Abstract
Hepatitis C virus (HCV) is a causative agent of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. HCV in circulating blood associates with lipoproteins such as very low density lipoprotein (VLDL) and low-density lipoprotein (LDL). Although these associations suggest that lipoproteins are important for HCV infectivity, the roles of lipoproteins in HCV production and infectivity are not fully understood. To clarify the roles of lipoprotein in the HCV life cycle, we analyzed the effect of apolipoprotein E (ApoE), a component of lipoprotein, on virus production and infectivity. The production of infectious HCV was significantly reduced by the knockdown of ApoE. When an ApoE mutant that fails to be secreted into the culture medium was used, the amount of infectious HCV in the culture medium was dramatically reduced; the infectious HCV accumulated inside these cells, suggesting that infectious HCV must associate with ApoE prior to virus release. We performed rescue experiments in which ApoE isoforms were ectopically expressed in cells depleted of endogenous ApoE. The ectopic expression of the ApoE2 isoform, which has low affinity for the LDL receptor (LDLR), resulted in poor recovery of infectious HCV, whereas the expression of other isoforms, ApoE3 and ApoE4, rescued the production of infectious virus, raising it to an almost normal level. Furthermore, we found that the infectivity of HCV required both the LDLR and scavenger receptor class B, member I (SR-BI), ligands for ApoE. These findings indicate that ApoE is an essential apolipoprotein for HCV infectivity.
Hepatitis C virus (HCV) infection is a major global health problem. More than 170 million people worldwide are infected with HCV. HCV causes chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (18). A member of the family Flaviviridae, HCV has a positive-sense, single-stranded RNA genome that is packaged into an enveloped viral particle. The genome encodes a large precursor polyprotein, which is cleaved by host and viral proteases to generate at least 10 functional viral proteins: core, envelope protein 1 (E1), E2, p7, nonstructural protein 2 (NS2), NS3, NS4A, NS4B, NS5A, and NS5B (12, 13). Core associates with the lipid droplet (LD). The role of this association remained elusive until robust HCV replication systems became available (32). We previously showed that the LD is an important organelle for HCV production (23). In hepatocytes, the LD is physiologically important as a lipid source for the production of lipoproteins such as very low density lipoprotein (VLDL) (11). VLDL is synthesized in the liver as a triglyceride/cholesterol ester-rich particle (diameter, 30 to 100 nm) surrounded by apolipoproteins such as apolipoprotein B100 (abbreviated as ApoB throughout), ApoC's, and ApoE. VLDL is released into blood vessels to be delivered as a lipid source to peripheral cells, and it is also readsorbed by liver cells after processing (5).
HCV particles circulating in the blood of HCV carriers associate with lipoproteins, such as low-density lipoprotein (LDL), VLDL, and chylomicrons; thus, these are termed lipo-viro particles (LVPs) (1, 26). Purified LVPs from circulating blood contain triglyceride, ApoB, ApoB48, ApoCII, ApoCIII, ApoE, and virus components such as HCV RNA and core (8), indicating that the LVP has dual viral and lipoprotein characteristics. The HCVcc strain, which contains a chimeric HCV-2a genome with a structural region from HCV-J6 and nonstructural/noncoding regions from an infectious JFH1 virus, can establish long-term infection in chimpanzees. Viruses recovered from the chimpanzee contain infectious virus particles with a slightly low density, suggesting that an in vivo association with low-density factors influences infectivity (19). However, the role of a lipoprotein-like component of LVPs in virus replication is not clear. Moreover, the mechanism by which LVPs are generated during HCV production is unknown.
When HCV-producing cells are treated with an inhibitor of microsomal triglyceride transfer protein (MTP) or with ApoB-specific small interfering RNA (siRNA), the production of HCV particles is suppressed (10, 14, 25). Therefore, lipoprotein biosynthesis appears to play an important role in the production of infectious HCV and its egress from infected cells. ApoB, ApoC1, and ApoE associate with infectious virus particles in the HCVcc infection/replication system (4, 6, 15, 22, 27). Furthermore, ApoE depletion suppresses the production of infectious HCV (4, 6, 15, 27). These reports strongly suggest the importance of lipoprotein function to the HCV life cycle. However, the precise roles of lipoproteins and apolipoproteins in virus production and infectivity are not fully understood.
