Abstract
Since dendritic cells may play a key role in defense against influenza virus infection, we examined the effects of recombinant hemagglutinin (HA) proteins derived from mouse-adapted H1N1 (A/WSN/1933), swine-origin 2009 pandemic H1N1 (A/Texas/05/2009), and highly pathogenic avian influenza H5N1 (A/Thailand/KAN-1/2004) viruses on mouse myeloid dendritic cells (mDCs). The results reveal that tumor necrosis factor alpha (TNF-α), interleukin-12 (IL-12) p70, and major histocompatibility complex class II (MHC-II) expression was increased in mDCs after treatment with recombinant HA proteins of H1N1 and H5N1. The specificity of recombinant HA treatments for mDC activation was diminished after proteinase K digestion. HA apparently promotes mDC maturation by enhancing CD40 and CD86 expression and suppressing endocytosis. No significant differences in mDC activation were observed among recombinant proteins of H1N1 and H5N1. The stimulation of mDCs by HA proteins of H1N1 and H5N1 was completely MyD88 dependent. These findings may provide useful information for the development of more-effective influenza vaccines.
Influenza viruses trigger seasonal epidemics or pandemics of contagious diseases with mild to severe consequences in human and poultry populations worldwide (28). Members of the Orthomyxoviridae family, influenza viruses consist of single-stranded, eight-segment, negative-sense genomic RNAs, helical viral ribonucleoprotein (RNP) complexes (RNA segments, NP, PB2, PB1, and PA) and four viral envelope proteins (hemagglutinin [HA], neuraminidase [NA], and M1 and M2 matrix proteins). Type A influenza viruses are further classified into various serotypes based on the antigenic characteristics of HA and NA glycoproteins (14).
In 2009, a swine-origin H1N1 strain emerged from the genetic reassortants of existing human, avian, and swine influenza viruses, resulting in a global pandemic marked by symptoms more severe than those associated with seasonal influenza virus (3, 24). According to comparative pathology in macaque monkeys, H5N1 induces greater cytokinemia, tissue damage, and interference with immune regulatory mechanisms than H1N1 infection (2). The HA spike protein of influenza virus is believed to play important roles in viral receptor binding, fusion, transmission, host range restriction, virulence, and pathogenesis (13, 27-30).
Dendritic cells (DCs), considered the most potent professional antigen-presenting cells, serve as links between innate and adaptive immunity (31). Upon encountering microbial pathogens, endogenous danger signals, or inflammatory mediators, DCs mature and elicit rapid and short-lived innate immune responses before migrating to secondary lymphoid organs and enhancing adaptive immunity (17). Two major subsets of DCs are recognized in mice and humans: (i) myeloid DCs (mDCs, also called conventional DCs), which participate most directly in antigen presentation and activation of naïve T cells, and (ii) plasmacytoid DCs (pDCs), which produce type I interferons in response to viral infection (16, 42) and are also capable of inducing immunotolerance under some conditions (9). mDCs and pDCs also comprise different heterologous subsets, with unique localizations, phenotypes, and functions (36). Due to their key role in immune regulation, DCs have been developed for immunotherapeutic agents or prophylactic or therapeutic vaccines for cancer, infectious diseases, and immune system-related diseases (32, 34).
DCs are essential in controlling the innate and adaptive immune responses against influenza virus infection (21). Viral RNA is recognized by various pattern recognition receptors (PRRs), including RIG-I-like receptors (RLRs), Toll-like receptors (TLRs), and nucleotide oligomerization domain (NOD)-like receptors (NLRs). TLRs play an especially important role in detecting virus invasion and activating DCs (18, 35). However, the mechanisms causing DC activation and maturation in response to influenza viruses are not clear. HA has been described as playing an important role in modulating influenza virus virulence and host immune responses (29). In this study, we examined the effects of several recombinant HA proteins (rHAs) derived from rHA of H1N1 (rH1HA) (A/WSN/1933) and (A/Texas/05/2009) and rHA of H5N1 (rH5HA) (A/Thailand/KAN-1/2004) viruses on the activation and maturation of the mDC subset derived from mouse bone marrow.
MATERIALS AND METHODS
HA plasmid construction.
The cDNA of the HA gene of influenza virus A/WSN/33 (H1N1) was obtained from virus stocks by using reverse transcription-PCR. The cDNA of the HA gene of A/Thailand/KAN-1/2004 (H5N1) was provided by Prasert Auewarakul, Siriraj Hospital, Mahidol University, Thailand. The cDNA of the HA gene of A/Texas/05/2009 (H1N1) (gi 255602259) was synthesized with human-optimized codon sequences using Mr. Gene (GeneArt, Regensburg, Germany). GCN4 (SGRLVPRGSPMKQIEDKIEEILSKIYHIENEIARIKKLIGEVG) residues and 6His (HHHHHH) tags were added to the C termini of the cDNA sequences of the three HA ectodomain strains for expression of soluble trimeric HA proteins, as previously reported (39). Next, the sequences were cloned into a pFastBac Dual baculovirus transfer vector (Invitrogen) and selected with ampicillin and a BamHI/XhoI restriction enzyme. All inserted sequences were confirmed by DNA sequence analyses performed by Mission Biotech Inc., Taipei, Taiwan.
