Abstract
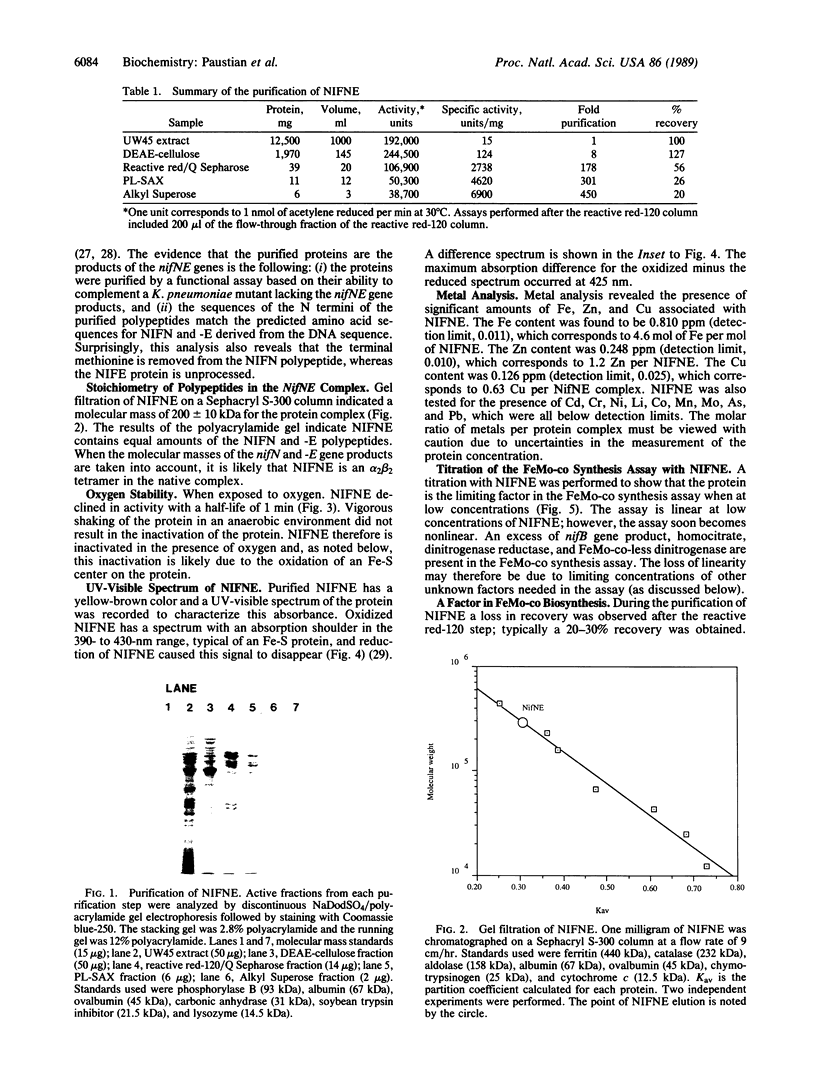
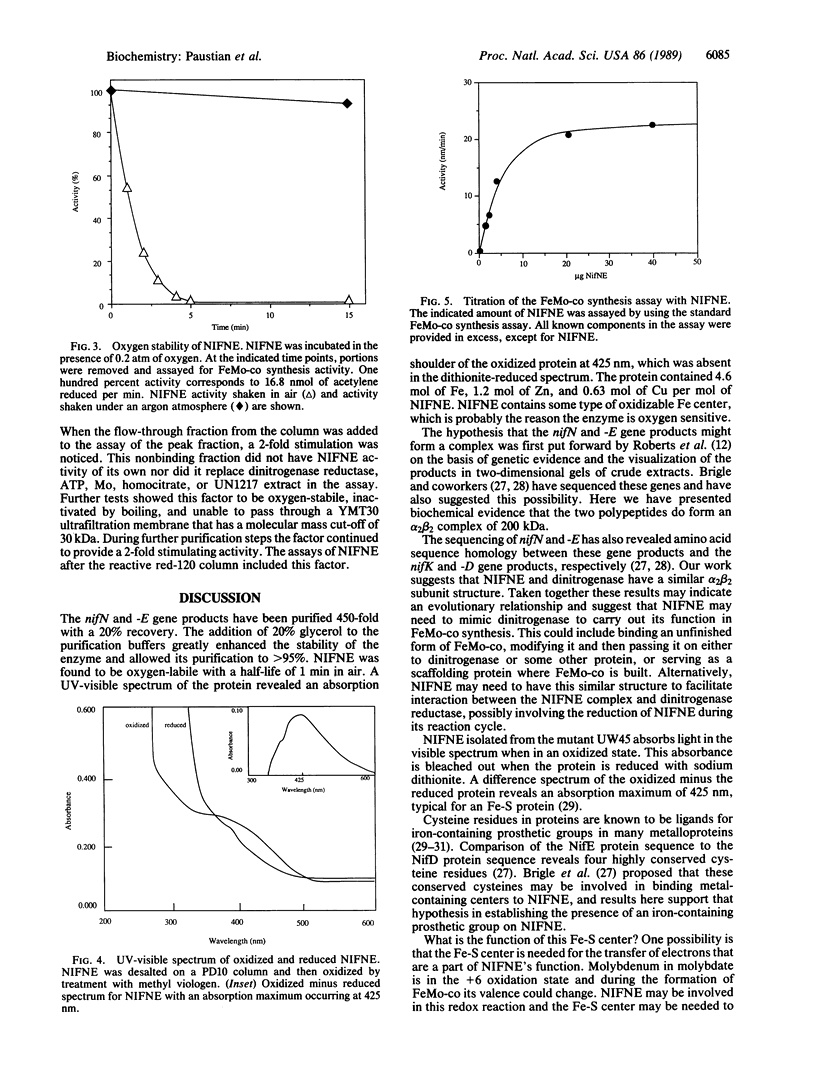
The nifN and -E gene products are involved in the synthesis of the iron-molybdenum cofactor of dinitrogenase, the enzyme responsible for the reduction of dinitrogen to ammonia. By using the in vitro iron-molybdenum cofactor biosynthesis assay, we have followed the purification of these gene products 450-fold to greater than 95% purity. An overall recovery of 20% was obtained with the purified protein having a specific activity of 6900 units/mg of protein. The protein (hereafter referred to as NIFNE) was found to contain equimolar amounts of the nifN and -E gene products and have a native molecular mass of 200 +/- 10 kDa, which indicates an alpha 2 beta 2 structure. NIFNE was oxygen labile with a half-life of 1 min in air. A UV-visible spectrum of the dye-oxidized protein showed an absorption maximum at 425 nm that could be bleached by reduction of NIFNE with sodium dithionite, suggesting the presence of an Fe center in NIFNE.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop P. E., Brill W. J. Genetic analysis of Azotobacter vinelandii mutant strains unable to fix nitrogen. J Bacteriol. 1977 May;130(2):954–956. doi: 10.1128/jb.130.2.954-956.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigle K. E., Weiss M. C., Newton W. E., Dean D. R. Products of the iron-molybdenum cofactor-specific biosynthetic genes, nifE and nifN, are structurally homologous to the products of the nitrogenase molybdenum-iron protein genes, nifD and nifK. J Bacteriol. 1987 Apr;169(4):1547–1553. doi: 10.1128/jb.169.4.1547-1553.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulen W. A., LeComte J. R. The nitrogenase system from Azotobacter: two-enzyme requirement for N2 reduction, ATP-dependent H2 evolution, and ATP hydrolysis. Proc Natl Acad Sci U S A. 1966 Sep;56(3):979–986. doi: 10.1073/pnas.56.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. R., Brigle K. E. Azotobacter vinelandii nifD- and nifE-encoded polypeptides share structural homology. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5720–5723. doi: 10.1073/pnas.82.17.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filler W. A., Kemp R. M., Ng J. C., Hawkes T. R., Dixon R. A., Smith B. E. The nifH gene product is required for the synthesis or stability of the iron-molybdenum cofactor of nitrogenase from Klebsiella pneumoniae. Eur J Biochem. 1986 Oct 15;160(2):371–377. doi: 10.1111/j.1432-1033.1986.tb09981.x. [DOI] [PubMed] [Google Scholar]
