Abstract
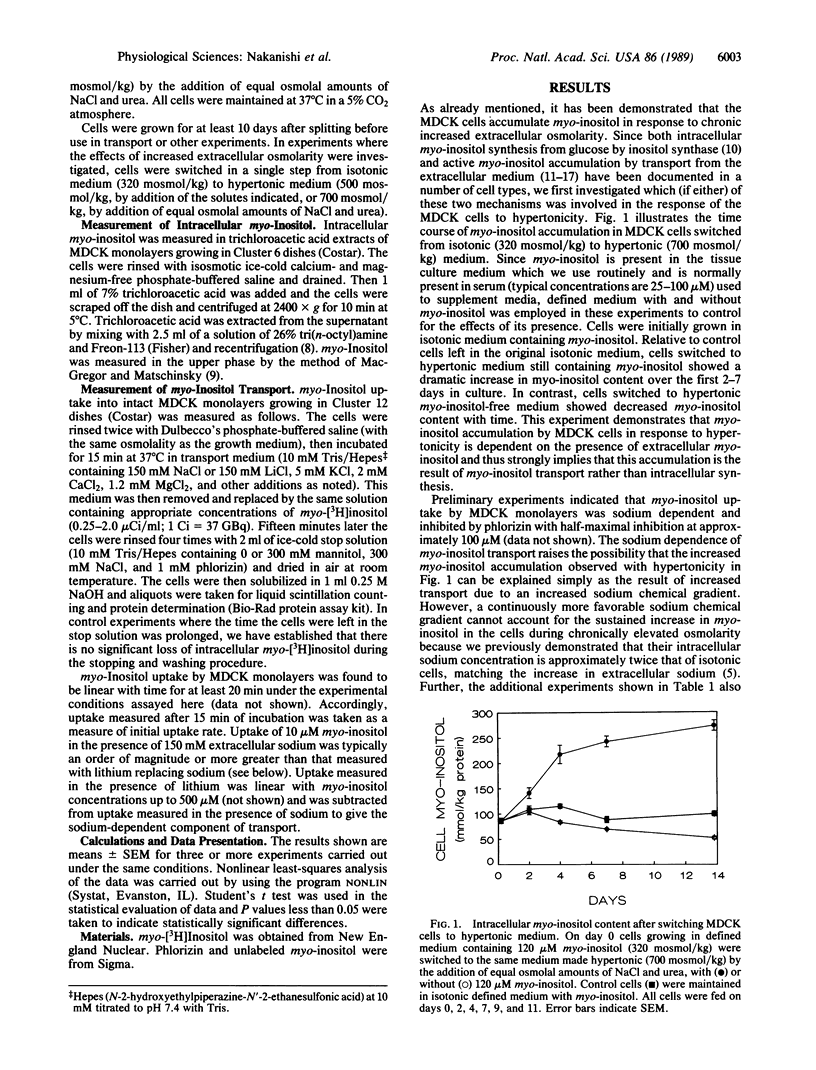
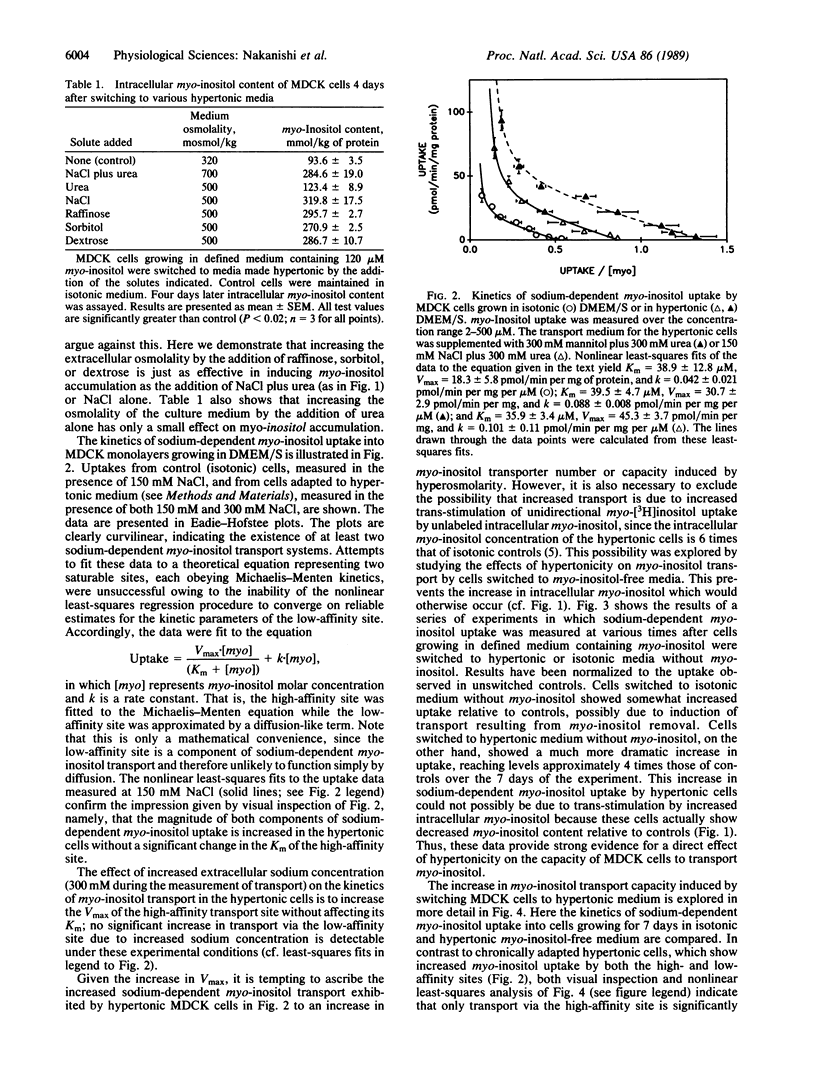
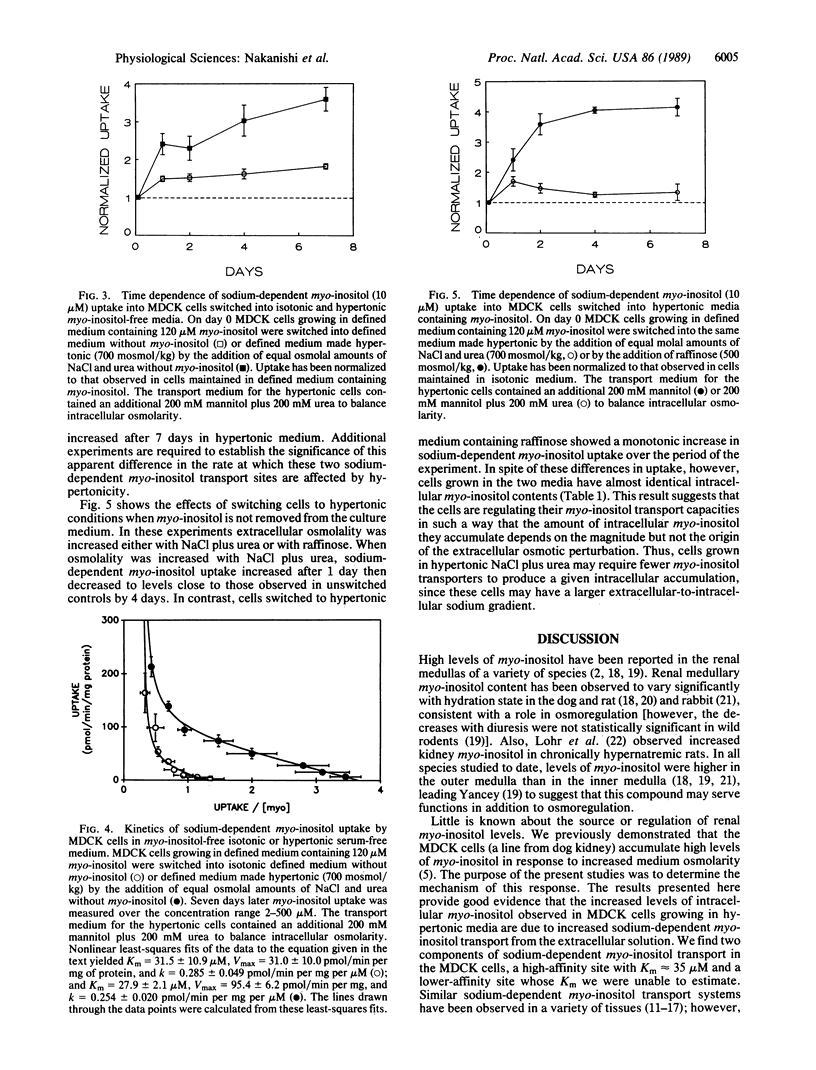
Renal medullary cells contain high concentrations of myo-inositol, sorbitol, betaine, and glycerophosphocholine, whose levels vary with urinary osmolality. Accumulation of these "compatible" organic osmolytes is believed to help the cells osmoregulate in response to the high extracellular osmolality that occurs as part of the urinary concentrating mechanism. MDCK cells (a line from dog kidney) were previously shown to accumulate myo-inositol in response to increased medium osmolality. We demonstrate here that this accumulation requires the presence of myo-inositol in the medium, implying that the myo-inositol is not synthesized by the cells but rather is transported into them from the extracellular solution. The MDCK cells contain sodium-dependent myo-inositol transporters. Relative to isotonic controls, sodium-dependent myo-inositol uptake is higher in cells exposed to increased osmolality either acutely (1-7 days) or chronically (greater than 1 year). Transport is further enhanced when the cells are cultured in myo-inositol-free medium. The transport has both high- and low-affinity components. The observed changes in transport involve changes in maximal velocity of the high-affinity component but not in its Km. We conclude that renal cells can osmoregulate by changing the number (or, less likely, the transport turnover rate) of functioning sodium-dependent myo-inositol transporters.