Recombinant HA protein generation and purification.
The inserted vectors were transformed into Escherichia coli strain DH10 Bac and selected with a combination of kanamycin, gentamicin, tetracycline, X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (Invitrogen), and IPTG (isopropyl-β-d-thiogalactopyranoside) (Bio-Rad). Recombinant bacmids were confirmed by PCR using M13 primers. Recombinant-bacmid-expressing secreted HA was transfected into Spodoptera frugiperda 9 (Sf9) cells by using Cellfectin reagent (Invitrogen) and amplified in the same cells according to the manufacturer's instructions. At 48 h postinfection (hpi), cells and supernatant were harvested, centrifuged, and filtered. HA proteins were purified by metal affinity chromatography using Ni-nitrilotriacetic acid (NTA) agarose (Invitrogen), as previously described (33).
Western blotting.
Purified rH1HA and rH5HA proteins were harvested, mixed with loading dye, heated at 100°C for 5 min, and separated onto 12% SDS-polyacrylamide gels. Gels were transferred to nitrocellulose (NC) membranes, blocked with 5% nonfat milk in 50 mM Tris, 0.14 M NaCl, and 0.05% Tween 20, pH 8.0 (TBST), and incubated with horseradish peroxidase (HRP)-conjugated rabbit anti-6His antibody (Ab) or anti-H1HA and anti-H5HA (Abcam) followed by HRP-conjugated goat anti-mouse or -rabbit Ab (Jackson ImmunoResearch). Immunoreactivity was measured using Plus-ECL chemiluminescence reagent (PerkinElmer, Inc.).
mDC generation.
C57BL/6 and C3H/HeN mice were purchased from the National Laboratory Animal Center, Taipei. MyD88−/− mice were gifts from Anthony DeFranco of the University of California at San Francisco. C3H/HeJ (TLR4 mutant) mice were kindly provided by Zaodung Ling of the Taiwan NHRI. All mice were housed at the NHRI barrier facility and cared for according to Institutional Animal Care and Use Committee-approved protocols. mDCs were generated from mouse bone marrow as previously described (8).
Cytokine production analysis.
Tumor necrosis factor alpha (TNF-α) production by DCs was detected by intracellular staining as previously described (44). Briefly, DCs were treated with phosphate-buffered saline (PBS), 20 ng/ml lipopolysaccharides (LPS) (from E. coli 0111:B4; Sigma), 200 nM CpG (InvivoGen), and 10 μg/ml of rH1HA (A/WSN/33), rH1HA (A/Texas/05/2009), or rH5HA (A/Thailand/KAN-1/2004) for 6 h. Brefeldin A (10 μg/ml; Biolegend) was added for the last 4 h. Cells were fixed, permeabilized, stained with anti-TNF-α monoclonal Ab (MAb) (Biolegend), and analyzed by flow cytometry (FACS Calibur; BD) with CellQuest software (BD Biosciences). To quantify cytokines, DC culture supernatants were collected following LPS or rHA treatment for either 6 h (TNF-α) or 24 h (interleukin-12 [IL-12]). Detection was performed with enzyme-linked immunosorbent assay (ELISA) kits (eBiosciences).
DC maturation assays.
As previously described (15), DC maturation was determined by major histocompatibility complex class II (MHC-II), CD40, and CD86 expression. The mDCs derived from wild-type (WT), MyD88−/−, C3H, and TLR4 mutant mice were treated with PBS, 50 ng/ml LPS, 50 μg/ml rH1HA (A/WSN/33), and either rH1HA (A/Texas/05/2009) or rH5HA (A/Thailand/KAN-1/2004) for 16 h, stained with anti-CD11c, -MHC-II, -CD40, or -CD86 antibodies (Biolegend), and analyzed by flow cytometry. Maturation was also detected at the endocytosis level. Stimulated mDCs were suspended in staining buffer (1% fetal bovine serum [FBS], 0.01% NaN3 dissolved in PBS) with 200 μg/ml dextran-fluorescein isothiocyanate (FITC) (Sigma) and incubated in darkness at 4°C or 37°C for 1.5 h, after which cells were washed with cold PBS and analyzed by flow cytometry.
Statistical analysis.
All results were analyzed using Student's t tests, with a P value of <0.05 indicating statistical significance.
RESULTS
Preparation and purification of rH1HA and rH5HA.
