Abstract
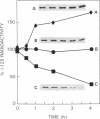
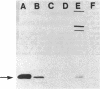
A soluble 27-kDa protein from Saccharomyces cerevisiae specifically prevents the inactivation of various enzymes caused by a nonenzymatic Fe3+/O2/thiol mixed-function oxidation system but not by mixed-function oxidation systems in which the thiol component is replaced by another electron donor-e.g., ascorbate. In this report, using a 125I-labeled monospecific antibody against the 27-kDa protein, we measured changes in the 27-kDa protector protein in response to changes in oxidative stress and heat shock. With a shift from an anaerobic (95% N2/5% CO2) to a hyperaerobic (95% O2/5% CO2) atmosphere, a 3-fold increase was observed. This increase was prevented by cycloheximide, indicating that the induction requires new protein synthesis. The antioxidant protein synthesis was also significantly enhanced by the addition of either 2-mercaptoethanol or Fe3+ to the growth medium. Radioimmunoassay results also show that the antioxidant protein is an abundant protein, as it constitutes 0.7% of total soluble protein from yeast grown aerobically. Immunoblotting experiments revealed that rat tissues also contain a 27-kDa protein that can be specifically recognized by antibodies against the yeast protein. These results suggest that in vivo induction in yeast of the 27-kDa protein may represent an adaptive response that evolved to protect cells against damage caused by thiol-dependent mixed-function oxidation systems, and the antioxidant protein is conserved in mammalian tissues. A heat shock applied to yeast did not cause any significant increases in the concentration of the 27-kDa protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P., Welch W. J. Characterization and purification of the small 28,000-dalton mammalian heat shock protein. J Biol Chem. 1987 Nov 15;262(32):15359–15369. [PubMed] [Google Scholar]
- Autor A. P. Biosynthesis of mitochondrial manganese superoxide dismutase in saccharomyces cerevisiae. Precursor form of mitochondrial superoxide dismutase made in the cytoplasm. J Biol Chem. 1982 Mar 10;257(5):2713–2718. [PubMed] [Google Scholar]
- Buettner G. R. Thiyl free radical production with hematoporphyrin derivative, cysteine and light: a spin-trapping study. FEBS Lett. 1984 Nov 19;177(2):295–299. doi: 10.1016/0014-5793(84)81303-4. [DOI] [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Finn G. J., Condon S. Regulation of catalase synthesis in Salmonella typhimurium. J Bacteriol. 1975 Aug;123(2):570–579. doi: 10.1128/jb.123.2.570-579.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I., Freeman B. Antioxidant defenses in the lung. Annu Rev Physiol. 1986;48:693–702. doi: 10.1146/annurev.ph.48.030186.003401. [DOI] [PubMed] [Google Scholar]
- Fucci L., Oliver C. N., Coon M. J., Stadtman E. R. Inactivation of key metabolic enzymes by mixed-function oxidation reactions: possible implication in protein turnover and ageing. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1521–1525. doi: 10.1073/pnas.80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiazzo F., Schiesser A., Rotilio G. Glutathione peroxidase in yeast. Presence of the enzyme and induction by oxidative conditions. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1200–1205. doi: 10.1016/s0006-291x(87)80197-3. [DOI] [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. Controls on the biosynthesis of the manganese-containing superoxide dismutase of Escherichia coli. Effects of thiols. J Biol Chem. 1987 Dec 25;262(36):17591–17595. [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Induction of superoxide dismutase by molecular oxygen. J Bacteriol. 1973 May;114(2):543–548. doi: 10.1128/jb.114.2.543-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Goscin S. A., Fridovich I. Superoxide dismutase and oxygen toxicity in a eukaryote. J Bacteriol. 1974 Feb;117(2):456–460. doi: 10.1128/jb.117.2.456-460.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass M. A., Frank L., Massaro D. The effect of bacterial endotoxin on synthesis of (Cu,Zn)superoxide dismutase in lungs of oxygen-exposed rats. J Biol Chem. 1982 Aug 25;257(16):9379–9383. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Kim I. H., Lee K. Y., Rhee S. G., Stadtman E. R. The isolation and purification of a specific "protector" protein which inhibits enzyme inactivation by a thiol/Fe(III)/O2 mixed-function oxidation system. J Biol Chem. 1988 Apr 5;263(10):4704–4711. [PubMed] [Google Scholar]
