion-deletion and immune ignorance has been described extensively (22,23).
Figure 2.
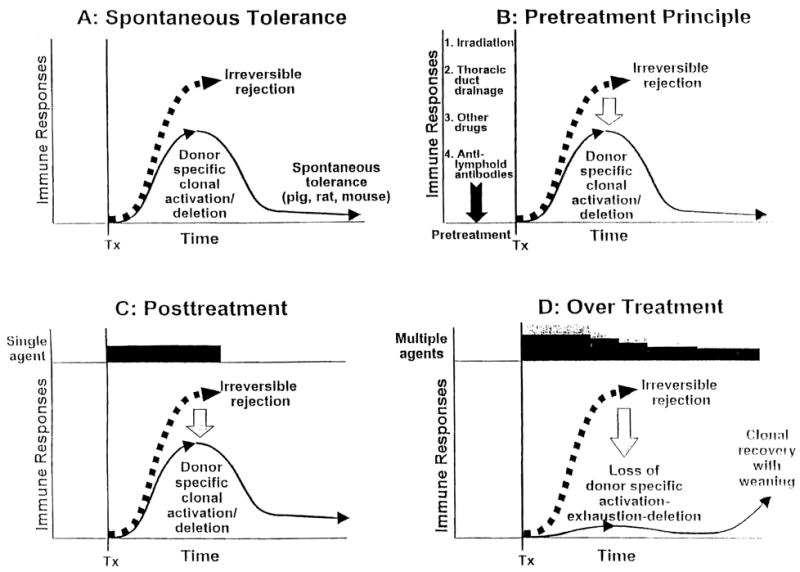
Rather than producing rejection (thick dashed arrows), the donor-specific immune response to allografts may be exhausted and deleted as depicted by the fall of the initially ascending continuous thin lines, (1) if the unmodified recipient response is too weak to eliminate the migratory donor cells (spontaneous tolerance models), (2) when recipient immune responsiveness is weakened in advance of transplantation (the pretreatment principle), or (3) when the recipient response is reduced into the deletable range after transplantation with just the right amount of immunosuppression. However, over treatment after transplantation (shown in D with multilayered bars), reduces the efficiency and extent of clonal exhaustion-deletion and is therefore antitolerogenic (see text for details). Tx, transplantation.
The ways by which immunosuppression can permit engraftment in otherwise rejecting experimental models and in humans are depicted in Figure 2(B and C). If immunosuppression is administered before transplantation, the antigraft response to the organ transplant’s passenger leukocytes can be reduced into a more readily deletable range (Fig. 2B). In some models, including that of irradiated human recipients of HLA-matched bone marrow cell allografts, deletional tolerance can be accomplished regularly with host pretreatment only. In retrospect, the kidney passenger leukocytes, combined with the total body irradiation used by Murray (1) and Hamburger (2) in their historical fraternal twin kidney recipients of 1959 were sufficient to induce sustained tolerance. However, less drastic conditioning without the risk of graft-versus-host disease is possible with thoracic duct drainage, total lymphoid irradiation, conventional antirejection drugs, and especially antilymphocyte serum, ALG, and other antilymphoid antibody preparations (reviewed in 24,25) (Fig. 2B).
The antigraft response also can be rendered deletable by immunosuppression after the arrival of the antigen, as shown in Figure 2C (23). However, this is a double-edged sword. To the extent that antigen-specific immune activation is prevented, the derivative event of exhaustion-deletion may be reduced (Fig. 2D). As global immune activity returns in an initially overimmunosuppressed host, the undeleted clone recovers with the rest of the immune repertoire. Continued graft survival is then dependent on continuous immunosuppression.
Two therapeutic principles of optimal immunosuppression derive from this view of drug-assisted tolerogenesis (23): recipient pretreatment and the use of the least posttransplantation immunosuppression consistent with graft survival and stable function. In the Colorado experience of 1962 to 1963, both principles were initially applied, but soon judged to be impractical because of the morbidity of pretreatment and the erratic control of acute rejection. When they were abandoned after 1963 and replaced by a philosophy of heavy immunosuppression, drug-free kidney recipients all but disappeared.
In contrast to the kidney, the liver (with its larger endowment of migratory passenger leukocytes) continued to induce such tolerance in some human recipients, first under relatively ineffective azathioprine-based immunosuppression. The additional cases that appeared with the introduction of cyclosporine (1980–1981) and tacrolimus (1989–1990, and more recently in Japan), were reported only when these “modern” drugs were used in steroid-sparing regimens: that is, in unknowing compliance with the principle of minimal posttransplantation immunosuppression.
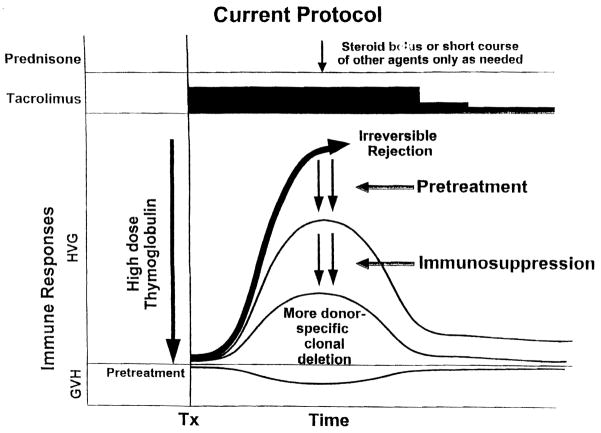
With the greater ability to modify, reverse, and control rejection with today’s armamentarium of potent immunosuppressants, we have combined both therapeutic principles for transplantation of the kidney, liver, pancreas, intestine, and lung (Fig. 3). The results suggest that recipients with a high, if not absolute, degree of sustained donor-specific nonreactivity (tolerance) can be systematically produced (26).