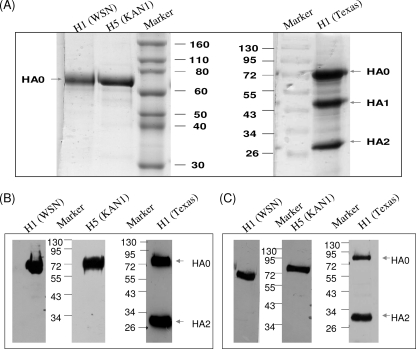
The cDNAs of the HA ectodomain genes of A/WSN/1933 (H1N1), A/Texas/05/2009 (H1N1), and A/Thailand/KAN-1/2004 (H5N1) influenza viruses were linked with GCN4 trimeric sequences and 6His residues to facilitate purification and stable expression, as previously reported (10, 39, 40). After recombinant baculoviruses were obtained to infect Sf9 insect cells, culture supernatants were harvested at 48 hpi and purified using Ni-NTA column chromatography. Purified rH1HA (A/WSN/1933) and rH5HA (A/Thailand/KAN-1/2004) displayed a unique protein band, as revealed by Coomassie blue staining. In contrast, purified rH1HA (A/Texas/05/2009) displayed three protein bands, indicating a cleavage of HA0 into HA1 and HA2 (Fig. 1 A). The molecular masses of these purified rHAs were approximately 72 kDa, as demonstrated by Western blotting with anti-HA antibody (Fig. 1B) and anti-His antibody (Fig. 1C). We also detected 2009 pandemic rH1HA [A/Texas/05/2009 (H1N1)] HA0 and HA2 expression (Fig. 1B and C).
FIG. 1.
Recombinant influenza virus HA proteins were expressed from baculovirus-infected Sf9 insect cells and purified by Ni-NTA resin. (A) Purified rH1HA (A/WSN/1933) [H1 (WSN)], rH5HA (A/Thailand/KAN-1/2004) [H5 (KAN1)], and rH1HA (A/Texas/05/2009) [H1 (Texas)] were subjected to SDS-PAGE and stained with Coomassie blue. (B) Purified rHAs were subjected to SDS-PAGE and stained with anti-H1HA or anti-H5HA antibody. (C) Purified rHAs were subjected to SDS-PAGE and stained using anti-His antibody. All of the data are representative of triplicate experiments. Molecular mass markers (in kilodaltons) are shown.
Stimulation of mDCs for cytokine production.
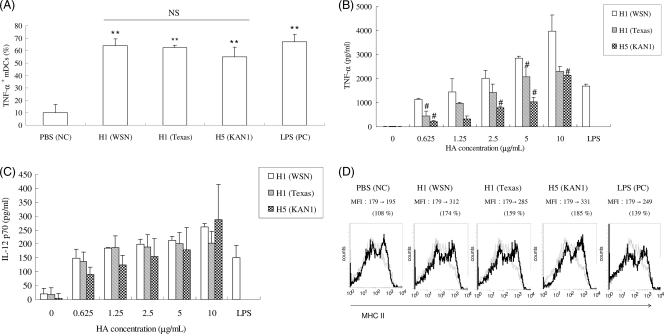
To understand the effects of rH1HA and rH5HA on mDC activation, we measured the levels of intracellular TNF-α production, a distinct mark of DC activation (19). We used the mDC isolation method described by Chu and Lowell (8), which selects exclusively CD11c+ DCs, i.e., the mDC subset. Our results indicate that rH1HA (A/WSN/1933) and rH1HA (A/Texas/05/2009) induced intracellular TNF-α production by mouse mDCs at higher levels than rH5HA (A/Thailand/KAN-1/2004) (Fig. 2 A). We also used an ELISA to detect TNF-α and IL-12 secretion in an attempt to quantify the levels of mDC-produced cytokines. The results confirmed that all rHAs promoted TNF-α and IL-12 p70 production by mDCs in a dose-dependent manner (Fig. 2B and C). Again, treatment with rH1HA (A/WSN/1933) induced higher levels of TNF-α production via mDCs than treatment with rH1HA (A/Texas/05/2009) or rH5HA (A/Thailand/KAN-1/2004) (Fig. 2B). No significant differences in IL-12 p70 production induced by any of these rHAs were observed (Fig. 2C). We measured the MHC-II levels in addition to intracellular TNF-α production to evaluate mDC activation. As the early marker for mDC activation, MHC-II is more functionally relevant to antigen presentation of mDCs. The results in Fig. 2D show that the changes in MHC-II level increased from 108% (PBS) to 174% [rH1HA (A/WSN/1933)], 159% [rH1HA (A/Texas/05/2009)], and 185% [rH5HA (A/Thailand/KAN-1/2004)]. According to these results, influenza virus rHA is capable of inducing mouse mDCs to generate proinflammatory cytokines.
FIG. 2.