We analyzed the production of HCV from cells in which apolipoprotein production was knocked down with siRNA. We found that ApoE is required for the infectivity of HCV, a finding consistent with other reports (4, 6, 15). ApoE is a polymorphic protein with three major isoforms: ApoE2, ApoE3, and ApoE4. The three isoforms differ by amino acid substitutions at one or two sites (residues 130 and 176) on the 317-amino-acid chain of the ApoE molecule. The polymorphism of ApoE influences its multiple functions due to isoform-dependent differences in receptor-binding activity and lipoprotein association preference. For example, ApoE2 has drastically lower LDL receptor (LDLR) binding activity than ApoE3 and ApoE4 (7). In the present study, we investigated the role of ApoE isoforms in virus production and infectivity.
(Part of this study was presented at the 16th International Symposium on Hepatitis C Virus and Related Viruses, Nice, France, 3 to 7 October 2009.)
MATERIALS AND METHODS
Cell culture and viruses.
The human hepatoma cell line HuH7.5 was grown in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (FBS), 100 U/ml nonessential amino acids (Invitrogen), and 100 μg/ml of both penicillin and streptomycin sulfate (Nacalai Tesque, Kyoto, Japan). Infectious HCV in cell culture (HCVcc) was produced by transfection of HuH7.5 cells with in vitro-transcribed RNA derived from JFH1 or TNS2J1.
Generation of ApoE knockdown cells.
Plasmids expressing short hairpin RNA (shRNA) targeting ApoE (5′-GCAGACACTGTCTGAGCAGGT-3′, 5′-CCGCCTCAAGAGCTGGTTCGA-3′, and 5′-GAAGGAGTTGAAGGCCTACAA-3′) or a control shRNA (5′-CAACAAGATGAAGAGCACCAA-3′) (Mission shRNA; Sigma) were transfected into HuH7.5 cells using TransIT-LT1 (Mirus). Cells were selected with puromycin (1.5 μg/ml; Invitrogen). Individual clones were screened by immunoblotting with an anti-ApoE antibody.
Plasmid construction and ectopic expression of ApoE in ApoE knockdown cells.
Human ApoE3 cDNA was amplified from human liver cDNA and cloned into the pCAG and pCAG-HA vectors. A hemagglutinin (HA) tag was fused with the C terminus of ApoE. ApoE mutants were constructed by using a mutagenesis kit according to the manufacturer's instructions (Toyobo, Osaka, Japan). The plasmids, primer sequences, and PCR templates, and the restriction enzyme sites used to construct the plasmids, are listed in Table 1. The stable ApoE knockdown cells (sh-#3) were transfected with ApoE expression plasmids using TransIT-LT1 (Mirus) to obtain HuH7.5 cells expressing ApoE isoforms.
TABLE 1.
Primers used for the construction of expression plasmidsa
| Plasmid name | Primer sequence (5′ to 3′) | Template DNA | Restriction enzyme | Vector |
|---|---|---|---|---|
| pCAG-ApoE2 | TGCCTGGCAGTGTACCAGGCCGGGGCCCGC | pCAG-ApoE3 | EcoRI | pCAG |
| pCAG-ApoE2-HA | CTTCTGCAGGTCATCGGCATCGCGGAGGAG | XhoI | pCAG-HA | |
| pCAG-ApoE3 | ATGAAGGTTCTGTGGGCTGCG | Human liver cDNA | EcoRI | pCAG |
| pCAG-ApoE3-HA | GTGATTGTCGCTGGGCACAGG | XhoI | pCAG-HA | |
| pCAG-ApoE4 | CGCGGCCGCCTGGTGCAGTACCGCGGCGAG | pCAG-ApoE3 | EcoRI | pCAG |
| pCAG-ApoE4-HA | CACGTCCTCCATGTCCGCGCCCAGCCGGGC | XhoI | pCAG-HA | |
| pCAG-ApoE3-KDEL | TAACAATTCACTCCTCAGGTGCAGGCTGCC | pCAG-ApoE3 | EcoRI | pCAG |
| CAGTTCATCTTTGTGATTGTCGCTGGGCAC | XhoI |
The sets of primers used to amplify the target genes, the template DNAs used in the PCRs, the restriction sites, and the destination plasmids into which the amplified DNA fragments were inserted are shown.
siRNA transfection.
siRNA transfection was performed using Silentfect (Bio-Rad) according to the manufacturer's protocol. Duplex nucleotides of siRNA specific to mRNA for ApoE (5′-AGACAGAGCCGGAGCCCGA-3′), the LDLR (5′-GGACAGAUAUCAUCAACGA-3′), or scavenger receptor class B, member I (SR-BI) (5′-GCAGCAGGUCCUUAAGAAC-3′), and a control siRNA, si-control, were purchased from Sigma.
