Abstract
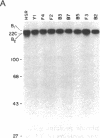
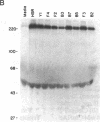
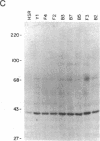
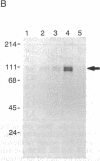
We have examined the role of a cell surface galactosyltransferase, laminin, and laminin-binding protein (receptor) in the invasion of clonal derivatives of a murine adrenal carcinoma cell line. Although a 10-fold variation was found in the ability to invade a reconstituted basement membrane matrix, levels of intracellular laminin and the laminin-binding protein were shown to be present and secreted equally in all lines. Of the eight lines tested, seven showed a correlation between invasion and the incorporation of [3H]galactose from UDP-[3H]galactose into a 90- to 110-kDa protein. One noninvasive line (clone HSR), however, retained high galactosyltransferase activity yet could not galactosylate the endogenous 90- to 110-kDa substrate. Interestingly, this clone was unable to attach to laminin. Although high galactosyltransferase activity can be consistent with cells of high invasiveness, our results suggest that the galactosylation status of a 90- to 110-kDa Y1 cell surface glycoprotein is most indicative of invasion potential.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Iwamoto Y., Kleinman H. K., Martin G. R., Aaronson S. A., Kozlowski J. M., McEwan R. N. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987 Jun 15;47(12):3239–3245. [PubMed] [Google Scholar]
- Bayna E. M., Shaper J. H., Shur B. D. Temporally specific involvement of cell surface beta-1,4 galactosyltransferase during mouse embryo morula compaction. Cell. 1988 Apr 8;53(1):145–157. doi: 10.1016/0092-8674(88)90496-5. [DOI] [PubMed] [Google Scholar]
- Carlsson S. R., Roth J., Piller F., Fukuda M. Isolation and characterization of human lysosomal membrane glycoproteins, h-lamp-1 and h-lamp-2. Major sialoglycoproteins carrying polylactosaminoglycan. J Biol Chem. 1988 Dec 15;263(35):18911–18919. [PubMed] [Google Scholar]
- Elices M. J., Goldstein I. J. Ehrlich ascites tumor cell UDP-Gal:N-acetyl-D-glucosamine beta(1,4)-galactosyltransferase. Purification, characterization, and topography of the acceptor-binding site. J Biol Chem. 1988 Mar 5;263(7):3354–3362. [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. L., Brew K., Vanaman T. C., Trayer I. P., Mattock P. The structure, function, and evolution of alpha-lactalbumin. Brookhaven Symp Biol. 1968 Jun;21(1):139–154. [PubMed] [Google Scholar]
- Juliano R. L. Membrane receptors for extracellular matrix macromolecules: relationship to cell adhesion and tumor metastasis. Biochim Biophys Acta. 1987 Nov 25;907(3):261–278. doi: 10.1016/0304-419x(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Cannon F. B., Laurie G. W., Hassell J. R., Aumailley M., Terranova V. P., Martin G. R., DuBois-Dalcq M. Biological activities of laminin. J Cell Biochem. 1985;27(4):317–325. doi: 10.1002/jcb.240270402. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Star V. L., Cannon F. B., Laurie G. W., Martin G. R. Basement membrane complexes with biological activity. Biochemistry. 1986 Jan 28;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Ogle R. C., Cannon F. B., Little C. D., Sweeney T. M., Luckenbill-Edds L. Laminin receptors for neurite formation. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1282–1286. doi: 10.1073/pnas.85.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Fambrough D. M. Cycling of the integral membrane glycoprotein, LEP100, between plasma membrane and lysosomes: kinetic and morphological analysis. Cell. 1987 Jun 5;49(5):669–677. doi: 10.1016/0092-8674(87)90543-5. [DOI] [PubMed] [Google Scholar]
- Lopez L. C., Bayna E. M., Litoff D., Shaper N. L., Shaper J. H., Shur B. D. Receptor function of mouse sperm surface galactosyltransferase during fertilization. J Cell Biol. 1985 Oct;101(4):1501–1510. doi: 10.1083/jcb.101.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R., Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- Passaniti A., Hart G. W. Sialic acids and penultimate oligosaccharides on metastatic tumour cell surfaces. Biochem Soc Trans. 1989 Feb;17(1):33–36. doi: 10.1042/bst0170033. [DOI] [PubMed] [Google Scholar]
- Porzig E. F. Galactosyltransferase activity of intact neural retinal cells from the embryonic chicken. Dev Biol. 1978 Nov;67(1):114–126. doi: 10.1016/0012-1606(78)90304-4. [DOI] [PubMed] [Google Scholar]
- Roseman S. The synthesis of complex carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem Phys Lipids. 1970 Oct;5(1):270–297. doi: 10.1016/0009-3084(70)90024-1. [DOI] [PubMed] [Google Scholar]
- Runyan R. B., Maxwell G. D., Shur B. D. Evidence for a novel enzymatic mechanism of neural crest cell migration on extracellular glycoconjugate matrices. J Cell Biol. 1986 Feb;102(2):432–441. doi: 10.1083/jcb.102.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan R. B., Versalovic J., Shur B. D. Functionally distinct laminin receptors mediate cell adhesion and spreading: the requirement for surface galactosyltransferase in cell spreading. J Cell Biol. 1988 Nov;107(5):1863–1871. doi: 10.1083/jcb.107.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Varmus H. E., Bishop J. M., George D. A cellular oncogene (c-Ki-ras) is amplified, overexpressed, and located within karyotypic abnormalities in mouse adrenocortical tumour cells. Nature. 1983 Jun 9;303(5917):497–501. doi: 10.1038/303497a0. [DOI] [PubMed] [Google Scholar]
- Shaper N. L., Hollis G. F., Douglas J. G., Kirsch I. R., Shaper J. H. Characterization of the full length cDNA for murine beta-1,4-galactosyltransferase. Novel features at the 5'-end predict two translational start sites at two in-frame AUGs. J Biol Chem. 1988 Jul 25;263(21):10420–10428. [PubMed] [Google Scholar]
- Shur B. D. Cell-surface glycosyltransferases in gastrulating chick embryos. I. Temporally and spatially specific patterns of four endogenous glycosyltransferase activities. Dev Biol. 1977 Jul 1;58(1):23–39. doi: 10.1016/0012-1606(77)90072-0. [DOI] [PubMed] [Google Scholar]
- Shur B. D. Evidence that galactosyltransferase is a surface receptor for poly(N)-acetyllactosamine glycoconjugates on embryonal carcinoma cells. J Biol Chem. 1982 Jun 25;257(12):6871–6878. [PubMed] [Google Scholar]
- Shur B. D., Neely C. A. Plasma membrane association, purification, and partial characterization of mouse sperm beta 1,4-galactosyltransferase. J Biol Chem. 1988 Nov 25;263(33):17706–17714. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewer U. M., Taraboletti G., Sobel M. E., Albrechtsen R., Liotta L. A. Role of laminin receptor in tumor cell migration. Cancer Res. 1987 Nov 1;47(21):5691–5698. [PubMed] [Google Scholar]
- Yannariello-Brown J., Wewer U., Liotta L., Madri J. A. Distribution of a 69-kD laminin-binding protein in aortic and microvascular endothelial cells: modulation during cell attachment, spreading, and migration. J Cell Biol. 1988 May;106(5):1773–1786. doi: 10.1083/jcb.106.5.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]