Stimulation of mDCs for cytokine production. (A) Percentages of TNF-α+ mDCs treated with PBS, rHAs, or LPS and stained with anti-TNF-α antibody, determined using flow cytometry. Results are expressed as means ± standard deviations (SD) from three independent experiments. **, P < 0.01 for rHA treatment compared to PBS treatment; NS, P > 0.05 between HA treatment groups (by Student's t test). NC, negative control; PC, positive control. (B) TNF-α levels measured from mDC culture supernatants and determined using an ELISA kit. Results are expressed as means ± SD from three independent experiments. #, P < 0.05 for rH5HA (A/Thailand/KAN-1/2004) and rH1HA (A/Texas/05/2009) compared to rH1HA (A/WSN/1933) (by Student's t test). (C) IL-12 p70 concentrations measured from mDC culture supernatants and determined using an ELISA kit. Results are expressed as means ± SD from three independent experiments. (D) Percentages of mDCs treated with PBS, HAs, or LPS and stained with anti-CD11c and anti-MHC-II antibodies, determined using flow cytometry. Changes in the mean fluorescence intensity (MFI) are indicated. All of the data are representative of at least three separate experiments.
Specificity of rH1HA and rH5HA for mDC activation.
Since DCs are sensitive to microbial components (especially endotoxin) that may be introduced during purification, the specificity of rHAs for mouse mDC activation was investigated using proteinase K treatment. As shown in Fig. 3, proteinase K-treated rH1HA (A/WSN/1933), rH1HA (A/Texas/05/2009), and rH5HA (A/Thailand/KAN-1/2004) reduced the levels of TNF-α production by mDCs from 64% to 0.9%, 55% to 6%, and 55% to 2.1%, respectively. Similar results were also found when the rHAs were heat inactivated (data not shown). These results reveal the specificity of rHAs for mDC activation.
FIG. 3.
Specificity of rH1HA and rH5HA for mDC activation. rHAs were treated with proteinase K (PK) (2 mg/ml) at 37°C for 1 h prior to being added to mDC cultures. Intracellular TNF-α production was measured after holding for an additional 6 h. Values shown on the graphs indicate percentages of fluorescent TNF-α+ DCs cultured with the indicated stimuli (black line) compared to percentages for the control (gray line). Data represent average values from three independent experiments. PE, phycoerythrin.
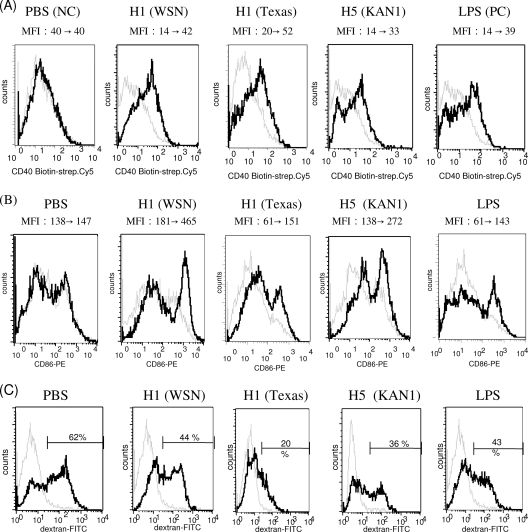
Enhancement of mDC maturation by rH1HA and rH5HA.
We evaluated the effects of the rHAs on mDC maturation, which is considered key to mDC function. Flow cytometry results indicate that the levels of expression of costimulatory molecules CD40 and CD86 in mouse mDCs were enhanced by rH1HA (A/WSN/1933), rH1HA (A/Texas/05/2009), and rH5HA (A/Thailand/KAN-1/2004), with no statistically significant difference observed among the various rHA treatments (Fig. 4 A and B). We also determined the induction of mDC endocytosis (7) and found that dextran-FITC uptake by mDCs decreased following treatment with any of the tested rHAs, indicating advanced maturity among the rHA-stimulated mDCs (Fig. 4C). According to these results, rHA promotes mDC maturation and likely enhances adaptive immunity.
FIG. 4.
rHA-enhanced mDC maturation. (A) mDCs were treated with rHAs, PBS, or LPS, stained with anti-CD11c and anti-CD40 antibodies, and analyzed by flow cytometry. Changes in MFI between PBS, LPS, or rHA treatment (black line) and the control (gray line) are indicated. strep., streptavidin. (B) mDCs were stained with anti-CD11c and anti-CD86 antibodies and analyzed by flow cytometry. (C) mDCs were incubated with dextran-FITC at 4°C (gray line) or 37°C (black line) and analyzed by flow cytometry. Percentages of dextran-FITC+ DCs are indicated. Data are representative of three separate experiments.
MyD88 and TLR4 involvement in rHA-induced mDC activation.
