Abstract
Alzheimer's disease (AD) is a chronic brain disorder characterized by cognitive impairment, cholinergic dysfunction, inflammation, tau and beta-amyloid pathology and vascular damage. Recent studies have shown, that high cholesterol levels are linked to the pathology of AD. The aim of our present work was to study the effects of hypercholesterolemia in adult rats. Five months after 5% cholesterol-enriched diet plasma cholesterol levels and total weight were significantly enhanced compared to controls. Spatial memory was studied in an 8-arm radial maze and cholesterol-treated rats showed an impaired learning and long-term memory. Hypercholesterolemia significantly reduced the number of cholinergic neurons in the basal nucleus of Meynert and decreased acetylcholine levels in the cortex. Nerve growth factor was only slightly enhanced in the cortex of cholesterol-treated animals. Levels of amyloid precursor protein, beta-amyloid(1–42), as well as tau and phospho-tau 181 were significantly enhanced in the cortex of cholesterol-fed rats. Hypercholesterolemia markedly increased several cerebral inflammatory markers and enhanced microglial CD11b-like immunoreactivity. Vascular density, stained by RECA-1 was not changed. However, cholesterol induced cortical microbleedings illustrated by intensive anti-rat IgG-positive spots in the cortex. In conclusion, our data demonstrate that hypercholesterolemia in rats caused memory impairment, cholinergic dysfunction, inflammation, enhanced cortical beta-amyloid and tau and microbleedings, all indications, which resemble an AD-like pathology.
Keywords: Cholesterol, Alzheimer's disease, Memory impairment, Cholinergic dysfunction, Inflammation, Hyperphosphorylated tau, Beta-amyloid(1–42), Microbleeding, In vivo
Introduction
Alzheimer´s disease (AD) is an age-dependent neurodegenerative disorder characterized by progressive loss of cognitive functions, damage of the cholinergic system, inflammatory processes, beta-amyloid deposition, tau-pathology, and vascular dysfunction. Besides, the causes for AD are unknown. Recent findings indicate that cerebrovascular dysfunction (Iadecola, 2004) and enhanced cholesterol may play a role in the pathology of AD (Kivipelto et al., 2001; Puglielli et al., 2003; Raffai and Weisgraber, 2003; Simons et al., 2001). This is supported by studies in vitro and in vivo, that demonstrate a possible beneficial protecting effect of statins, inhibitors of the cholesterol synthesis, against AD (Kandiah and Feldman, 2009; Tong et al., 2009). Indeed, statins lowered serum beta-amyloid levels in humans with elevated cholesterol levels (Buxbaum et al., 2002; Friedhoff et al., 2001) although other studies were conflicting (Kandiah and Feldman, 2009; Ishii et al., 2003; Serrano-Pozo et al., 2010). The exact mechanism of high blood cholesterol in AD brains is not known, because cholesterol is not able to pass the blood-brain barrier (BBB) (Bojanic et al., 2010; Pfrieger, 2003; Xie et al., 2003). It is assumed that cholesterol is involved in the processing of amyloid-precursor protein (APP) within lipid rafts, which might result in enhanced processing to beta-amyloid(1–42) (Ehehalt et al., 2003; Simons et al., 2001). In fact, in vitro and in vivo studies have shown that hypercholesterolemia results in enhanced levels of beta-amyloid (Ghribi et al., 2006; Levin-Allerhand et al., 2002; Prasanthi et al., 2008; Refolo et al., 2000; Wellington, 2004). In addition, tau pathology has recently been associated with membrane cholesterol and beta-amyloid-induced neurotoxicity (Nicholson and Ferreira, 2009). Furthermore, in AD brains chronic inflammatory responses including microglial activation have been detected (Akiyama et al., 2000; Eikelenboom and van Gool, 2004; Lucas et al., 2006; Rogers, 2008). The role of cholesterol in inflammation is not clear, however oxygenated derivates of cholesterol, oxysterols, upregulate the expression of various inflammatory cytokines and chemokines (Lemaire-Ewing et al., 2005; Rosklint et al., 2002; Dugas et al., 2010; Prunet et al., 2006; Morello et al., 2009; Trousson et al., 2009; Vejux et al., 2008; Sottero et al., 2009).
The effect of hypercholesterolemia in vivo has been studied with different animal species. Sparks et al. (1994) reported for the first time that hypercholesterolemia affects beta-amyloid in rabbits and displayed some neuropathological changes similar to those seen in AD (Ghribi et al., 2006; Prasanthi et al., 2008; Sharma et al., 2008; Sparks et al., 2000; Xue et al., 2007). In recent years, hypercholesterolemic studies were performed using transgenic AD mouse models (e.g. Apolipoprotein E, low density lipoprotein receptor knockout mice and transgenic mice expressing familial AD mutant human APP), which exhibited cognitive dysfunction, increased APP processing and beta-amyloid accumulation, as well as an inflammatory response (Levin-Allerhand et al., 2002; Rahman et al., 2005; Refolo et al., 2000; Thirumangalakudi et al., 2008). Recently, Granholm and colleagues (2008) demonstrated that hypercholesterolemia in rats increase the number of working memory errors, but identified no changes in the level of beta-amyloid and nerve growth factor (NGF).
The aim of our present study was to explore if hypercholesterolemia in rats affects the cholinergic system and displays further AD-like pathologies, such as beta-amyloid dysfunction, cognitive impairments and inflammation. Our data reveal that a cholesterol-enriched diet resulted in cholinergic dysfunction in rats and lead to enhanced levels of cortical beta-amyloid, tau, different inflammatory markers, resulted in microbleedings in the brain and markedly reduced spatial memory in the 8-arm radial maze.
Results
Hypercholesterolemia increases plasma cholesterol and weight gain
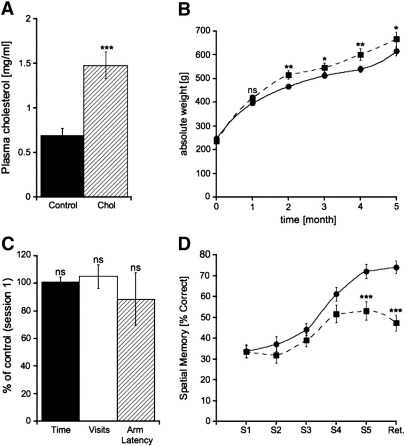
Plasma cholesterol levels, measured by HPLC and UV detection, were significantly increased in hypercholesterolemic animals (1.48 ± 0.15 mg/ml, n = 5), compared to control rats (0.69 ± 0.08 mg/ml, n = 9) after 5 months of treatment (Fig. 1A). The weight of cholesterol-treated rats significantly increased after 2 months compared to control animals (Fig. 1B).
Fig. 1.
Hypercholesterolemia increases plasma cholesterol levels and decreases spatial memory. Sprague Dawley rats were treated with control or high cholesterol diet for 5 months. (A) Measurement of plasma cholesterol levels by HPLC and UV detection demonstrated increased plasma cholesterol concentrations in cholesterol-treated animals (Chol) compared to controls. (B) After 2 months cholesterol-fed rats (■) exhibited significantly enhanced gain in weight compared to controls (●). (C) Cholesterol-treated animals showed no differences in (1) the time needed to find all 4 baits (filled bar), (2) the number of total visits (open bar) and (3) the length of time in the visited arm (hatched bar). (D) Hypercholesterolemic rats (■) showed an impaired ability of spatial learning and storage of long-term memory compared to controls (●). Statistical analysis for plasma cholesterol and absolute weight gain was performed by one way ANOVA with a Fisher LSD posthoc test. Behavioral testings were statistically analyzed within each group by using one-way ANOVA with repeated measures and between control and cholesterol-fed animals by using student T-test. *** p < 0.001; ** p < 0.01; * p < 0.05; ns, not significant.
Hypercholesterolemia affects spatial memory
In order to assess spatial memory, the rats were tested in an 8-arm radial maze after 4 months. Cholesterol-treated rats showed an impaired spatial learning performance at session 5 (Fig. 1D). In addition, long-term memory (retention) was markedly reduced (Fig. 1D). In order to exclude a decreased mobility of the cholesterol-treated animals, the time to find all baits, the number of total visits and the time spent in the visited arm (latency) were examined, but no differences between control and hypercholesterolemic rats were found (Fig. 1C).