- Hageman R. V., Burris R. H. Nitrogenase and nitrogenase reductase associate and dissociate with each catalytic cycle. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2699–2702. doi: 10.1073/pnas.75.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes T. R., McLean P. A., Smith B. E. Nitrogenase from nifV mutants of Klebsiella pneumoniae contains an altered form of the iron-molybdenum cofactor. Biochem J. 1984 Jan 1;217(1):317–321. doi: 10.1042/bj2170317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover T. R., Shah V. K., Roberts G. P., Ludden P. W. nifV-dependent, low-molecular-weight factor required for in vitro synthesis of iron-molybdenum cofactor of nitrogenase. J Bacteriol. 1986 Sep;167(3):999–1003. doi: 10.1128/jb.167.3.999-1003.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial J., Ugalde R. A., Shah V. K., Brill W. J. Role of the nifQ gene product in the incorporation of molybdenum into nitrogenase in Klebsiella pneumoniae. J Bacteriol. 1984 Apr;158(1):187–194. doi: 10.1128/jb.158.1.187-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil T., MacNeil D., Roberts G. P., Supiano M. A., Brill W. J. Fine-structure mapping and complementation analysis of nif (nitrogen fixation) genes in Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):253–266. doi: 10.1128/jb.136.1.253-266.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani H. H., Shah V. K., Brill W. J. Activation of inactive nitrogenase by acid-treated component I. J Bacteriol. 1974 Nov;120(2):697–701. doi: 10.1128/jb.120.2.697-701.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. J., Levy M. A., Orme-Johnson W. H. Metal and sulfur composition of iron-molybdenum cofactor of nitrogenase. Proc Natl Acad Sci U S A. 1983 Jan;80(1):147–150. doi: 10.1073/pnas.80.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieva-Gómez D., Roberts G. P., Klevickis S., Brill W. J. Electron transport to nitrogenase in Klebsiella pneumoniae. Proc Natl Acad Sci U S A. 1980 May;77(5):2555–2558. doi: 10.1073/pnas.77.5.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme-Johnson W. H. Iron-sulfur proteins: structure and function. Annu Rev Biochem. 1973;42(0):159–204. doi: 10.1146/annurev.bi.42.070173.001111. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson W. H. Molecular basis of biological nitrogen fixation. Annu Rev Biophys Biophys Chem. 1985;14:419–459. doi: 10.1146/annurev.bb.14.060185.002223. [DOI] [PubMed] [Google Scholar]