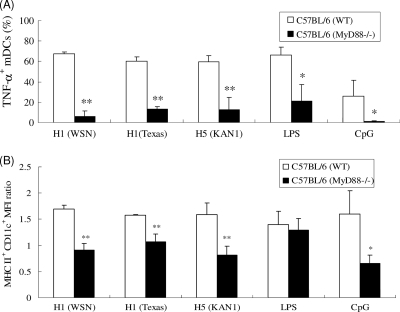
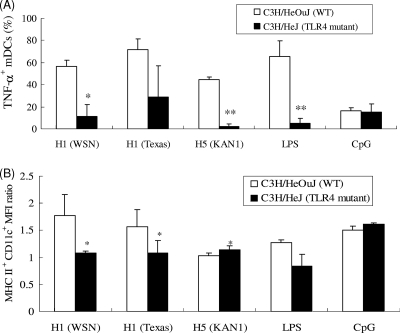
Since TLRs are essential for viral recognition and the induction of innate and acquired immune responses (35), we examined whether TLRs are involved in mDC activation via rHAs. Since MyD88 is necessary for TLR function (with the exception of TLR3), we obtained mDCs from WT (C57BL/6) and MyD88−/− mice for rHA stimulation and found that MyD88 was required for activating the effects of rHAs on mDCs, as indicated by poor TNF-α production by MyD88−/− mDCs following rHA treatment (Fig. 5 A). Similarly, the levels of MHC-II expression in MyD88−/− mDCs following rHA treatments were all reduced compared to those in WT (C57BL/6) mDCs (Fig. 5B). We also tested for TLR4 involvement by using mDCs derived from TLR4 mutant (C3H/HeJ) mice and found that rHA-induced TNF-α production was diminished in TLR4 mutant mDCs compared to that in WT cells (Fig. 6 A). The levels of MHC-II expression in TLR4 mutant mDCs decreased following treatments with rH1HA (A/WSN/1933) and rH1HA (A/Texas/05/2009) but slightly increased following treatment with rH5HA (A/Thailand/KAN-1/2004) (Fig. 6B). These results suggest that, following recognition by TLR4, rH1HA may differ from rH5HA in activating mDCs but that the stimulation by these rHAs is completely MyD88 dependent.
FIG. 5.
MyD88 involvement in rHA-induced mDC activation. mDCs were obtained from C57BL/6 and MyD88−/− mice and treated with rHAs, LPS, or CpG, and intracellular TNF-α production and MHC-II expression were determined. (A) Percentages of TNF-α+ mDCs. (B) MFI ratios of MHC-II+ CD11c+ mDCs. Results are expressed as means ± SD from three independent experiments. *, P < 0.05, and **, P < 0.01, comparing MyD88−/− mice to control (by Student's t test).
FIG. 6.
TLR4 involvement in rHA-induced mDC activation. mDCs were obtained from C3H/HeOuJ and TLR4 mutant mice and treated with rHAs, LPS, or CpG, and intracellular TNF-α production and MHC-II expression were determined. (A) Percentages of TNF-α+ mDCs. (B) MFI ratios of MHC-II+ CD11c+ mDCs. Results are expressed as means ± SD from three independent experiments. *, P < 0.05, and **, P < 0.01, comparing MyD88−/− mice to control (by Student's t test).
DISCUSSION
Our main findings are that (i) rHAs generated by the baculovirus-insect cell expression system are capable of inducing mDC activation and maturation and (ii) TLR4 and MyD88 are required for the stimulatory effect of HA on mDCs. We hypothesize that specific strains of H1N1 and H5N1 may alter the stimulatory effects of HA on mDCs, because HA is considered a key determinant of influenza virus pathogenesis. It was reported that a minor change in the HA receptor domain is capable of modifying H5N1 virus virulence and systemic spread in mice (43). The 2009 pandemic H1N1 virus was recently found to trigger a form of pneumonia in ferrets more severe than that triggered by seasonal H1N1 virus but less severe than that triggered by the highly pathogenic avian influenza (HPAI) H5N1 virus (37), probably due to different HAs. However, our present study demonstrates no significant differences in mDC activation by rH5HA (A/Thailand/KAN-1/2004), rH1HA (A/Texas/05/2009), and rH1HA (A/WSN/1933). To our knowledge, this is the first description of rHA and mDC self-adjuvanticity.
Specific CD8+ T cell priming against influenza virus infection requires mDC-activated antigen presentation to promote T cell proliferation and differentiation (1). Here we demonstrate that rH1HA and rH5HA are capable of inducing IL-12 p70 secretion by mDCs, suggesting that TH1 or cytotoxic T lymphocyte (CTL) adaptive immune responses can be elicited by activated mDCs, as reported previously (19). This result was contradictory to another report using HA extracted from purified H3N2 influenza virus to selectively inhibit the release of LPS-induced IL-12 p35 at the transcription level (23). The HA obtained from the purified H3N2 influenza virus is monomeric, due to the bromelain treatment used in the preparation (23); however, the rHAs used in this study are trimeric (data not shown). The suppression of IL-12 production in mDCs may be due to a lack of the HA conformational structure. An experimental animal model previously revealed an increase in IL-12 p70 in bronchoalveolar lavage fluids from influenza virus-infected BALB/c mice (22). Additionally, distinct DC subsets are known to release differential cytokines and to regulate adaptive immunity following influenza virus infection (1, 4). Different DC subsets can induce specific TLR pathways following influenza virus infection. For instance, mDCs are activated via TLR3 and TLR9 (41), whereas pDCs are activated via TLR7 (11). The CD8α+ conventional DC subset specifically initiates CTL immunity in response to influenza virus infection (4). The pDC subsets produce large amounts of type I interferon (IFN-α/β) and trigger specific antiviral immunity (16). Although the functions of individual DC subsets remain unclear, we found that mDCs can still play an important role in modulating immune responses initiated by influenza virus rHAs.