Hypercholesterolemia decreases cholinergic activity in the nBM
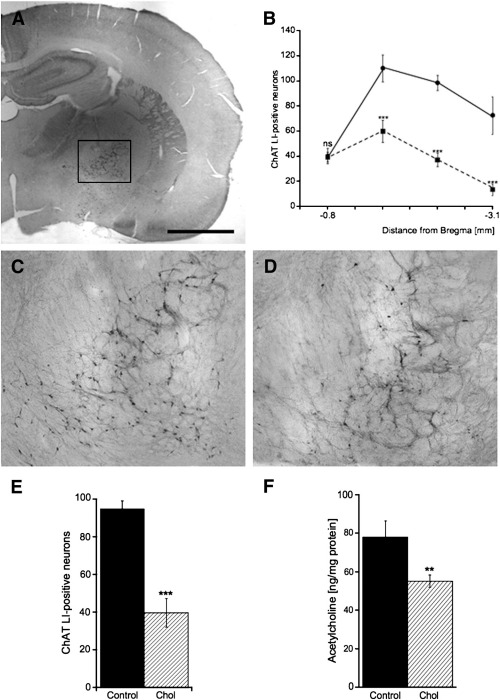
Cholinergic neurons were immunohistochemically stained for choline acetyltransferase (ChAT) (Figs. 2A, C, and D) and ChAT-positive neurons were analyzed in the nBM between Bregma −0.8 mm and −3.1 mm (B). Hypercholesterolemic animals (Fig. 2D) exhibited a decreased number of ChAT-positive neurons in the nBM (Figs. 2B and E) compared to control rats (Figs. 2A and C). Acetylcholine levels in the cortex were significantly reduced in rats treated with cholesterol-enriched diet compared to controls (Fig. 2F).
Fig. 2.
Hypercholesterolemia impairs the cholinergic activity in the brain. (A) The number of choline acetyltransferase (ChAT) positive neurons in the basal nucleus of Meynert was counted between Bregma −0.8 mm and −3.1 mm (B) and revealed a decreased number of ChAT-positive neurons in cholesterol-fed rats (■) (D) compared to control animals (●) (C). Hypercholesterolemic rats (E) exhibited a decreased number of ChAT-positive neurons in the nBM (hatched bar), compared to controls (filled bar). (F) The level of cortical acetylcholine was diminished in cholesterol-fed rats (hatched bar), compared to controls (filled bar). Statistical analysis was performed by one way ANOVA with a Fisher LSD posthoc test. *** p < 0.001; ** p < 0.01; ns, not significant. Scale bar in A = 2300 μm (A), 500 μm (C, D).
Hypercholesterolemia affects APP and beta-amyloid
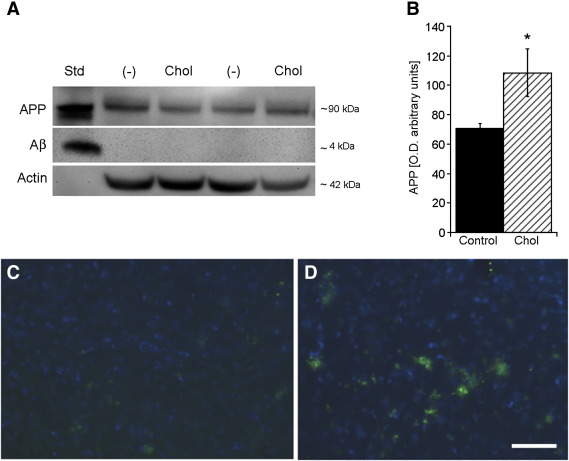
Western Blot analysis clearly demonstrated a single band of APP standard at approximately 90 kDa, as well as a single band of beta-amyloid standard at approximately 4 kDa (Fig. 3A). Beta-amyloid was not detectable in the cortex of control or cholesterol-fed rats by Western Blot analysis (Fig. 3A). APP was clearly seen in cortex of controls and cholesterol treated rats (Fig. 3A). Densitometric analysis (correlated to actin, Fig. 3B) displayed significantly enhanced concentrations of APP protein in cholesterol-fed animals compared to controls (Fig. 3B). Beta-amyloid(1–42) levels as measured by ELISA were significantly enhanced in the cortex of hypercholesterolemic rats (Table 1). Immunohistochemical staining against beta-amyloid in the cortex of cholesterol-fed rats (Fig. 3D) and controls (Fig. 3C) displayed an apparent increased beta-amyloid-like immunoractivity in hypercholesterolemic rats.
Fig. 3.
Hypercholesterolemia affects APP and beta-amyloid in rats. (A) Western Blot analysis was performed for amyloid precursor protein (APP) and beta-amyloid (Aβ) in controls (−) and cholesterol-fed (Chol) animals. (A) Standards (Std) for APP (100 ng) and beta-amyloid (100 ng) were clearly visualized at ~ 90 kDa (APP) or ~4kDa (Aβ). (A) Cortical APP was detected in controls and rats fed with high cholesterol diet, while beta-amyloid was not detectable. (B) Densitometric analysis (corrected for total actin levels) exhibited an enhanced concentration of APP protein in cholesterol-treated animals (hatched bar) compared to controls (filled bar). Immunohistochemical detection of beta-amyloid-like immunoreactivity in the cortex was visualized by fluorescence microscopy (C, D, Alexa-488, green; counterstained against nuclear DAPI, blue). Hypercholesterolemic rats (D) exhibited an enhanced beta-amyloid-positive staining compared to control animals (C). Statistical analysis was performed by one way ANOVA with a Fisher PLSD posthoc test. Values are given as mean ± S.E.M. optical units. * p < 0.05. Scale bar in D = 230 μm (C, D).
Table 1.
Effects of hypercholesterolemia on cortical NGF, beta-amyloid(1–42), tau and phospho-tau 181.
| [pg/mg tissue] | Control | Cholesterol | p |
|---|---|---|---|
| Nerve growth factor | 146 ± 30 | 259 ± 61 | ns |
| Beta-amyloid (1–42) | 84 ± 14 | 134 ± 7 | ** |
| Tau | 2492 ± 455 | 5755 ± 953 | ** |
| Phospho-Tau 181 | 24 ± 6 | 51 ± 7 | * |
Sprague Dawley rats were administered to control and cholesterol-enriched diet for 5 months. Brains were removed, the cortices extracted and the levels of nerve growth factor (NGF), beta-amyloid(1–42), tau and phospho-tau 181 were measured by ELISA assays. Values are given as mean ± S.E.M. in [pg/mg tissue]. Statistical analysis was performed by one way ANOVA with a Fisher LSD posthoc test. ** p < 0.01; * p < 0.05; ns, not significant.
Hypercholesterolemia increases several cortical proteins
The levels of tau and phospho-tau 181 were significantly enhanced in hypercholesterolemic rats compared to controls as analyzed by ELISA (Table 1). NGF in the cortex was slightly, but not significantly increased in hypercholesterolemic animals (Table 1). A large number of inflammation markers were measured in the cortex by Multiplex SearchLight® ELISA and significantly increased levels of different cytokines (GM-CSF, IL-1α, IL-6, IL-10, TNFα), chemokines (MCP-1, MIP-1α, MIP-2, MIP-3α), matrix metalloproteinase-2 (MMP-2) and of the growth factor PDGF-BB were observed in hypercholesterolemic rats (Table 2). Only the chemokine RANTES did not change in control and cholesterol-treated animals (Table 2).
Table 2.
Hypercholesterolemia enhances several cortical inflammation markers.
| [pg/mg tissue] | Control | Cholesterol | p |
|---|---|---|---|
| GM-CSF | 146 ± 53 | 538 ± 89 | * |
| IL-1α | 37 ± 13 | 153 ± 61 | * |
| IL-6 | 194 ± 56 | 802 ± 205 | * |
| IL-10 | 7.6 ± 2.3 | 36 ± 6 | * |
| MCP-1 | 15 ± 4.2 | 77 ± 15 | *** |
| MIP-1α | 9.4 ± 2.3 | 18 ± 4 | * |
| MIP-2 | 1.8 ± 0.5 | 8.3 ± 1.7 | ** |
| MIP-3α | 51 ± 11 | 140 ± 22 | ** |
| MMP-2 | 264 ± 88 | 1134 ± 159 | *** |
| PDGF-BB | 104 ± 23 | 298 ± 64 | ** |
| TNFα | 35 ± 11 | 100 ± 18 | * |
| RANTES | 27 ± 6 | 35 ± 6 | ns |
Sprague Dawley rats were administered to control and cholesterol-enriched diet for 5 months. Brains were removed and cortical extracts were analyzed by Multiplex SearchLight® ELISA for granulocyte macrophage colony-stimulatory factor (GM-CSF), various interleukins (IL-1α, IL-6, IL-10), monocyte chemotactic protein-1 (MCP-1), different macrophage inflammatory proteins (MIP-1α, MIP-2, MIP-3α), matrix metalloproteinase-2 (MMP-2), platelet-derived growth factor subunit B (PDGF-BB), tumor necrosis factor-alpha (TNFα), and RANTES. Values are given as mean ± S.E.M. in [pg/mg tissue]. Statistical analysis was performed by one way ANOVA with a Fisher LSD posthoc test. *** p < 0.001; ** p < 0.01; * p < 0.05; ns, not significant.