Antibodies and reagents.
Rat anti-HA (3F10; Roche Applied Science), mouse anti-Flag (M2; Sigma), mouse anti-actin (AC-40; Sigma), goat anti-α1-antitrypsin (K15600G; Biodesign International), mouse anti-ApoE (13F45; Autogen Bioclear), goat anti-ApoE (AB947; Chemicon International), sheep anti-ApoB (K90086C; Biodesign International), mouse anti-core (CP11; Institute of Immunology), goat anti-LDLR (AF2148; R&D Systems), rabbit anti-SR-BI (EP1556Y; Abcam), mouse anti-CD81 (JS-81; BD Biosciences), and normal goat IgG (sc-2028; Santa Cruz Biotechnology) antibodies were purchased commercially. Rat anti-claudin 1 (anti-CLDN1) antibodies have been described previously (16). Rabbit polyclonal antibodies specific for NS5A were raised against a bacterially expressed glutathione S-transferase (GST)-NS5A (amino acids [aa] 1 to 406) fusion protein. Horseradish peroxidase-linked donkey antibodies to goat IgG (Santa Cruz Biotechnology) and donkey antibodies to sheep IgG (Jackson ImmunoResearch) were used. Horseradish peroxidase-linked goat antibodies to rat IgG, sheep antibodies to mouse IgG, and donkey antibodies to rabbit IgG were purchased from Amersham Biosciences. Human recombinant ApoE3 (A2331) was purchased from Sigma.
Focus-forming unit assay.
The infectivity titer of HCV was determined on HuH7.5 cells by endpoint dilution and immunostaining of infected cells. Each sample was serially diluted 5-fold in DMEM, and 100 μl was used to inoculate 6 × 103 naïve HuH7.5 cells in a 96-well plate. Infection was examined 48 h postinoculation by immunofluorescence using a rabbit polyclonal anti-NS5A antibody and an Alexa 488-conjugated anti-rabbit IgG antibody (Invitrogen). Infectious foci were counted, and the titer was calculated and expressed in focus-forming units (FFU) per milliliter. The imaging analysis was conducted with an Axiovert 200 microscope (Carl Zeiss).
Intracellular and extracellular infectivity experiments.
HCV-infected cells were washed twice with phosphate-buffered saline (PBS), collected by centrifugation, and then suspended in distilled water. Cells were sheared by 10 strokes with a 27-gauge needle (Terumo, Tokyo, Japan) before incubation at room temperature for 15 min. The lysate supernatant was collected after centrifugation, filtered through a 0.45-μm-pore-size filter (Iwaki, Tokyo, Japan), and concentrated with an Amicon Ultra-15 centrifugal filter (Millipore). The solvent was changed to DMEM, and the resulting solution was used as the intracellular HCV source. The cell culture medium was collected and filtered through a 0.45-μm-pore-size filter. The filtrate was concentrated with an Amicon Ultra-15 centrifugal filter and was then used as the extracellular virus source.
Quantification of HCV core protein.
HCV core protein in the culture medium was quantified by using the Ortho HCV antigen enzyme-linked immunosorbent assay (ELISA) kit (Ortho-Clinical Diagnostics) according to the manufacturer's protocol.
Isopycnic gradient centrifugation of HCV particles.
The 20-times-concentrated HCVcc was layered on top of 14 to 54% iodixanol gradients prepared in PBS. Gradients were centrifuged in an RPS40T rotor (Hitachi, Tokyo, Japan) at 36,000 rpm for 16 h at 4°C. Ten fractions (700 μl each) were collected from the top of the tube. The buoyant density of each fraction was calculated from the refractive index data, measured with an Abbe refractometer (Atago, Tokyo, Japan).
Real-time RT-PCR.