TLRs are required for sensing influenza virus RNA and activating innate and adaptive immune responses (18). Note that influenza virus H2 HA also induces innate immune responses in murine B lymphocytes via MyD88-dependent pathways (20), meaning that TLR and MyD88 are both required for activating innate immune responses against influenza virus infection. Further, the inflammatory signaling pathways of DCs are regulated by NF-κB, mitogen-activated protein kinase (MAPK), and IFN-α/β activation via the TRIF and TLR-MyD88 pathways (5). Here, we have demonstrated that TLR4/MyD88 are involved in regulating initial interactions between mDCs and both rH1HA and rH5HA. The TLR signaling pathway was also reported to regulate the activation of NS5A of hepatitis C virus and A52R of vaccinia virus (6). The NS1 protein of the A/PR8/8/34 (H1N1) influenza virus strain binds with RIG-1 and suppresses the RIG-1 pathway (25). However, the TLR signaling mechanism between DCs and influenza virus rHAs requires further clarification.
The N-linked glycans of influenza virus HA have been shown to regulate receptor binding and neutralization activity against influenza virus infection (38) and also to be important in influenza virus recognition by the innate immune system (26). We speculate that N-linked glycans may affect DC modulation by rH1HA and rH5HA. Removal of N-linked glycans by endo-β-N-acetylglucosaminidase H (endo H) glycosidase of rH5HA (A/Thailand/KAN-1/2004) but not rH1HA (A/Texas/05/2009) or rH1HA (A/WSN/1933) resulted in a significant decrease in TNF-α production by mDCs (data not shown). Further studies that use rH5HAs from different clades of H5N1 influenza viruses and from mammalian glycosyltransferase-engineered insect cells (12) or mammalian cells that contain complex-type N-linked glycans, rather than the rH5HA with high-mannose-type glycans used in this study, are required.
In summary, we found that rHAs derived from human H1N1 (A/WSN/1933), novel swine-origin 2009 pandemic H1N1 (A/Texas/05/2009), and avian H5N1 (A/Thailand/KAN-1/2004) influenza viruses are capable of activating mouse mDCs via the MyD88 signaling pathway. However, no significant differences in mDC activation among rH1HA (A/WSN/1933), rH1HA (A/Texas/05/2009), and rH5HA (A/Thailand/KAN-1/2004) were observed. These findings may provide useful information for the development of more-effective influenza vaccines.
Acknowledgments
We thank Prasert Auewarakul, Siriraj Hospital, Mahidol University, for providing the HA gene of A/Thailand/KAN-1/2004 (H5N1).
This work was supported by the National Science Council (NSC98-2321-B-007-003), the Research Booster Program of National Tsing Hua University (99N2550E1), and Yeastern Biotech Co. (for C.-L.C.), Taiwan.
Footnotes
Published ahead of print on 15 September 2010.
REFERENCES
- 1.Ballesteros-Tato, A., B. Leon, F. E. Lund, and T. D. Randall. 2010. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza. Nat. Immunol. 11:216-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baskin, C. R., H. Bielefeldt-Ohmann, T. M. Tumpey, P. J. Sabourin, J. P. Long, A. Garcia-Sastre, A. E. Tolnay, R. Albrecht, J. A. Pyles, P. H. Olson, L. D. Aicher, E. R. Rosenzweig, K. Murali-Krishna, E. A. Clark, M. S. Kotur, J. L. Fornek, S. Proll, R. E. Palermo, C. L. Sabourin, and M. G. Katze. 2009. Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus. Proc. Natl. Acad. Sci. U. S. A. 106:3455-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastien, N., N. A. Antonishyn, K. Brandt, C. E. Wong, K. Chokani, N. Vegh, G. B. Horsman, S. Tyler, M. R. Graham, F. A. Plummer, P. N. Levett, and Y. Li. 2010. Human infection with a triple-reassortant swine influenza A(H1N1) virus containing the hemagglutinin and neuraminidase genes of seasonal influenza virus. J. Infect. Dis. 201:1178-1182. [DOI] [PubMed] [Google Scholar]
- 4.Belz, G. T., C. M. Smith, D. Eichner, K. Shortman, G. Karupiah, F. R. Carbone, and W. R. Heath. 2004. Cutting edge: conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J. Immunol. 172:1996-2000. [DOI] [PubMed] [Google Scholar]