Effects on cortical microglia, vascular structures and IgG
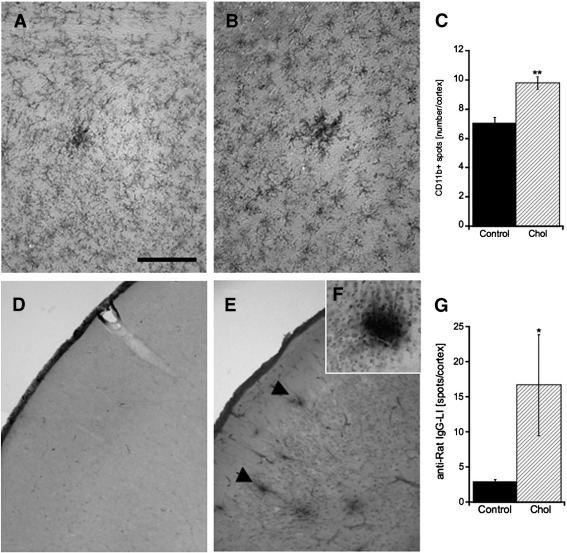
Microglia in cortex were immunohistochemically stained for CD11b in controls (Fig. 4A) and hypercholesterolemic rats (Fig. 4B). Quantitative analysis showed that the number of CD11b-positive spots was significantly enhanced in cholesterol-fed rats compared to control animals (Fig. 4C). Vascular structures in the cortex were immunohistochemically stained by RECA-1. Quantitative measurement of RECA-1-positive crossings per grid in the cortex showed no alterations in the vascular density between control and cholesterol-fed rats (data not shown). Microbleedings were identified by immunohistochemical analysis against anti-rat IgG in control (Fig. 4D) and hypercholesterolemic rats (Figs. 4E and F). Quantitative analysis displayed an enhanced number of anti-rat IgG-positive spots in the cortex of cholesterol-treated animals compared to controls (Fig. 4G).
Fig. 4.
Immunohistochemical staining in the cortex for microglial CD11b and anti-rat IgG of controls and cholesterol-fed rats. Controls (A) and hypercholesterolemic (B) animals were immunohistochemically stained for the microglial marker CD11b. (C) Quantitative analysis revealed an increased number of CD11b-positive spots in the cortex of cholesterol-fed rats. Microbleedings in cortex of control (D) and cholesterol-fed animals (E, F) were immunohistochemically visualized by anti-rat IgG staining. A magnification of an anti-rat IgG-positive spot is shown in F. (G) The number of anti-rat IgG-positive spots in the cortex was significantly enhanced in cholesterol-treated animals (hatched bar) compared to controls (filled bar). Statistical analysis was performed by one way ANOVA with a Fisher LSD posthoc test. **p < 0.01; * p < 0.05; ns, not significant. Scale bar in A = 200 μm (A , B); 800 μm (D, E); 360 μm (F).
Discussion
In the present study we show for the first time that hypercholesterolemia decreased the number of cholinergic neurons in the nBM and the cortical acetylcholine level, which might result in a dysfunction of the cholinergic system. Furthermore, cholesterol-enriched diet reduced spatial learning and the ability to store long-term memory in an 8-am radial maze, enhanced the inflammation process, elevated cortical beta-amyloid, tau and phospho-tau 181 and increased microbleedings in the cortex.
Cholesterol has been demonstrated to affect the APP processing and linked to the pathology of AD (Raffai and Weisgraber, 2003; Simons et al., 2001; Shobab et al., 2005). Various animal studies have been shown that hypercholesterolemia displays some characteristics of AD in vivo (Granholm et al., 2008; Refolo et al., 2000). The current study was performed in male rats to exclude hormonal influences, because cholesterol homeostasis of females is mediated by the hypolipodemic properties of estrogens (de Marinis et al., 2008). Our present study revealed that a cholesterol-enriched diet enhanced plasma cholesterol concentration. However, it is not clear how plasma cholesterol is able to affect brain functions, since cholesterol does not pass the BBB. However, oxysterols, oxygenated derivates of cholesterol are able to pass lipophilic membranes (Björkhem et al., 2002; Björkhem, 2002). In fact, increased brain levels of 27-hydroxycholesterol have been measured in AD patients (Björkhem et al., 2006). Although, not proven enhanced blood cholesterol may be metabolized to its oxysterols, which can easier enter the brain.
The 8-arm radial maze is well established to test learning and memory in a controlled environment (Jarrard et al., 1984). Our study revealed severe spatial learning and long-term memory deficits in hypercholesterolemic rats. Spatial memory was tested in a partially baited 8-arm radial maze. Three weeks after a 5-day acquisition period, rats were again tested to assess long-term memory performance (retention). Hypercholesterolemic rats were significantly heavier in weight and to exclude decreased mobility, we monitored various parameters and found no differences in motivation and mobility between control and hypercholesterolemic rats. We conclude that a cholesterol-enriched diet results in spatial memory impairment, which is in line with previous studies (Anstey et al., 2008; Liu et al., 2008; Foster, 2006). Indeed, rats fed with higher amount of dietary fat showed widespread cognitive deficits on various tasks of learning and memory such as, Olton's radial arm maze, a non-spatial test of conditional associative learning, the Hebb–Williams complex maze series, and a variable-interval delayed alternation test that highlighted deficits in rule-learning and specific memory function (Greenwood and Young, 2002; Molteni et al., 2002; Winocur and Greenwood, 1999). The mechanisms of effects of dietary fat on cognitive function are not completely understood, however, it seems possible that high fat diet may cause glucose intolerance and/or insulin resistance in rat brains (Srinivasan et al., 2004; Greenwood and Young, 2002; Molteni et al., 2002; Winocur and Greenwood, 1999; Greenwood and Winocur, 2001).
AD is characterized by a substantial loss of cholinergic innervation in the cerebral cortex and a significant reduction of cholinergic basal forebrain neurons can be observed (Mesulam, 2010; Mufson et al., 2003; Vogels et al., 1990). As a result, the concentrations of acetylcholine and ChAT are markedly reduced in the cortex of AD patients (Bowen et al., 1976; Davies and Maloney, 1976). The clinically outcome of AD is represented by progressive memory impairment and cognitive dysfunctions (McKhann et al., 1984). Accordingly, it has been shown that the basal forebrain cholinergic complex is closely associated with learning and memory (McKinney, 2005; Winkler et al., 1995) and systemic disturbances lead to mnemonic impairment or loss (McLin et al., 2002; Pizzo et al., 2002). Cholinergic neurons in the nBM have been demonstrated as the most sensitive neurons to age-related neurofibrillary degeneration (Sassin et al., 2000) and might be associated with cognitive impairments in aging and AD (Wenk, 1997). Our present data demonstrate that a cholesterol-enriched diet decreased the number of ChAT-positive neurons in the nBM resulting in a reduction of acetylcholine in the cortex. We suggest that hypercholesterolemia leads to a disrupted cholinergic system in the basal forebrain, which may contribute to the spatial memory impairment. Cholesterol may interact with the membrane environment of cholinergic receptors (muscarinic as well as nicotinic) and may thus modulate their activity (Bohr, 2004). It is also very likely that there are functional relationships between cholinergic receptors and lipid rafts-specialized plasmalemmal structures enriched in cholesterol (Feron and Kelly, 2001). Indeed, high cholesterol levels reduce the activity of plasmalemmal receptors, such as certain monoaminergic receptors, e.g. beta-adrenergic receptors (Barnett et al., 1989), which can modulate the cholinergic neurons.
Nerve growth factor (NGF) is the most prominent molecule to protect cholinergic neurons against neurodegeneration (Schliebs and Arendt, 2006; Winkler et al., 1998). In AD brains, a loss of NGF and its receptor TrkA was identified in the basal forebrain, whereas the concentration of NGF in target regions is increased (Schindowski et al., 2008). This alteration is caused by an impaired retrograde transport, accumulation of NGF in target regions of cholinergic neurons and a loss of NGF in the basal forebrain (Longo and Massa, 2004; Schindowski et al., 2008; Schliebs and Arendt, 2006). Moreover, various studies exhibited that administration of exogenous NGF can rescue memory deficits (Covaceuszach et al., 2009; de Rosa et al., 2005). In the present study, we discovered a slight but not significant increase of NGF in the cortex of cholesterol-treated animals. Thus, we suggest that a damaged cholinergic system in hypercholesterolemia enhances NGF in the cortex.
It is well established that inflammation is seen in AD brains and in hypercholesterolemic animal models (Rahman et al., 2005; Thirumangalakudi et al., 2008; Xue et al., 2007). Our data demonstrate that a cholesterol-enriched diet induced an elevated immunohistochemical-positive staining for the microglial marker CD11b. It has been shown, that activated microglia produce and secrete various cytokines and chemokines, e.g. IL-1, MCP-1, MIP-1α, or TNFα (Akiyama et al., 2000; Lucas et al., 2006; Rogers, 2008). The cytokine IL-1 has been linked to APP regulation and it may promote beta-amyloid production (Akiyama et al., 2000; Eikelenboom and van Gool, 2004) possibly by affecting the acetylcholinesterase expression (Akiyama et al., 2000) or by activation of neurodegeneration (Akiyama et al., 2000; Stuchbury and Münch, 2005). In agreement with previous in vivo studies, we found an activation of microglial immunoreactivity (Granholm et al., 2008; Rahman et al., 2005; Thirumangalakudi et al., 2008; Xue et al., 2007) and elevations of different inflammation markers (Rahman et al., 2005; Thirumangalakudi et al., 2008). The mechanism of microglial activation and the role of cholesterol in the process of inflammation is not fully known. Different studies revealed that oxygenated derivates of cholesterol (24-hydroxycholesterol, 25-hydroxycholesterol or 27-hydroxycholesterol) are involved in the upregulation of a number of inflammatory markers especially 24-hydroxycholesterol (Lemaire-Ewing et al., 2005; Rosklint et al., 2002; Dugas et al., 2010; Prunet et al., 2006; Morello et al., 2009; Trousson et al., 2009; Vejux et al., 2008; Sottero et al., 2009; Joffre et al., 2007). We have recently shown that oxysterol treatment differentially regulate cholinergic nBM neurons in organotypic brain slices (Ullrich and Humpel, 2010). Thus, in our hypercholesterolemic rat model, we observed strong activation of several inflammation markers, which are implicated in pathophysiological alterations similar to AD and could be mediated by different cholesterol oxysterols.