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagnasco S. M., Uchida S., Balaban R. S., Kador P. F., Burg M. B. Induction of aldose reductase and sorbitol in renal inner medullary cells by elevated extracellular NaCl. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1718–1720. doi: 10.1073/pnas.84.6.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnasco S., Balaban R., Fales H. M., Yang Y. M., Burg M. Predominant osmotically active organic solutes in rat and rabbit renal medullas. J Biol Chem. 1986 May 5;261(13):5872–5877. [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B. Active transport of myo-inositol in rat pancreatic islets. Biochem J. 1986 Jun 15;236(3):889–893. doi: 10.1042/bj2360889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary W. F., Crane R. K. Active transport of myo-inositol and its relation to the sugar transport system in hamster small intestine. Biochim Biophys Acta. 1970 Apr 21;203(2):308–316. doi: 10.1016/0005-2736(70)90145-8. [DOI] [PubMed] [Google Scholar]
- Cohen M. A., Hruska K. A., Daughaday W. H. Free myo-inositol in canine kidneys: selective concentration in the renal medulla. Proc Soc Exp Biol Med. 1982 Mar;169(3):380–385. doi: 10.3181/00379727-169-41361. [DOI] [PubMed] [Google Scholar]
- Coon H. G., Weiss M. C. A quantitative comparison of formation of spontaneous and virus-produced viable hybrids. Proc Natl Acad Sci U S A. 1969 Mar;62(3):852–859. doi: 10.1073/pnas.62.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder C. N., Collins J. G., Brannan T. S., Sharma J. Aldose reductase and sorbitol dehydrogenase distribution in rat kidney. J Histochem Cytochem. 1977 Jan;25(1):1–8. doi: 10.1177/25.1.401844. [DOI] [PubMed] [Google Scholar]