- Roberts G. P., MacNeil T., MacNeil D., Brill W. J. Regulation and characterization of protein products coded by the nif (nitrogen fixation) genes of Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):267–279. doi: 10.1128/jb.136.1.267-279.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. C., Dean D. R., Burgess B. K. Iron-molybdenum cofactor biosynthesis in Azotobacter vinelandii requires the iron protein of nitrogenase. J Biol Chem. 1987 Oct 15;262(29):14327–14332. [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Chisnell J. R., Brill W. J. Acetylene reduction by the iron-molybdenum cofactor from nitrogenase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):232–236. doi: 10.1016/0006-291x(78)91654-6. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Davis I. C., Gordon J. K., Orme-Johnson W. H., Brill W. J. Nitrogenase. 3. Nitrogenaseless mutants of Azotobacter vinelandii: activities, cross-reactions and EPR spectra. Biochim Biophys Acta. 1973 Jan 18;292(1):246–255. doi: 10.1016/0005-2728(73)90269-7. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Davis L. C., Brill W. J. Nitrogenase. I. Repression and derepression of the iron-molybdenum and iron proteins of nitrogenase in Azotobacter vinelandii. Biochim Biophys Acta. 1972 Feb 28;256(2):498–511. doi: 10.1016/0005-2728(72)90078-3. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Imperial J., Ugalde R. A., Ludden P. W., Brill W. J. In vitro synthesis of the iron-molybdenum cofactor of nitrogenase. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1636–1640. doi: 10.1073/pnas.83.6.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Ugalde R. A., Imperial J., Brill W. J. Molybdenum in nitrogenase. Annu Rev Biochem. 1984;53:231–257. doi: 10.1146/annurev.bi.53.070184.001311. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Strandberg G. W., Wilson P. W. Formation of the nitrogen-fixing enzyme system in Azotobacter vinelandii. Can J Microbiol. 1968 Jan;14(1):25–31. doi: 10.1139/m68-005. [DOI] [PubMed] [Google Scholar]
- Thompson S. T., Cass K. H., Stellwagen E. Blue dextran-sepharose: an affinity column for the dinucleotide fold in proteins. Proc Natl Acad Sci U S A. 1975 Feb;72(2):669–672. doi: 10.1073/pnas.72.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugalde R. A., Imperial J., Shah V. K., Brill W. J. Biosynthesis of iron-molybdenum cofactor in the absence of nitrogenase. J Bacteriol. 1984 Sep;159(3):888–893. doi: 10.1128/jb.159.3.888-893.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. S., Pan W. H., Friesen G. D., Burgess B. K., Corbin J. L., Stiefel E. I., Newton W. E. Iron-molybdenum cofactor from nitrogenase. Modified extraction methods as probes for composition. J Biol Chem. 1982 Jul 25;257(14):8042–8048. [PubMed] [Google Scholar]
- Yoch D. C., Carithers R. P. Bacterial iron-sulfur proteins. Microbiol Rev. 1979 Sep;43(3):384–421. doi: 10.1128/mr.43.3.384-421.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]