Beta-amyloid depositions and neurofibrillary tangles are distinctive hallmarks of AD. A disturbed cholesterol homeostasis within lipid rafts might influence APP processing, which result in increased beta-aymloid(1–42) production and deposition (Ghribi, 2008; Simons et al., 2001). Whereas, little is known about the function of cholesterol in the formation of neurofibrillary tangles. Our data reveal that hypercholesterolemia enhanced APP and beta-amyloid(1–42) levels, as well as tau and phospho-tau 181 levels in the cortex. This was also confirmed by descriptive immunohistochemical analysis showing enhanced beta-amyloid-like immunoreactivity in the cortex of cholesterol-fed rats. For quantitative determination of tau and phospho-tau 181 we used a human specific ELISA assay, which has not been tested for rats. However, due to high homology between human and rat tau, we think that rat tau can be detected. Our findings are in line with previous studies showing elevated concentrations of beta-amyloid(1–42) (Sharma et al., 2008; Prasanthi et al., 2008; Ghribi et al., 2006; Refolo et al., 2000) and an enhanced level of hyperphosphorylated tau (Ghribi et al., 2006) after cholesterol-enriched diet. Various studies exhibited that 27-hydroxycholesterol supported beta-amyloid aggregation in the brain (Sharma et al., 2008; Prasanthi et al., 2009). In addition, APP can be upregulated due to NGF accumulation in the cortex of AD patients (Schindowski et al., 2008). Ghribi and colleagues (2006) suggested that increased beta-amyloid trigger phosphorylation of tau by activation of extracellular signal-regulated protein kinase (ERK) and Nicholson and Ferreira (2009) described an association between membrane cholesterol and beta-amyloid-induced tau toxicity in AD. Thus, we suggest that a cholesterol-enriched diet affected the cleavage of APP, which results in the production of beta-amyloid(1–42) and that hypercholesterolemia upregulates tau and phospho-tau 181 in the cortex. However, we could not observe beta-amyloid plaque deposition and neurofibrillary tangles in hypercholesterolemic rats. This findings may either need longer times of cholesterol diet or the combination with other parameters, e.g. low pH, apolipoprotein E or metals (Marksteiner and Humpel, 2008).
It is well established that vascular risk factors and neurovascular dysfunction play integral roles in the pathogenesis of AD (Bell and Zlokovic, 2009; Dickstein et al., 2010; Iadecola, 2004; Stolp and Dziegielewska, 2009). Furthermore, AD is often associated with cerebral hypoperfusion, which may lead to cortical microinfarcts and microbleedings (Suter et al., 2002; Miklossy, 2003). It has been shown that hypercholesterolemia affects the BBB integrity and leads to increase in IgG extravasation in cholesterol-fed rabbits (Chen et al., 2008; Sparks et al., 2000). Nevertheless, recent studies have shown that normal rat brains contain low IgG, but the levels dramatically increase after head injury (Aihara et al., 1994; Hazama et al., 2005). In agreement, we exhibited an increased anti-rat IgG-positive immunoreactivity in the cortex of cholesterol-treated animals compared to controls indicating microbleedings and microinfarcts. In contrast, RECA-1-positive immunohistochemistry, a well-known marker for capillaries (Moser et al., 2003) demonstrated no changes in the vascular structure of controls and hypercholesterolemic rats. However, the immunohistochemical staining against rat IgG or RECA-1 does not demonstrate changes to small molecules of the vascular system. Consequently, further studies are necessary to evaluate microchanges and the integrity of the BBB. Furthermore, it might be possible that elevated MMP-2 levels in our cholesterol-treated rats contribute to the disruption of the basal lamina and tight junctions of the BBB (Candelario-Jalil et al., 2009). Finally, a dysfunctional transport across the BBB may also affect efflux of metabolic waste or influx of energy substrates. An example of diminished transport could be insulin, which has an important role in CNS physiology like modulation of glucose utilization in specific brain regions or modulation of synaptic levels of neurotransmitters (Biessels et al., 2004; Hoyer, 2004). Thus, a decreased insulin transport might diminish many beneficial effects of insulin in the brain. Taken together we suggest that a hypercholesterolemic diet induces small microbleedings in the cortex associated with microglial activation and dysfunctional metabolic changes of the BBB similar to AD pathology.
Taken together, our results suggest that hypercholesterolemia causes a dysfunction of the cholinergic system, cognitive deficits, inflammation, beta-amyloid and tau-pathology and microbleedings. Thus, hypercholesterolemic rats resemble some AD-like pathologies and demonstrate for the first time an impaired cholinergic system due to high cholesterol diet.
Experimental methods
Controls and hypercholesterolemia
Male Sprague Dawley rats (aged 6 months) were housed at the Animal Department of the Medical University Innsbruck and had free access to food and water with a 12/12 h light–dark circle. Animals were fed with a special diet for 5 months and randomly assigned to the following groups: group 1: controls, normal diet (n = 10) and group 2: hypercholesterolemia, diet supplemented with 5% cholesterol (n = 10). The diet contains the following ingredients: 450 g/kg cornstarch, 140 g/kg casein, 155 g/kg maltodextrin, 100 g/kg sucrose, 40 g/kg soybean oil, 50 g/kg fiber, 35 g/kg mineral mix, 1.8 g/kg L-cystine, 1.4 g/kg choline chloride, 0.008 g/kg butylhydroxytoluol, 10 g/kg vitamin mix (without folic acid), 1 g/kg chocolate aroma, 0.002 g/kg folic acid and additional 50 g/kg cholesterol in the cholesterol-treated group (Ssniff special diet GmbH; Soest Germany). The animals were weighed every month during the experiment. All animal experiments were approved by the Austrian Ministry of Science.
Spatial memory testing in the 8-arm radial maze
Four months after start of the experiment spatial memory was assessed using the 8-arm radial maze (PanLab, Spain). The maze consists of eight identical open dark plexiglas arms with side panels and sunk-in-food cups at the end radiating from a circular platform. To facilitate spatial navigation small high contrast visual cues (triangle, vertical bars, X and square) were placed above the doors of four arms and in a higher magnification on the corresponding walls. Before memory testing, all animals were food deprivated (2 g food pellets/animal/day) and habituated to the maze and the experimental set-up. The spatial memory testing consisted of five sessions with five trials per day. Four arms were baited with food pellets (chocolate) and the trials ended when all baits were found or after 10 min. To exclude any olfactory effects additional baits were placed under the food cups of all arms. After 3 weeks the animals were again tested (retention) for one session with five trials. The whole experiment was automatically controlled and monitored by a computer with Mazesoft Software (Version 8.1.9).
Collection of blood and brains
One day after retention, animals were anesthetized by subcutaneous injection of sodium thiopental (Sandoz; 12.5 mg/ml). Brains (n = 5) for brain extracts were removed, the parietal cortex dissected and immediately frozen in CO2 snow and the rest of the brain was frozen on a cork in the CO2 snow. From the same animals 1–2 ml blood was collected from the heart, immediately centrifuged by 2300 × g and the plasma frozen at −80 °C. For immunohistochemistry, rats (n = 5) were perfused with 4% paraformaldehyde (PFA) in PBS, the brains removed and postfixed for 30 min in 4% PFA and stored in 20% sucrose/sodium azide. Brains were then frozen in a CO2 stream and sectioned with a cryostat (Leica Jung CM3000) into 60 μm sections (immunohistochemistry).
Measurement of cholesterol in plasma
Cholesterol was analyzed by HPLC and UV detection as described by Webb et al. (1982). Briefly, 50 μl of plasma or standards (0–5 mg/ml cholesterol in 70% ethanol) were mixed with 1 ml of alcoholic potassium hydroxide and incubated for 30 min at 75 °C. After cooling to room temperature, 1 ml of de-ionized water and 2 ml of n-hexane were added to the tubes. The tubes were agitated for 15 min and centrifuged for 5 min at 200 × g. One ml of the n-hexane (upper) layer was transferred into a glass tube and evaporated at 75 °C. Residues were dissolved in 500 μl mobile phase (44% acetonitrile, 54% isopropanol, 2% a.d.) and 100 μl were injected onto the column (RP C18 100 × 3 mm, Beckman) 0.6 ml/min. Cholesterol was monitored at 205 nm with an UV-detector (Beckman detector 166). Sample values were calculated from the standard curve in a linear range.