- Eisenberg F., Jr D-myoinositol 1-phosphate as product of cyclization of glucose 6-phosphate and substrate for a specific phosphatase in rat testis. J Biol Chem. 1967 Apr 10;242(7):1375–1382. [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Sodium- and energy-dependent uptake of myo-inositol by rabbit peripheral nerve. Competitive inhibition by glucose and lack of an insulin effect. J Clin Invest. 1982 Nov;70(5):1009–1018. doi: 10.1172/JCI110688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullans S. R., Blumenfeld J. D., Balschi J. A., Kaleta M., Brenner R. M., Heilig C. W., Hebert S. C. Accumulation of major organic osmolytes in rat renal inner medulla in dehydration. Am J Physiol. 1988 Oct;255(4 Pt 2):F626–F634. doi: 10.1152/ajprenal.1988.255.4.F626. [DOI] [PubMed] [Google Scholar]
- Hammerman M. R., Sacktor B., Daughaday W. H. myo-Inositol transport in renal brush border vesicles and it inhibition by D-glucose. Am J Physiol. 1980 Aug;239(2):F113–F120. doi: 10.1152/ajprenal.1980.239.2.F113. [DOI] [PubMed] [Google Scholar]
- Johnstone R. M., Sung C. P. Transport of myo-inositol in Ehrlich ascites cells. Biochim Biophys Acta. 1967;135(5):1052–1055. doi: 10.1016/0005-2736(67)90074-0. [DOI] [PubMed] [Google Scholar]
- Khym J. X. An analytical system for rapid separation of tissue nucleotides at low pressures on conventional anion exchangers. Clin Chem. 1975 Aug;21(9):1245–1252. [PubMed] [Google Scholar]
- MacGregor L. C., Matschinsky F. M. An enzymatic fluorimetric assay for myo-inositol. Anal Biochem. 1984 Sep;141(2):382–389. doi: 10.1016/0003-2697(84)90058-7. [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Balaban R. S., Burg M. B. Survey of osmolytes in renal cell lines. Am J Physiol. 1988 Aug;255(2 Pt 1):C181–C191. doi: 10.1152/ajpcell.1988.255.2.C181. [DOI] [PubMed] [Google Scholar]