Figure 3.
Current protocol of immunosuppression in which pre-treatment is given with a large dose of a potent ALG (thymoglobulin) followed by tacrolimus monotherapy to which other agents are added only for rejection. The inverted curve at the bottom shows the usually silent graft-versus-host (GVH) reaction. HVG, host-versus-graft.
Footnotes
The work was supported by National Institutes of Health Grant DK 29961 and DK 64207-01.
References
- 1.Murray JE, Merrill JP, Dammin GJ, et al. Study of transplantation immunity after total body irradiation: clinical and experimental investigation. Surgery. 1960;48:272–284. [PubMed] [Google Scholar]
- 2.Hamburger J, Vaysse J, Crosnier J, et al. Renal homotransplantation in man after radiation of the recipient. Am J Med. 1962;32:854–871. doi: 10.1016/0002-9343(62)90032-3. [DOI] [PubMed] [Google Scholar]
- 3.Küss R, Legrain M, Mathé G, et al. Homologous human kidney transplantation: experience with six patients. Postgrad Med J. 1962;38:528–531. doi: 10.1136/pgmj.38.443.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray JE, Merrill JP, Harrison JH, et al. Prolonged survival of human-kidney homografts by immunosuppressive drug therapy. N Engl J Med. 1963;268:1315–1323. doi: 10.1056/NEJM196306132682401. [DOI] [PubMed] [Google Scholar]
- 5.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]
- 6.Billingham RE, Brent L, Medawar PB. “Actively acquired tolerance” of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 7.Main JM, Prehn RT. Successful skin homografts after the administration of high dosage X radiation and homologous bone marrow. J Natl Cancer Inst. 1955;15:1023–1029. [PubMed] [Google Scholar]
- 8.Cecka JM, Terasaki PI. Clinical transplants 2001. Los Angeles: UCLA Immunogenetics Center; 2002. World transplant records; p. 279. [Google Scholar]
- 9.Starzl TE. Experience in renal transplantation. Philadelphia: W. B. Saunders Co; 1964. Anitrejection therapy; pp. 132–133. [Google Scholar]
- 10.Starzl TE. Experience in renal transplantation. Philadelphia: W. B. Saunders Co; 1964. Pretreatment with prednisone; pp. 171–178. [Google Scholar]
- 11.McGeown M, Douglas JF, Donaldson RA, et al. Ten-year results of renal transplantation with azathioprine and prednisolone as only immunosuppression. Lancet. 1988;1:983–985. doi: 10.1016/s0140-6736(88)91792-8. [DOI] [PubMed] [Google Scholar]
- 12.Calne RY, Friend PJ, Moffatt S, et al. Prope tolerance perioperative campath IH, and low-dose cyclosporin monotherapy in renal allograft recipients. Lancet. 1998;351:1701–1702. doi: 10.1016/S0140-6736(05)77739-4. [DOI] [PubMed] [Google Scholar]
- 13.Starzl TE, Marchioro TL, Porter KA, et al. Factors determining short- and long-term survival after orthotopic liver homotransplantation in the dog. Surgery. 1965;58:131–155. [PMC free article] [PubMed] [Google Scholar]
- 14.Starzl TE, Marchioro TL, Porter KA, et al. The use of heterologous anti-lymphoid agents in canine renal and liver homotransplantation and in human renal homotransplantation. Surg Gynecol Obstet. 1967;124:301–318. [PMC free article] [PubMed] [Google Scholar]
- 15.Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–474. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 16.Murase N, Starzl TE, Tanabe M, et al. Variable chimerism, graft-versus-host disease, and tolerance after different kinds of cell and whole organ transplantation from Lewis to Brown Norway rats. Transplantation. 1995;60:158–171. doi: 10.1097/00007890-199507000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 18.Starzl TE, Demetris AJ, Murase N, et al. The lost chord: microchimerism. Immunol Today. 1996;17:577–584. 588. doi: 10.1016/s0167-5699(96)10070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramos HC, Reyes J, Abu-Elmagd K, et al. Weaning of immunosuppression in long term liver transplant recipients. Transplantation. 1995;59:212–217. doi: 10.1097/00007890-199501270-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takatsuki M, Uemoto SH, Inomata Y, et al. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation. 2001;72:449–454. doi: 10.1097/00007890-200108150-00016. [DOI] [PubMed] [Google Scholar]
- 21.Starzl TE, Demetris AJ, Murase N, et al. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Starzl TE, Zinkernagel R. Antigen localization and migration in immunity and tolerance. N Engl J Med. 1998;339:1905–1913. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starzl TE, Zinkernagel R. Transplantation tolerance from a historical perspective. Nat Rev Immunol. 2001;1:233–239. doi: 10.1038/35105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starzl TE, Weil R, III, Koep LJ. The pretreatment principle in renal transplantation as illustrated by thoracic duct drainage. In: Cummings NB, Klahr S, editors. Chronic renal disease. New York: Plenum Publishing Corporation; 1985. pp. 507–515. [Google Scholar]
- 25.Starzl TE. The saga of liver replacement, with particular reference to the reciprocal influence of liver and kidney transplantation (1955–1967) J Am Coll Surg. 2002;195:587–610. doi: 10.1016/s1072-7515(02)01498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starzl T, Murase N, Abu-Elmagd K, et al. Tolerogenic immunosuppression for organ transplantation. Lancet. 2003;361:1502–1510. doi: 10.1016/s0140-6736(03)13175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]