Measurement of acetylcholine in the cortex
Acetylcholine (Ach) levels were analyzed by HPLC and electrochemical detection as described by us (Fischer et al., 1998). Briefly, the tissue was homogenized in 150 μl ice-cold sodium-phosphate buffer (PBS + 5 μM neostigmine, pH 5.3) using an ultrasonic device (Branson sonifier 250, Danburry) and centrifuged at 16,000 × g at 4 °C for 5 min. The supernatant (100 μl) was rapidly injected onto the analytical column (BASI MF6150), connected to an “immobilized-enzyme-reactor” (IMER; BASI MF6151). This IMER hydrolyzes Ach and generates hydrogen peroxide, which can electrochemically detected via oxidation at a platinum electrode (+ 500 mV vs. Ag/AgCl2) of an electrochemical detector (Antel Leiden Decade II). The mobile phase consisted of 50 mM sodium phosphate, 0.01% sodium azide, pH 8.5. The amount of Ach in the homogenates was quantified using calibration curves of external Ach standards (Sigma, St. Louis).
Immunohistochemistry
Immunohistochemistry was performed as described previously (Ullrich and Humpel, 2009). All incubations were performed at 4 °C for 2 days including 0.1% Triton, such that the antibodies can penetrate into the brain sections. Slices were washed with 0.1% Triton/PBS at room temperature for 30 min and pretreated for 20 min with 20% methanol/1% H2O2/PBS (only for 3,3´-diaminobenzidine labeling). After thorough rinsing, the sections were blocked with 20% horse serum/0.2% bovine serum albumine/PBS and then incubated for 2 days at 4 °C with primary antibodies. Antibodies used were as follows: goat anti-choline acetyltransferase (Millipore; 1:750); mouse anti-rat beta-amyloid(17–42) (Millipore; 1:100); mouse anti-CD11b (Millipore; 1:500); and mouse anti-RECA-1 (Serotec; 1:100). The sections were again washed with PBS and incubated with secondary biotinylated anti-goat, or anti-mouse (1:200; Vector Lab., Szabo, Vienna, Austria) for 1 h at room temperature. After being washed, sections were incubated in an avidin–biotin complex solution (ABC-Elite Vectastain reagent; Vector Lab., USA) for 1 h, washed in 50 mM Tris-buffered saline (TBS), and then the signal was detected using 0.5 mg/ml 3,3´-diaminobenzidine including 0.003% H2O2 as a substrate in TBS. The sections were mounted on glass slides, air-dried and coverslipped with Entellan (Merck, Darmstadt, Germany). Unspecific labeling was defined by omitting the primary antibody. When fluorescence immunohistochemistry was performed, the methanol pretreatment was omitted and as a secondary antibody Alexa-488 (Invitrogen, Austria; 1:400) was used. Immunolabeling was visualized with a Leica DMIRB fluorescence inverse microscope equipped with an Apple computer and Improvison DarkLab software.
Evaluation of BBB disruption
Breakdown of the BBB permeability was analyzed using immunohistochemistry for rat IgG (Schmidt-Kastner et al., 1993). Briefly, sections were incubated for 2 h in biotinylated rabbit anti-rat IgG (Vector, 1:400). After being washed, sections were incubated in an avidin-biotin complex solution (ABC-Elite Vectastain reagent; Vector Lab., USA) for 1 h. Visualization of IgG-immunoreactivity was identical to that described above for immunohistochemistry.
ELISA for NGF
NGF analysis was performed by using a commercial ELISA (Promega) as described previously (Zassler and Humpel, 2006). Briefly, ELISA plates (96 wells) were coated with an anti-NGF monoclonal antibody diluted in carbonate coating buffer (pH 9.7) and incubated overnight at 4 °C. Plates were blocked for 1 h at room temperature with 1x blocking buffer (200 μl/well), then NGF standards (0–100 pg/well) or diluted homogenates (100 μl) were added and incubated at room temperature for 6 h on a shaker. After washing, the plates were incubated with monoclonal rat anti-NGF overnight at 4 °C, again washed and then horseradish peroxidase-conjugated anti-rat antibody (1:4,000) was added to the plates and incubated at room temperature for 2 h on a shaker. Plates were again washed and the enzyme substrate (TMB One solution, Promega) was added and incubated for 15 min at room temperature. The enzyme reaction was stopped by adding 1N HCl and the absorbance was measured at 450 nm in a microplate reader. Sample values were calculated from the standard curve in a linear range.
ELISA for rat beta-amyloid, tau and phospho-tau 181
Rat beta-amyloid(1–42) levels were measured using a commercial high sensitive ELISA Kit (WAKO, Neuss, Germany). ELISAs for tau and phospho-tau 181 (Innogenetics; NV, Gent, Belgium) were performed as described by us (Blasko et al., 2006). Briefly, standards or extracts (100 μl) were added and incubated at 4 °C overnight. After washing, the plates were incubated with 100 μl HRP-conjugate at 4 °C for 1 h. Plates were again washed and the enzyme substrate (TMB-solution) was added and incubated for 30 min in the dark. The enzyme reaction was stopped and the absorbance was measured at 450 nm in a microplate reader. Sample values were calculated from the standard curve in a linear range.
Measurement of inflammation markers
Inflammation markers were analyzed using a Multiplex rat 6-plex ELISA (SearchLight®, Aushon Biosystems) as described by us recently (Marksteiner et al., 2009). Standards or extracts (50 μl) were added to the pre-spotted plates and incubated for 3 h at room temperature on a shaker. After washing, the plates were incubated with 50 μl of biotinylated antibody at room temperature for 30 min. Plates were again washed and 50 μl of streptavidin-HRP reagent was added to each well and incubated for 30 min. After the final washing step, 50 μl of SuperSignal® chemiluminescent substrate was added and the luminescent signal was detected with a cooled CCD camera equipped with the SearchLight® CCD imaging and analysis system. Sample values were calculated from the standard curve in a linear range.
Western Blot analysis
Western Blots were performed as described by us (Böttger et al., 2010; Marksteiner and Humpel, 2008). Briefly, samples were heated at 70 °C for 10 min and then loaded onto 10% Bis-Tris polyacrylamide gels (NuPage, Invitrogen) and electrophoresed for 35 min at 200 V. Samples were electrotransferred to nylon-PVDF Immobilon-PSQ membranes (Millipore) for 90 min at 30 V with a 20% methanol blotting buffer (Invitrogen). For detection, the Western Breeze Chemiluminescent System (Invitrogen) was used. Briefly, blots were blocked for 30 min with blocking buffer, then incubated for 90 min with the primary mouse anti-amyloid precursor protein A4 antibody (Chemicon; 1:2000), mouse anti-rat beta-amyloid(17–24) antibody (Chemicon; 1:1000) or rabbit anti-actin antibody (Sigma-Aldrich; 1:1000), washed and incubated with alkaline phosphatase-conjugated anti-mouse or anti-rabbit antibodies for 30 min at room temperature. After being washed, blots were incubated in CDP-Star chemiluminescent substrate solution (Invitrogen) and the signal was visualized with a cooled CCD camera (SearchLight®, Thermoscience).
Quantitative analysis and statistics
Behavioral testing was statistically analyzed within each group by using one-way ANOVA with repeated measures and between control and cholesterol-fed animals by using student T-test. The number of ChAT-positive neurons was counted in the basal nucleus of Meynert (nBM) between Bregma −0.8 mm and −3.2 mm in control and hypercholesterolemic rats visualized under a 20× objective according to the rat brain atlas (Paxinos and Watson, 1986). Rat anti-IgG and CD11b-positive spots were counted in the cortex in 6 fields per section and with 4 sections per brain under a 20× objective in control and cholesterol-fed rats. The capillary network was assessed according to Moser et al. (2003) by counting the number of crossings of capillaries, which cross the lines of a grid (150 μm × 150 μm per single square). Sections were photographed under the microscope and digitized pictures overlaid in NIH ImageJ software with a grid. A quantitative analysis of Western Blot was performed by densitometry. Optical density of bands were measured using NIH ImageJ software. Obtained values were corrected according to background and band size and compared to actin values (internal control). Quantitative data are presented as mean values ± S.E.M. The significance of differences between the control and cholesterol-fed group was assessed by using one way ANOVA, followed by Fisher PLSD posthoc test by comparing controls against cholesterol treatment, where p < 0.05 represents statistical significance.
Acknowledgments
This study was supported by the Austrian Science Fonds (P19122-B05). We thank Ursula Kirzenberger-Winkler for her excellent technical help.