RNA was extracted from 10-times-concentrated HCVcc for real-time reverse transcription-PCR (RT-PCR). Quantitative real-time RT-PCR analysis of the 5′ untranslated region of the HCV genome was performed as described previously (30). The forward and reverse primers were 5′-CCCTCCCGGGAGAGCCATAGTG-3′ and 5′-GTCTCGCGGGGGCACGCCCAAAT-3′, respectively. The TaqMan probe was 5′-6-carboxyfluorescein (FAM)-TCTGCGGAACCGGTGAGTACAC-BHQ1-3′.
Statistical analysis.
Data are expressed as means and standard deviations (SD). Statistical analyses were performed using Student's t test, and a P value of <0.05 was considered statistically significant.
RESULTS
The production of infectious HCVcc from ApoE-depleted cells is suppressed.
To clarify the roles of ApoE in HCV production, we infected ApoE knockdown cells with HCVcc and measured the amount of infectious HCV released into the culture medium. siRNA targeting ApoE or randomized control siRNA was introduced into HuH7.5 cells, and then the cells were infected with JFH1 4 h after transfection. The culture medium was inoculated into naïve HuH7.5 cells for infectivity analysis. The effect of ApoE knockdown was verified by Western blot analysis. ApoE siRNA treatment efficiently reduced the levels of ApoE in HuH7.5 cells, whereas the levels of actin, α1-antitrypsin, and ApoB remained unchanged (see Fig. S1A in the supplemental material). HCV genome replication, as determined by the amounts of virus proteins (core and NS5A) in cell lysates, was not affected by ApoE knockdown (see Fig. S1A). To determine if ApoE affects the secretion of HCV into culture medium, the amount of core in the medium was measured by a core-specific ELISA. We observed that there is no gross difference in the ratio of HCV core and HCV RNA between culture media harvested at different time points after virus infection, indicating that measurement of the level of core is relevant for representing HCV. The knockdown of endogenous ApoE reduced the level of core to 53% of that in control siRNA-treated cells (see Fig. S1B in the supplemental material). Next, we assessed the infectivity of extracellular virus particles. The infectivity of HCV in the culture medium of ApoE knockdown cells was strongly suppressed compared to that of HCV from control siRNA-treated cells (see Fig. S1B), a finding consistent with previous reports (4, 6, 15). Downregulated virus release and reduced production of infectious virus were also observed when the infectious chimeric HCV genome, TNS2J1 (30), which contains the HCV-1b-derived structural region and the JFH1-derived nonstructural region, was examined (see Fig. S1B, right). These results indicate that ApoE is a cellular factor essential for the production of infectious HCV.
To further clarify the role of ApoE in the HCV life cycle, we established HuH7.5 cells in which ApoE was stably knocked down with ApoE-specific short hairpin RNA and a control cell line that expressed normal control shRNA (NC). In the resultant two ApoE knockdown cell clones (sh-#3 and sh-#12), ApoE was barely detected in the cell lysate and medium (see Fig. S1C, top, in the supplemental material). The amounts of actin, α1-antitrypsin, and ApoB proteins were not affected compared to normal control shRNA-expressing cells (sh-NC) (see Fig. S1C in the supplemental material).
We examined whether or not the downregulation of ApoE influences the infection efficiency of HCV using these cell lines. Cells were infected with HCVcc, and infectivity was analyzed by a focus-forming unit assay 48 h after infection (see Fig. S1D in the supplemental material). We found no substantial difference in HCV infectivity in these cells. Therefore, endogenous ApoE is not required for HCV entry or for the establishment of infection. However, the production of infectious HCV from sh-ApoE cells was remarkably reduced, although the level of HCV core was only about 50% reduced (see Fig. S1E in the supplemental material). This result is consistent with the results obtained for cells in which ApoE was transiently knocked down (see Fig. S1B).