- 5.Bohnenkamp, H. R., K. T. Papazisis, J. M. Burchell, and J. Taylor-Papadimitriou. 2007. Synergism of Toll-like receptor-induced interleukin-12p70 secretion by monocyte-derived dendritic cells is mediated through p38 MAPK and lowers the threshold of T-helper cell type 1 responses. Cell. Immunol. 247:72-84. [DOI] [PubMed] [Google Scholar]
- 6.Bowie, A. G., and L. Unterholzner. 2008. Viral evasion and subversion of pattern-recognition receptor signalling. Nat. Rev. Immunol. 8:911-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow, A., D. Toomre, W. Garrett, and I. Mellman. 2002. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature 418:988-994. [DOI] [PubMed] [Google Scholar]
- 8.Chu, C. L., and C. A. Lowell. 2005. The Lyn tyrosine kinase differentially regulates dendritic cell generation and maturation. J. Immunol. 175:2880-2889. [DOI] [PubMed] [Google Scholar]
- 9.Coquerelle, C., and M. Moser. 2010. DC subsets in positive and negative regulation of immunity. Immunol. Rev. 234:317-334. [DOI] [PubMed] [Google Scholar]
- 10.Cornelissen, L. A. H. M., R. P. de Vries, E. A. de Boer-Luijtze, A. Rigter, P. J. M. Rottier, and C. A. M. de Haan. 2010. A single immunization with soluble recombinant trimeric hemagglutinin protects chickens against highly pathogenic avian influenza virus H5N1. PloS One 5:e10645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 12.Harrison, R. L., and D. L. Jarvis. 2006. Protein N-glycosylation in the baculovirus-insect cell expression system and engineering of insect cells to produce “mammalianized” recombinant glycoproteins. Adv. Virus Res. 68:159-191. [DOI] [PubMed] [Google Scholar]
- 13.Harrison, S. C. 2008. Viral membrane fusion. Nat. Struct. Mol. Biol. 15:690-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horimoto, T., and Y. Kawaoka. 2005. Influenza: lessons from past pandemics, warnings from current incidents. Nat. Rev. Microbiol. 3:591-600. [DOI] [PubMed] [Google Scholar]
- 15.Huang, R. Y., Y. L. Yu, W. C. Cheng, C. N. Ouyang, E. Fu, and C. L. Chu. 2010. Immunosuppressive effect of quercetin on dendritic cell activation and function. J. Immunol. 184:6815-6821. [DOI] [PubMed] [Google Scholar]
- 16.Jewell, N. A., N. Vaghefi, S. E. Mertz, P. Akter, R. S. Peebles, Jr., L. O. Bakaletz, R. K. Durbin, E. Flano, and J. E. Durbin. 2007. Differential type I interferon induction by respiratory syncytial virus and influenza A virus in vivo. J. Virol. 81:9790-9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joffre, O., M. A. Nolte, R. Sporri, and C. Reis e Sousa. 2009. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 227:234-247. [DOI] [PubMed] [Google Scholar]
- 18.Kumar, H., T. Kawai, and S. Akira. 2009. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 388:621-625. [DOI] [PubMed] [Google Scholar]
- 19.Liu, Y. J. 2001. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell 106:259-262. [DOI] [PubMed] [Google Scholar]
- 20.Marshall-Clarke, S., L. Tasker, O. Buchatska, J. Downes, J. Pennock, S. Wharton, P. Borrow, and D. Z. Wiseman. 2006. Influenza H2 haemagglutinin activates B cells via a MyD88-dependent pathway. Eur. J. Immunol. 36:95-106. [DOI] [PubMed] [Google Scholar]
- 21.McGill, J., J. W. Heusel, and K. L. Legge. 2009. Innate immune control and regulation of influenza virus infections. J. Leukoc. Biol. 86:803-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monteiro, J. M., C. Harvey, and G. Trinchieri. 1998. Role of interleukin-12 in primary influenza virus infection. J. Virol. 72:4825-4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noone, C. M., E. A. Lewis, A. B. Frawely, R. W. Newman, B. P. Mahon, K. H. Mills, and P. A. Johnson. 2005. Novel mechanism of immunosuppression by influenza virus haemagglutinin: selective suppression of interleukin 12 p35 transcription in murine bone marrow-derived dendritic cells. J. Gen. Virol. 86:1885-1890. [DOI] [PubMed] [Google Scholar]
- 24.Peiris, J. S., W. W. Tu, and H. L. Yen. 2009. A novel H1N1 virus causes the first pandemic of the 21st century. Eur. J. Immunol. 39:2946-2954. [DOI] [PubMed] [Google Scholar]
- 25.Pichlmair, A., O. Schulz, C. P. Tan, T. I. Naslund, P. Liljestrom, F. Weber, and C. Reis e Sousa. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314:997-1001. [DOI] [PubMed] [Google Scholar]