References
- Aihara N., Tanno H., Hall J.J., Pitts L.H., Noble L.J. Immunocytochemical loclization of immunoglobulins in the rat brain: relationship to the blood-brain barrier. J. Comp. Neurol. 1994;342:481–496. doi: 10.1002/cne.903420402. [DOI] [PubMed] [Google Scholar]
- Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., Finch C.E., Frautschy S., Griffin W.S.T., Hampel H., Hull M., Landreth G., Lue L.-F., Mrak R., Mackenzie I.R., McGeer P.L., O'Bannion M.K., Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van Muiswinkel F.L., Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey K.J., Lipnicki D.M., Low L.F. Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am. J. Geriatr. Psychiatry. 2008;16:343–354. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- Barnett J.V., Haigh L.S., Marsh J.D., Galper J.B. Effects of low density lipoproteins and mevinolin on sympathetic responsiveness in cultured chick atrial cells. J. Biol. Chem. 1989;264:10779–10786. [PubMed] [Google Scholar]
- Bell R.D., Zlokovic B.V. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels G.J., Bravenboer B., Gispen W.H. Glucose, insulin and the brain: modulation of cognition and synaptic plasticity in helath and disease: a preface. Eur. J. Pharmacol. 2004;490:1–4. doi: 10.1016/j.ejphar.2004.02.057. [DOI] [PubMed] [Google Scholar]
- Björkhem I. Do oxysterols control cholesterol homeostasis? J. Clin. Invest. 2002;110:725–730. doi: 10.1172/JCI16388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkhem I., Meaney S., Diczfalusy U. Oxysterols in human circulation: which role do they have? Curr. Opin. Lipidol. 2002;13:247–253. doi: 10.1097/00041433-200206000-00003. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Heverin M., Leoni V., Meaney S., Diczfalusy U. Oxysterols and Alzheimer's disease. Acta Neurol. Scand. 2006;114:43–49. doi: 10.1111/j.1600-0404.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- Blasko I., Lederer W., Oberbauer H., Walch T., Kemmler G., Hinterhuber H., Marksteiner J., Humpel C. Measurement of thirteen biological markers in CSF of patients with Alzheimer´s disease and other dementias. Dement. Geriatr. Cogn. Disord. 2006;21:9–15. doi: 10.1159/000089137. [DOI] [PubMed] [Google Scholar]
- Bohr I. Hypercholesterolemic diet applied to rat dams protects their offspring against cognitive deficits. Simulated neonatal anoxia model. Physiol. Behav. 2004;82:703–711. doi: 10.1016/j.physbeh.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Bojanic D.D., Tarr P.T., Gale G.D., Smith D.J., Bok D., Chen B., Nusinowitz S., Lövgren-Sandblom A., Björkhem I., Edwards P.A. Differential expression and function of ABCG1 and ABCG4 during development and ageing. J. Lipid Res. 2010;5:169–181. doi: 10.1194/jlr.M900250-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttger D., Ullrich C., Humpel C. Monocytes deliver bioactive nerve growth factor through a brain capillary endothelial cell-monolayer in vitro and counteract degeneration of cholinergic neurons. Brain Res. 2010;1312:108–119. doi: 10.1016/j.brainres.2009.11.062. [DOI] [PubMed] [Google Scholar]
- Bowen D.M., Smith C.B., White P., Davison A.N. Neutoransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976;99:459–496. doi: 10.1093/brain/99.3.459. [DOI] [PubMed] [Google Scholar]
- Buxbaum J.D., Cullen E.I., Friedhoff L.T. Pharmacological concentrations of the HMG-CoA reductase inhibitor lovostatin decrease the formation of the Alzheimer beta-amyloid peptide in vitro and in patients. Front. Biosci. 2002;7:50–59. doi: 10.2741/A739. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E., Yang Y., Rosenberg G.A. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Gawryluk J.W., Wagener J.F., Ghribi O., Geiger J.D. Caffeine blocks disruption of blood brain barrier in a rabbit model of Alzheimer's disease. J. Neuroinflammation. 2008;5:12. doi: 10.1186/1742-2094-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covaceuszach S., Capsoni S., Ugolini G., Spirito F., Vignone D., Cattaneo A. Development of a non invasive NGF-based therapy for Alzheimer's disease. Curr. Alzheimer Res. 2009;6:158–170. doi: 10.2174/156720509787602870. [DOI] [PubMed] [Google Scholar]
- Davies P., Maloney A.J. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- De Marinis E., Martini C., Trentalance A., Pallottini V. Sex differences in hepatic regulation of cholesterol homeostasis. J. Endocrinol. 2008;198:635–643. doi: 10.1677/JOE-08-0242. [DOI] [PubMed] [Google Scholar]
- De Rosa R., Garcia A.A., Braschi C., Capsoni S., Maffei L., Berardi N., Cattaneo A. Intranasal administration of nerve growth factor (NGF) rescues recognition memory deficits in AD11 anti-NGF transgenic mice. Proc. Natl Acad. Sci. USA. 2005;102:3811–3816. doi: 10.1073/pnas.0500195102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein D.l., Walsh J., Brautigam H., Stockton S.D., Gandy S., Hof P. Role of vascular risk factors and vascular dysfunction in Alzheimer's disease. J. Mt. Sinai Hosp. NY. 2010;77:82–102. doi: 10.1002/msj.20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas B., Charbonnier S., Baarine M., Ragot K., Delmas D., Ménétrier F., Lherminier J., Malvitte L., Khalfaoui T., Bron A., Creuzot-Garcher C., Latruffe N., Lizard G. Effects of oxysterols on cell viability, inflammatory cytokines, VEGF, and reactive oxygen species production on human retinal cells: cytoprotective effects and prevention of VEGF secretion by resveratrol. Eur. J. Nutr. 2010 doi: 10.1007/s00394-010-0102-2. PMID: 20339855 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Ehehalt R., Keller P., Haass C., Thiele C., Simons K. Amyloidogenic processing of the Alzheimer β-amyloid precursor protein depends on lipid rafts. J. Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom P., van Gool W.A. Neuroinflammatory perspectives on the two faces of Alzheimer´s disease. J. Neural Transm. 2004;111:281–294. doi: 10.1007/s00702-003-0055-1. [DOI] [PubMed] [Google Scholar]
- Feron O., Kelly R.A. The caveolar paradox. Suppressing, inducing, and terminating eNOS signaling. Circ. Res. 2001;88:129–131. doi: 10.1161/01.res.88.2.129. [DOI] [PubMed] [Google Scholar]
- Fischer H.P., Marksteiner J., Ransmayer G., Saria A., Humpel C. NGF but not GDNF or Neurturin enhance acetylcholine tissue levels in striatal organotypic brain slices. Int. J. Dev. Neurosci. 1998;16:391–401. doi: 10.1016/s0736-5748(98)00039-2. [DOI] [PubMed] [Google Scholar]
- Foster T.C. Biological markers of age-related memory deficits: treatment of senescent physiology. CNS Drugs. 2006;20:153–166. doi: 10.2165/00023210-200620020-00006. [DOI] [PubMed] [Google Scholar]
- Friedhoff L.T., Cullen E.I., Geoghagen N.S., Buxbaum J.D. Treatment with controlled-release lovastatin decreases serum concentrations of human beta-amyloid (A beta) peptide. Int. J. Neuropsychopharmacol. 2001;4:127–130. doi: 10.1017/S1461145701002310. [DOI] [PubMed] [Google Scholar]
- Ghribi O. Potential mechanisms linking cholesterol to Alzheimer's disease-like pathology in rabbit brain, hippocampal organotypic slices, and skeletal muscle. J. Alzheimers Dis. 2008;15:673–684. doi: 10.3233/jad-2008-15412. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ghribi O., Larsen B., Schrag M., Herman M. High cholesterol content in neurons increases BACE, β-amyloid, and phosphorylated tau levels in rabbit hippocampus. Exp. Neurol. 2006;200:460–467. doi: 10.1016/j.expneurol.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Granholm A.-C., Bimonte-Nelson H.A., Moore A.B., Nelson M.E., Freeman L.R., Sambamurti K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J. Alzheimers Dis. 2008;14:133–145. doi: 10.3233/jad-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood C.E., Winocur G. Glucose treatment reduces memory deficits in young adult rats fed high-fat diets. Neurobiol. Learn. Mem. 2001;75:179–189. doi: 10.1006/nlme.2000.3964. [DOI] [PubMed] [Google Scholar]
- Greenwood C.E., Young S.N. Dietary fat intake and the brain: a developing frontier in biological psychiatry. J. Psychiatry Neurosci. 2002;26:182–184. [PMC free article] [PubMed] [Google Scholar]