- Roy G., Sauvé R. Effect of anisotonic media on volume, ion and amino-acid content and membrane potential of kidney cells (MDCK) in culture. J Membr Biol. 1987;100(1):83–96. doi: 10.1007/BF02209143. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Tsumita T. Properties of scyllitol transport in rat kidney slices. Biochim Biophys Acta. 1974 Dec 24;373(3):490–494. doi: 10.1016/0005-2736(74)90028-5. [DOI] [PubMed] [Google Scholar]
- Taub M., Chuman L., Saier M. H., Jr, Sato G. Growth of Madin-Darby canine kidney epithelial cell (MDCK) line in hormone-supplemented, serum-free medium. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3338–3342. doi: 10.1073/pnas.76.7.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Garcia-Perez A., Murphy H., Burg M. Signal for induction of aldose reductase in renal medullary cells by high external NaCl. Am J Physiol. 1989 Mar;256(3 Pt 1):C614–C620. doi: 10.1152/ajpcell.1989.256.3.C614. [DOI] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- Yancey P. H. Osmotic effectors in kidneys of xeric and mesic rodents: corticomedullary distributions and changes with water availability. J Comp Physiol B. 1988;158(3):369–380. doi: 10.1007/BF00695336. [DOI] [PubMed] [Google Scholar]
- Yorek M. A., Dunlap J. A., Ginsberg B. H. Myoinositol uptake by four cultured mammalian cell lines. Arch Biochem Biophys. 1986 May 1;246(2):801–807. doi: 10.1016/0003-9861(86)90336-x. [DOI] [PubMed] [Google Scholar]