- Kim K., Rhee S. G., Stadtman E. R. Nonenzymatic cleavage of proteins by reactive oxygen species generated by dithiothreitol and iron. J Biol Chem. 1985 Dec 15;260(29):15394–15397. [PubMed] [Google Scholar]
- Levine R. L., Oliver C. N., Fulks R. M., Stadtman E. R. Turnover of bacterial glutamine synthetase: oxidative inactivation precedes proteolysis. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2120–2124. doi: 10.1073/pnas.78.4.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. L. Oxidative modification of glutamine synthetase. II. Characterization of the ascorbate model system. J Biol Chem. 1983 Oct 10;258(19):11828–11833. [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Xuong N. H., Geiduschek E. P. Quantitative analysis of the heat shock response of Saccharomyces cerevisiae. J Bacteriol. 1982 Jul;151(1):311–327. doi: 10.1128/jb.151.1.311-327.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra H. P. Generation of superoxide free radical during the autoxidation of thiols. J Biol Chem. 1974 Apr 10;249(7):2151–2155. [PubMed] [Google Scholar]
- Morgan R. W., Christman M. F., Jacobson F. S., Storz G., Ames B. N. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver C. N., Levine R. L., Stadtman E. R. A role of mixed-function oxidation reactions in the accumulation of altered enzyme forms during aging. J Am Geriatr Soc. 1987 Oct;35(10):947–956. doi: 10.1111/j.1532-5415.1987.tb02297.x. [DOI] [PubMed] [Google Scholar]
- Piper P. W., Curran B., Davies M. W., Lockheart A., Reid G. Transcription of the phosphoglycerate kinase gene of Saccharomyces cerevisiae increases when fermentative cultures are stressed by heat-shock. Eur J Biochem. 1986 Dec 15;161(3):525–531. doi: 10.1111/j.1432-1033.1986.tb10474.x. [DOI] [PubMed] [Google Scholar]
- Pritchard G. G., Wimpenny J. W., Morris H. A., Lewis M. W., Hughes D. E. Effects of oxygen on Propionibacterium shermanii grown in continuous culture. J Gen Microbiol. 1977 Oct;102(2):223–233. doi: 10.1099/00221287-102-2-223. [DOI] [PubMed] [Google Scholar]
- Privalle C. T., Fridovich I. Inductions of superoxide dismutases in Escherichia coli under anaerobic conditions. Accumulation of an inactive form of the manganese enzyme. J Biol Chem. 1988 Mar 25;263(9):4274–4279. [PubMed] [Google Scholar]
- Privalle C. T., Gregory E. M. Superoxide dismutase and O2 lethality in Bacteroides fragilis. J Bacteriol. 1979 Apr;138(1):139–145. doi: 10.1128/jb.138.1.139-145.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puget K., Michelson A. M. Isolation of a new copper-containing superoxide dismutase bacteriocuprein. Biochem Biophys Res Commun. 1974 Jun 4;58(3):830–838. doi: 10.1016/s0006-291x(74)80492-4. [DOI] [PubMed] [Google Scholar]
- Richter H. E., Loewen P. C. Induction of catalase in Escherichia coli by ascorbic acid involves hydrogen peroxide. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1039–1046. doi: 10.1016/0006-291x(81)91928-8. [DOI] [PubMed] [Google Scholar]
- Rivett A. J. Preferential degradation of the oxidatively modified form of glutamine synthetase by intracellular mammalian proteases. J Biol Chem. 1985 Jan 10;260(1):300–305. [PubMed] [Google Scholar]
- Ross D., Albano E., Nilsson U., Moldéus P. Thiyl radicals--formation during peroxidase-catalyzed metabolism of acetaminophen in the presence of thiols. Biochem Biophys Res Commun. 1984 Nov 30;125(1):109–115. doi: 10.1016/s0006-291x(84)80341-1. [DOI] [PubMed] [Google Scholar]
- Ross D., Norbeck K., Moldéus P. The generation and subsequent fate of glutathionyl radicals in biological systems. J Biol Chem. 1985 Dec 5;260(28):15028–15032. [PubMed] [Google Scholar]
- Saez G., Thornalley P. J., Hill H. A., Hems R., Bannister J. V. The production of free radicals during the autoxidation of cysteine and their effect on isolated rat hepatocytes. Biochim Biophys Acta. 1982 Oct 28;719(1):24–31. doi: 10.1016/0304-4165(82)90302-6. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by aerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):344–350. doi: 10.1128/jb.154.1.344-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside C., Hassan H. M. Induction and inactivation of catalase and superoxide dismutase of Escherichia coli by ozone. Arch Biochem Biophys. 1987 Sep;257(2):464–471. doi: 10.1016/0003-9861(87)90591-1. [DOI] [PubMed] [Google Scholar]