- Hazama G.-I., Yasuhara O., Morita H., Aimi Y., Tooyama I., Kimura H. Mouse brain IgG-like immunoreactivity: strain-specific occurrence in microglia and biochemical identification of IgG. J. Comp. Neurol. 2005;492:234–249. doi: 10.1002/cne.20710. [DOI] [PubMed] [Google Scholar]
- Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur. J. Pharmacol. 2004;490:115–125. doi: 10.1016/j.ejphar.2004.02.049. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Neuroscience. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Ishii K., Tokuda T., Matsushima T., Miya F., Shoji S., Ikeda S., Tamaoka A. Pravastatin at 10 mg/day does not decrease plasma levels of either amyloid-beta (Abeta) 40 or Abeta 42 in humans. Neurosci. Lett. 2003;350:161–164. doi: 10.1016/s0304-3940(03)00895-4. [DOI] [PubMed] [Google Scholar]
- Jarrard L.E., Okaichi H., Steward O., Goldschmidt R.B. On the role of hippocampal connections in the performance of place and cue tasks: comparisons with damage to hippocampus. Behav. Neurosci. 1984;98:946–954. doi: 10.1037//0735-7044.98.6.946. [DOI] [PubMed] [Google Scholar]
- Joffre C., Leclère L., Buteau B., Martine L., Cabaret S., Malvitte L., Acar N., Lizard G., Bron A., Creuzot-Garcher C., Bretillon L. Oxysterols induced inflammation and oxidation in primary porcine retinal pigment epithelial cells. Curr. Eye Res. 2007;32:271–280. doi: 10.1080/02713680601187951. [DOI] [PubMed] [Google Scholar]
- Kandiah N., Feldman H.H. Therapeutic potential of statin in Alzheimer's disease. J. Neurol. Sci. 2009;283:230–234. doi: 10.1016/j.jns.2009.02.352. [DOI] [PubMed] [Google Scholar]
- Kivipelto M., Helkala E.-L., Laakso M.P., Hänninen T., Hallikainen M., Alhainen K., Soininen H., Tuomilehto J., Nissien A. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire-Ewing S., Prunet C., Montange T., Vejux A., Berthier A., Bessède G., Corcos L., Gambert P., Nèel D., Lizard G. Comparison of the cytotoxic, pro-oxidant and pro-inflammatory characteristics of different oxysterols. Cell Biol. Toxicol. 2005;21:97–114. doi: 10.1007/s10565-005-0141-2. [DOI] [PubMed] [Google Scholar]
- Levin-Allerhand J.A., Lominska C.E., Smith J.D. Increased amyloid-β levels in APPSWE transgenic mice treated chronically with a physiological high-fat high-cholesterol diet. J. Nutr. Health Aging. 2002;6:315–319. [PubMed] [Google Scholar]
- Liu R.Y., Gu R., Qi X.L., Zhang T., Zhao Y., He Y., Pei J.J., Guan Z.Z. Decreased nicotinic receptors and cognitive deficit in rats intracerebroventricularly injected with beta-amyloid peptide(1–42) and fed a high-cholesterol diet. J. Neurosci. Res. 2008;86:183–193. doi: 10.1002/jnr.21463. [DOI] [PubMed] [Google Scholar]
- Longo F.M., Massa S.M. Neuroprotective strategies in Alzheimer´s disease. NeuroRx. 2004;1:117–127. doi: 10.1602/neurorx.1.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas S.-M., Rothwell N.J., Gibson R.M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006;147:232–240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marksteiner J., Humpel C. Beta-amyloid expression, release and extracellular deposition in aged rat brain slices. Mol. Psychiatry. 2008;13:939–952. doi: 10.1038/sj.mp.4002072. [DOI] [PubMed] [Google Scholar]
- Marksteiner J., Kemmler G., Weiss E.M., Knaus G., Ullrich C., Mechteriakov S., Oberbauer H., Auffinger S., Hinterhölzl J., Hinterhuber H., Humpel C. Five out of 16 plasma signaling proteins are enhanced in plasma of patients with mild cognitive impairment and Alzheimer´s disease. Neurobiol. Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.03.011. PMID: 19395124 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKinney M. Brain cholinergic vulnerability: relevance to behavior and disease. Biochem. Pharmacol. 2005;70:1115–1124. doi: 10.1016/j.bcp.2005.05.019. [DOI] [PubMed] [Google Scholar]
- McLin D.E., Miasnikov A.A., Weinberger N.M. Induction of behavioral associative memory by stimulation of the nucleus basalis. PNAS. 2002;99:4002–4007. doi: 10.1073/pnas.062057099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M. The cholinergic lesion of Alzheimer's disease: pivotal factor or side show? Learn. Mem. 2010;11:43–49. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- Miklossy J. Cerebral hypoperfusion induces cortical watershed microinfarcts which may further aggravate cognitive decline in Alzheimer's disease. Neurol. Res. 2003;25:605–610. doi: 10.1179/016164103101202048. [DOI] [PubMed] [Google Scholar]
- Molteni R., Barnard R.J., Ying Z., Roberts C.K., Gómez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- Morello F., Saglio E., Noghero A., Schiavone D., Williams T.A., Verhove A., Bussolino F., Veglio F., Mulatero P. LXR-activating oxysterols induce the expression of inflammatory markers in endothelial cells through LXR-independent mechanisms. Atherosclerosis. 2009;207:38–44. doi: 10.1016/j.atherosclerosis.2009.04.001. d. [DOI] [PubMed] [Google Scholar]
- Moser K.V., Schmidt-Kastner R., Hinterhuber H., Humpel C. Brain capillaries and cholinergic neurons persist in organotypic brain slices in the absence of blood flow. Eur. J. Neurosci. 2003;18:85–94. doi: 10.1046/j.1460-9568.2003.02728.x. [DOI] [PubMed] [Google Scholar]
- Mufson E.J., Ginsberg S.D., Ikonomovic M.D., DeKosky S.T. Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J. Chem. Neuroanat. 2003;26:233–242. doi: 10.1016/s0891-0618(03)00068-1. [DOI] [PubMed] [Google Scholar]
- Nicholson A.M., Ferreira A. Increased membrane cholesterol might render mature hippocampal neurons more susceptible to β-amyloid-induced calpain activation and tau toxicity. J. Neurosci. 2009;29:4640–4651. doi: 10.1523/JNEUROSCI.0862-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Watson C. 2nd ed. Academic Press; Australia: 1986. The rat brain in stereotaxic coordinates. [Google Scholar]
- Pfrieger F.W. Role of cholesterol in synapse formation and function. Biochem. Biophys. Acta. 2003;1610:271–280. doi: 10.1016/s0005-2736(03)00024-5. [DOI] [PubMed] [Google Scholar]
- Pizzo D.P., Thal L.J., Winkler J. Mnemonic deficits in animals depend upon the degree of cholinergic deficit and task complexicity. Exp. Neurol. 2002;177:292–305. doi: 10.1006/exnr.2002.7993. [DOI] [PubMed] [Google Scholar]
- Prasanthi J., Schommer E., Thomasson S., Thompson A., Feist G., Ghribi O. Regulation of β-amyloid levels in the brain of cholesterol-fed rabbit, a model system for sporadic Alzheimer's disease. Mech. Ageing Dev. 2008;129:649–655. doi: 10.1016/j.mad.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanthi J., Huls A., Thomasson S., Thomspon A., Schommer E., Ghribi O. Differential effects of 24-hydroxycholesterol and 27-hydroxycholesterol on β-amyloid precursor protein levels and processing in human neuroblastoma SH-SY5Y cells. Mol. Neurodegener. 2009;4:1. doi: 10.1186/1750-1326-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunet C., Montange T., Véjux A., Laubriet A., Rohmer J.-F., Riedinger J.-M., Athias A., Lemaire-Ewing S., Néel D., Petit J.-M., Steinmetz E., Brenot R., Gambert P., Lizard G. Multiplexed flow cytometric analysis of pro- and anti-inflammatory cytokines in the culture media of oxysterol-treated human monocytic cells and in the sera of Atherosclerotic patients. Cytom. A. 2006;69:359–373. doi: 10.1002/cyto.a.20272. [DOI] [PubMed] [Google Scholar]
- Puglielli L., Tanzi R.E., Kovacs D.M. Alzheimer's disease: the cholesterol connection. Nat. Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- Raffai R.L., Weisgraber K.H. Cholesterol: from heart attack to Alzheimer's disease. J. Lipid Res. 2003;44:1423–1430. doi: 10.1194/jlr.R300007-JLR200. [DOI] [PubMed] [Google Scholar]
- Rahman S.M.A., Van Dam A.-M., Schultzberg M., Crisby M. High cholesterol diet results in increased expression of interleukin-6 and caspase-1 in the brain of apolipoprotein E knockout and wild type mice. J. Neuroimmunol. 2005;169:59–67. doi: 10.1016/j.jneuroim.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Refolo L.M., Pappolla M.A., Malester B., LaFrancois J., Bryant-Thomas T., Wang R., Tint G.S., Sambamurti K., Duff K. Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol. Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- Rogers J. The inflammatory response in Alzheimer's disease. J. Periodontol. 2008;79:1535–1543. doi: 10.1902/jop.2008.080171. [DOI] [PubMed] [Google Scholar]