- 26.Reading, P. C., M. D. Tate, D. L. Pickett, and A. G. Brooks. 2007. Glycosylation as a target for recognition of influenza viruses by the innate immune system. Adv. Exp. Med. Biol. 598:279-292. [DOI] [PubMed] [Google Scholar]
- 27.Rogers, G. N., J. C. Paulson, R. S. Daniels, J. J. Skehel, I. A. Wilson, and D. C. Wiley. 1983. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature 304:76-78. [DOI] [PubMed] [Google Scholar]
- 28.Salomon, R., and R. G. Webster. 2009. The influenza virus enigma. Cell 136:402-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skehel, J. 2009. An overview of influenza haemagglutinin and neuraminidase. Biologicals 37:177-178. [DOI] [PubMed] [Google Scholar]
- 30.Steinhauer, D. A. 1999. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology 258:1-20. [DOI] [PubMed] [Google Scholar]
- 31.Steinman, R. M. 2007. Lasker Basic Medical Research Award. Dendritic cells: versatile controllers of the immune system. Nat. Med. 13:1155-1159. [DOI] [PubMed] [Google Scholar]
- 32.Steinman, R. M., and J. Banchereau. 2007. Taking dendritic cells into medicine. Nature 449:419-426. [DOI] [PubMed] [Google Scholar]
- 33.Stevens, J., O. Blixt, T. M. Tumpey, J. K. Taubenberger, J. C. Paulson, and I. A. Wilson. 2006. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science 312:404-410. [DOI] [PubMed] [Google Scholar]
- 34.Tacken, P. J., I. J. de Vries, R. Torensma, and C. G. Figdor. 2007. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat. Rev. Immunol. 7:790-802. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi, O., and S. Akira. 2009. Innate immunity to virus infection. Immunol. Rev. 227:75-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueno, H., A. K. Palucka, and J. Banchereau. 2010. The expanding family of dendritic cell subsets. Nat. Biotechnol. 28:813-815. [DOI] [PubMed] [Google Scholar]
- 37.van den Brand, J. M., K. J. Stittelaar, G. van Amerongen, G. F. Rimmelzwaan, J. Simon, E. de Wit, V. Munster, T. Bestebroer, R. A. Fouchier, T. Kuiken, and A. D. Osterhaus. 2010. Severity of pneumonia due to new H1N1 influenza virus in ferrets is intermediate between that due to seasonal H1N1 virus and highly pathogenic avian influenza H5N1 virus. J. Infect. Dis. 201:993-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang, C. C., J. R. Chen, Y. C. Tseng, C. H. Hsu, Y. F. Hung, S. W. Chen, C. M. Chen, K. H. Khoo, T. J. Cheng, Y. S. Cheng, J. T. Jan, C. Y. Wu, C. Ma, and C. H. Wong. 2009. Glycans on influenza hemagglutinin affect receptor binding and immune response. Proc. Natl. Acad. Sci. U. S. A. 106:18137-18142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei, C. J., L. Xu, W. P. Kong, W. Shi, K. Canis, J. Stevens, Z. Y. Yang, A. Dell, S. M. Haslam, I. A. Wilson, and G. J. Nabel. 2008. Comparative efficacy of neutralizing antibodies elicited by recombinant hemagglutinin proteins from avian H5N1 influenza virus. J. Virol. 82:6200-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weldon, W. C., B. Z. Wang, M. P. Martin, D. G. Koutsonanos, I. Skountzou, and R. W. Compans. 2010. Enhanced immunogenicity of stabilized trimeric soluble influenza hemagglutinin. PloS One 5:e12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong, J. P., M. E. Christopher, S. Viswanathan, N. Karpoff, X. Dai, D. Das, L. Q. Sun, M. Wang, and A. M. Salazar. 2009. Activation of Toll-like receptor signaling pathway for protection against influenza virus infection. Vaccine 27:3481-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu, L., and Y. J. Liu. 2007. Development of dendritic-cell lineages. Immunity 26:741-750. [DOI] [PubMed] [Google Scholar]
- 43.Yen, H. L., J. R. Aldridge, A. C. Boon, N. A. Ilyushina, R. Salomon, D. J. Hulse-Post, H. Marjuki, J. Franks, D. A. Boltz, D. Bush, A. S. Lipatov, R. J. Webby, J. E. Rehg, and R. G. Webster. 2009. Changes in H5N1 influenza virus hemagglutinin receptor binding domain affect systemic spread. Proc. Natl. Acad. Sci. U. S. A. 106:286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu, Y. L., I. H. Chen, K. Y. Shen, R. Y. Huang, W. R. Wang, C. J. Chou, T. T. Chang, and C. L. Chu. 2009. A triterpenoid methyl antcinate K isolated from Antrodia cinnamomea promotes dendritic cell activation and Th2 differentiation. Eur. J. Immunol. 39:2482-2491. [DOI] [PubMed] [Google Scholar]