Abstract
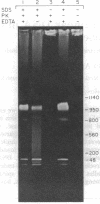
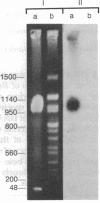
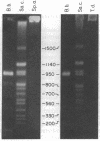
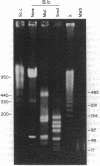
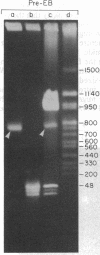
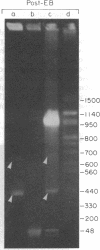
Using pulsed-field gel electrophoresis we examined the genome of Borrelia burgdorferi, a eubacterium of the spirochete phylum and the agent of Lyme disease. A population of this species' cells was lysed in situ in agarose blocks. An abundant DNA form that behaved as a linear duplex molecule under different electrophoretic conditions was found. The estimated size of the molecule was 950 kilobases. DNA from two other genera of spirochetes did not enter the gel under these conditions. These studies indicate that Borrelia spirochetes, perhaps uniquely among prokaryotic organisms, have linear chromosomes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G., Garon C. F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987 Jul 24;237(4813):409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F. Biology of Borrelia species. Microbiol Rev. 1986 Dec;50(4):381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J Clin Microbiol. 1988 Mar;26(3):475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M. Characterization of the 'unusual' mobility of large circular DNAs in pulsed field-gradient electrophoresis. Nucleic Acids Res. 1988 Feb 11;16(3):925–939. doi: 10.1093/nar/16.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darai G., Zöller L., Matz B., Delius H., Speck P. T., Flügel R. M. Analysis of Mycoplasma hyorhinis genome by use of restriction endonucleases and by electron microscopy. J Bacteriol. 1982 May;150(2):788–794. doi: 10.1128/jb.150.2.788-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner K., Laas W., Patterson D. Fractionation of large mammalian DNA restriction fragments using vertical pulsed-field gradient gel electrophoresis. Somat Cell Mol Genet. 1986 Mar;12(2):185–195. doi: 10.1007/BF01560665. [DOI] [PubMed] [Google Scholar]
- Garvey E. P., Santi D. V. Stable amplified DNA in drug-resistant Leishmania exists as extrachromosomal circles. Science. 1986 Aug 1;233(4763):535–540. doi: 10.1126/science.3726545. [DOI] [PubMed] [Google Scholar]
- Greenberg E. P., Canale-Parola E. Chemotaxis in Spirochaeta aurantia. J Bacteriol. 1977 Apr;130(1):485–494. doi: 10.1128/jb.130.1.485-494.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S. F., Burgdorfer W., Barbour A. G. Bacteriophage in the Ixodes dammini spirochete, etiological agent of Lyme disease. J Bacteriol. 1983 Jun;154(3):1436–1439. doi: 10.1128/jb.154.3.1436-1439.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. H., Grossman L. I. Electrophoresis of DNA in agarose gels. Optimizing separations of conformational isomers of double- and single-stranded DNAs. Biochemistry. 1977 Sep 20;16(19):4217–4225. doi: 10.1021/bi00638a014. [DOI] [PubMed] [Google Scholar]
- Lee J. J., Smith H. O. Sizing of the Haemophilus influenzae Rd genome by pulsed-field agarose gel electrophoresis. J Bacteriol. 1988 Sep;170(9):4402–4405. doi: 10.1128/jb.170.9.4402-4405.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J. T., Simon M. I., Barbour A. G. Antigenic variation is associated with DNA rearrangements in a relapsing fever Borrelia. Cell. 1985 Jun;41(2):403–409. doi: 10.1016/s0092-8674(85)80013-1. [DOI] [PubMed] [Google Scholar]
- Mickel S., Arena V., Jr, Bauer W. Physical properties and gel electrophoresis behavior of R12-derived plasmid DNAs. Nucleic Acids Res. 1977;4(5):1465–1482. doi: 10.1093/nar/4.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J., Hoch J. A. Revised genetic linkage map of Bacillus subtilis. Microbiol Rev. 1985 Jun;49(2):158–179. doi: 10.1128/mr.49.2.158-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasterk R. H., Simon M. I., Barbour A. G. Transposition of structural genes to an expression sequence on a linear plasmid causes antigenic variation in the bacterium Borrelia hermsii. Nature. 1985 Nov 21;318(6043):257–263. doi: 10.1038/318257a0. [DOI] [PubMed] [Google Scholar]
- Pyle L. E., Corcoran L. N., Cocks B. G., Bergemann A. D., Whitley J. C., Finch L. R. Pulsed-field electrophoresis indicates larger-than-expected sizes for mycoplasma genomes. Nucleic Acids Res. 1988 Jul 11;16(13):6015–6025. doi: 10.1093/nar/16.13.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley M., Anilionis A. Evolution of the bacterial genome. Annu Rev Microbiol. 1978;32:519–560. doi: 10.1146/annurev.mi.32.100178.002511. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Sela M. N., Kornman K. S., Ebersole J. L., Holt S. C. Characterization of treponemes isolated from human and non-human primate periodontal pockets. Oral Microbiol Immunol. 1987 Mar;2(1):21–29. doi: 10.1111/j.1399-302x.1987.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Serwer P., Allen J. L. Conformation of double-stranded DNA during agarose gel electrophoresis: fractionation of linear and circular molecules with molecular weights between 3 X 10(6) and 26 X 10(6). Biochemistry. 1984 Feb 28;23(5):922–927. doi: 10.1021/bi00300a020. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]