- Rosklint T., Ohlsson B.G., Wiklund O., Norén K., Hultén L.M. Oxysterols induce interleukin-1β production in human macrophages. Eur. J. Clin. Invest. 2002;32:35–42. doi: 10.1046/j.1365-2362.2002.00931.x. [DOI] [PubMed] [Google Scholar]
- Sassin I., Schultz C., Thal D.R., Rüb U., Arai K., Braak E., Braak H. Evolution of Alzheimer's disease-related cytoskeletal changes in the basal nucleus of Meynert. Acta Neuropathol. 2000;100:259–269. doi: 10.1007/s004019900178. [DOI] [PubMed] [Google Scholar]
- Schindowski K., Belarbi K., Buée L. Neurotrophic factors in Alzheimer's disease: role of axonal transport. Genes Brain Behav. 2008;7:43–56. doi: 10.1111/j.1601-183X.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliebs R., Arendt T. The significance of the cholinergic system in the brain during aging and in Alzheimer´s disease. J. Neural Transm. 2006;113:1625–1644. doi: 10.1007/s00702-006-0579-2. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R., Meller D., Bellander B.M., Strömberg I., Olson L., Ingvar M. A one-step immunohistochemical method for detection of blood-brain barrier disturbances for immunoglobulins in lesioned rat brain with special reference to false-positive labelling in immunohistochemistry. J. Neurosci. Methods. 1993;46:121–132. doi: 10.1016/0165-0270(93)90147-j. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A., Vega G.L., Lütjohann D., Lacascio J.J., Tennis M.K., Deng A., Atri A., Hyman B.T., Irizarry M.C., Growdon J.H. Effects of simvastatin on cholesterol metabolism and Alzheimer disease biomarkers. Alzheimer Dis. Assoc. Disord. 2010 doi: 10.1097/WAD.0b013e3181d61fea. PMID:20473136 [Epub ehead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Prasanthi J., Schommer E., Feist G., Ghribi O. Hypercholesterolemia-induced Aβ accumulation in rabbit brain is associated with alteration in IGF-1 signaling. Neurobiol. Dis. 2008;32:426–432. doi: 10.1016/j.nbd.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Shobab L.A., Hsiung G.-Y.R., Feldman H.H. Cholesterol in Alzheimer's disease. Lancet Neurol. 2005;4:841–852. doi: 10.1016/S1474-4422(05)70248-9. [DOI] [PubMed] [Google Scholar]
- Simons M., Keller P., Dichgans J., Schulz J.B. Cholesterol and Alzheimer's disease. Is there a link. Neurology. 2001;57:1089–1093. doi: 10.1212/wnl.57.6.1089. [DOI] [PubMed] [Google Scholar]
- Sottero B., Gamba P., Garguilo S., Leonarduzzi G., Poli G. Cholesterol oxidation products and disease: an emerging topic of interest in medical chemistry. Curr. Med. Chem. 2009;16:685–705. doi: 10.2174/092986709787458353. [DOI] [PubMed] [Google Scholar]
- Sparks D.L., Scheff S.W., Hunsaker J.C., Liu H., Landers T., Gross D.R. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp. Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- Sparks D.L., Kuo Y.-M., Roher A., Martin T., Lukas R. Alterations of Alzheimer's disease in the cholesterol-fed rabbit, including vascular inflammation. Ann. NY Acad. Sci. 2000;903:335–344. doi: 10.1111/j.1749-6632.2000.tb06384.x. [DOI] [PubMed] [Google Scholar]
- Srinivasan K., Patole P.S., Kaul C.L., Ramarao P. Reversal of glucose intolerance by pioglitazone in high fat diet-fed rats. Methods Find. Exp. Clin. Pharmacol. 2004;26:327–333. doi: 10.1358/mf.2004.26.5.831322. [DOI] [PubMed] [Google Scholar]
- Stolp H.B., Dziegielewska K.M. Review: role of developmental inflammation and blood-brain barrier dysfunction in neurodevelopmental and neurodegenerative disease. Neuropathol. Appl. Neurobiol. 2009;35:132–146. doi: 10.1111/j.1365-2990.2008.01005.x. [DOI] [PubMed] [Google Scholar]
- Stuchbury G., Münch G. Alzheimer's associated inflammation, potential drug targets and future therapies. J. Neural Transm. 2005;112:429–453. doi: 10.1007/s00702-004-0188-x. [DOI] [PubMed] [Google Scholar]
- Suter O.C., Sunthorn T., Kraftsik R., Straubel J., Darekar P., Khalili K., Miklossy J. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke. 2002;33:1986–1992. doi: 10.1161/01.str.0000024523.82311.77. [DOI] [PubMed] [Google Scholar]
- Thirumangalakudi L., Prakasam A., Zhang R., Bimonte-Nelson H., Sambamurti K., Kindy M.S., Bhar N.R. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J. Neurochem. 2008;106:475–485. doi: 10.1111/j.1471-4159.2008.05415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X.K., Nicolakakis N., Fernandes P., Ongali B., Brouilette J., Quirion R., Hamel E. Simvastatin improves cerebrovascular function and counters soluble amyloid-beta, inflammation and oxidative stress in aged APP mice. Neurobiol. Dis. 2009;35:406–414. doi: 10.1016/j.nbd.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Trousson A., Bernard S., Petit P.X., Liere P., Pianos A., El Hadri K., Lobaccaro J.-M.A., Ghandour M.S., Raymondjean M., Schumacher M., Massaad C. 25-hydroxycholesterol provokes oligodendrocyte cell line apoptosis and stimulates the secreted phospholipase A2 type IIA via LXR beta and PXR. J. Neurochem. 2009;109:945–958. doi: 10.1111/j.1471-4159.2009.06009.x. [DOI] [PubMed] [Google Scholar]
- Ullrich C., Humpel C. The pro-apoptotic substance thapsigargin selectively stimulates re-growth of brain capillaries. Curr. Neurovasc. Res. 2009;6:171–180. doi: 10.2174/156720209788970063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich C., Humpel C. Effects of cholesterol and its 24S-OH and 25-OH oxysterols on choline acetyltransferase-positive neurons in brain slices. Pharmacology. 2010;86:15–21. doi: 10.1159/000314333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vejux A., Malvitte L., Lizard G. Side effects of oxysterols: cytotoxicity, oxidation, inflammation, and phospholipidosis. Braz. J. Med. Biol. Res. 2008;41:545–556. doi: 10.1590/s0100-879x2008000700001. [DOI] [PubMed] [Google Scholar]
- Vogels O.J., Broere C.A., ter Laak H.J., ten Donelaar H.J., Nieuwenhuys R., Schulte B.P. Cell loss and shrinkage in the nucleus basalis Meynert complex in Alzheimer's disease. Neurobiol. Aging. 1990;11:3–13. doi: 10.1016/0197-4580(90)90056-6. [DOI] [PubMed] [Google Scholar]
- Webb L.E., Lengle E., Izzo R., Woods E. Micromethod for liquid-chromatographic determination of cholesterol in lipemic sera. Clin. Chem. 1982;28:1769–1772. [PubMed] [Google Scholar]
- Wellington C.L. Cholesterol at the crossroads: Alzheimer's disease and lipid metabolism. Clin. Genet. 2004;66:1–16. doi: 10.1111/j.0009-9163.2004.00280.x. [DOI] [PubMed] [Google Scholar]
- Wenk G.L. The nucleus basalis magnocellularis cholinergic system: one hundred years of progress. Neurobiol. Learn. Mem. 1997;67:85–95. doi: 10.1006/nlme.1996.3757. [DOI] [PubMed] [Google Scholar]
- Winkler J., Suhr S.T., Gage F.H., Thal L.J., Fisher L.J. Essential role of neocortical acetylcholine in spatial memory. Nature. 1995;375:484–487. doi: 10.1038/375484a0. [DOI] [PubMed] [Google Scholar]
- Winkler J., Thal L.J., Gage F.H., Fisher L.J. Cholinergic strategies for Alzheimer´s disease. J. Mol. Med. 1998;76:555–567. doi: 10.1007/s001090050250. [DOI] [PubMed] [Google Scholar]
- Winocur G., Greenwood C.E. The effects of high fat diets and environmental influences on cognitive performance in rats. Behav. Brain Res. 1999;101:153–161. doi: 10.1016/s0166-4328(98)00147-8. [DOI] [PubMed] [Google Scholar]
- Xie C., Lund E.G., Turley S.D., Russell D.W., Dietschy J.M. Quantitation of two pathways for cholesterol excretion from the brain in normal mice and mice with neurodegeneration. J. Lipid Res. 2003;44:1780–1789. doi: 10.1194/jlr.M300164-JLR200. [DOI] [PubMed] [Google Scholar]
- Xue Q.-S., Sparks D.L., Streit W. Microglial activation in the hippocampus of hypercholesterolemic rabbits occurs independent of increased amyloid production. J. Neuroinflammation. 2007;4:20. doi: 10.1186/1742-2094-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zassler B., Humpel C. Transplantation of NGF secreting primary monocytes counteracts NMDA-induced cell death of rat cholinergic neurons in vivo. Exp. Neurol. 2006;198:391–400. doi: 10.1016/j.expneurol.2005.12.009. [DOI] [PubMed] [Google Scholar]