To exclude the possibility that the suppressed production of infectious HCV was caused by an off-target effect of shRNA, we examined whether or not HCV production was rescued by the ectopic expression of ApoE in the sh-ApoE cell lines. We inoculated infectious HCVcc into sh-ApoE cells that were either left untransfected or transfected with the ApoE expression plasmid. Forty-eight hours later, the culture medium and cell lysates were harvested in order to measure infectivity and the production of HCV proteins, respectively. Ectopic expression of ApoE or ApoE-HA increased ApoE protein levels in the cells, and ApoE was secreted into the medium. There were no changes in the levels of NS5A, actin, ApoB, and α1-antitrypsin in these cells (see Fig. S2A in the supplemental material). Interestingly, the ectopic expression of ApoE or ApoE-HA did not restore the secretion of HCV core to the level in control HCV-infected HuH7.5 cells (see Fig. S2B). However, HCV infectivity was restored to a level similar to that for sh-NC (see Fig. S2C). These results indicate that ApoE shRNA directly affects ApoE gene expression and that ApoE itself is an essential host factor for HCV infectivity.
ApoE interacts with infectious HCV particles released into the culture medium.
To clarify the mechanisms underlying the role of ApoE in HCV infectivity, we examined the interaction between ApoE and HCV particles by performing coimmunoprecipitation experiments. The culture medium from cells bearing infectious JFH1 replicons was incubated either with an anti-ApoE antibody or with normal goat IgG. Immunocomplexes were recovered with protein G-Sepharose. RNA was extracted from the complex and was analyzed by quantitative RT-PCR. HCV RNA was detected mainly in the complex precipitated with the anti-ApoE antibody; only a little HCV RNA was detected with normal goat IgG (see Fig. S3A in the supplemental material). There is no substantial difference between the levels of HCV precipitated by different sources of anti-ApoE antibodies (data not shown). The fraction not precipitated by the anti-ApoE antibody had little infectivity, while the supernatant of the reaction mixture with normal goat IgG had significantly high infectivity (see Fig. S3B). These results provide evidence of a direct interaction between ApoE and HCV that is important for infectivity.
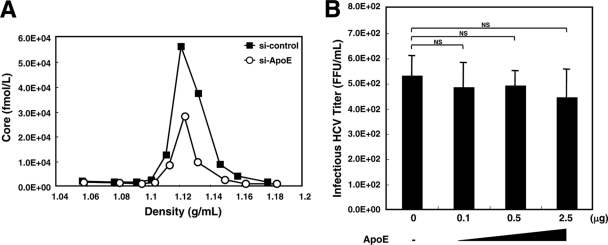
We conducted an iodixanol density gradient assay to find out if there was any qualitative difference between HCV produced from HuH7.5 cells and HCV produced from ApoE knockdown HuH7.5 cells (Fig. 1A). We could not see any difference in their buoyant densities to explain the difference in infectivity. This indicates that association with ApoE does not cause a drastic physical change in HCV.
FIG. 1.
Nature of HCV produced from ApoE knockdown HuH7.5 cells. (A) Buoyant density analysis. HuH7.5 cells were seeded onto 60-mm-diameter dishes. Cells were transfected with siRNA (si-control or si-ApoE). Four hours after transfection, cells were infected with HCVcc. Forty-eight hours after inoculation, the concentrated culture medium was fractionated using 14 to 54% iodixanol density gradient centrifugation at 36,000 rpm for 16 h at 4°C. The buoyant density profile is represented by the amount of core protein (in femtomoles per liter) in each fraction. Data from a representative of three experiments are shown. (B) Analysis of the infectivity of HCVcc produced from ApoE knockdown HuH7.5 cells after incubation with recombinant ApoE. HCVcc from cells in which ApoE expression was silenced was incubated alone or with different doses of human recombinant ApoE3 at 37°C for 2 h. Then the reaction mixtures were inoculated into naïve HuH7.5 cells. Forty-eight hours after infection, titers of infectious HCV were quantified by a focus-forming unit assay. The average values for three independent experiments are shown; error bars, standard deviations of the means. P values were determined by comparison (by Student's t test) with HCVcc that was not treated with recombinant ApoE3. NS, not significant (P > 0.05).
Release of infectious HCV into the culture medium depends on the secretion of ApoE.
Our results and those of other groups clearly indicate the importance of the association of HCV with ApoE for infectivity (4, 6, 15, 27). However, it is uncertain when ApoE associates with HCV during the processes of morphogenesis and the secretion of infectious HCV particles. ApoE by itself can be released from ApoE-producing cells; thus, it is possible that HCV associates with ApoE after being secreted into the culture medium. However, this possibility is less likely, because the infectivity of HCV secreted from cells lacking ApoE expression was not rescued by incubation with different doses of recombinant ApoE (Fig. 1B).
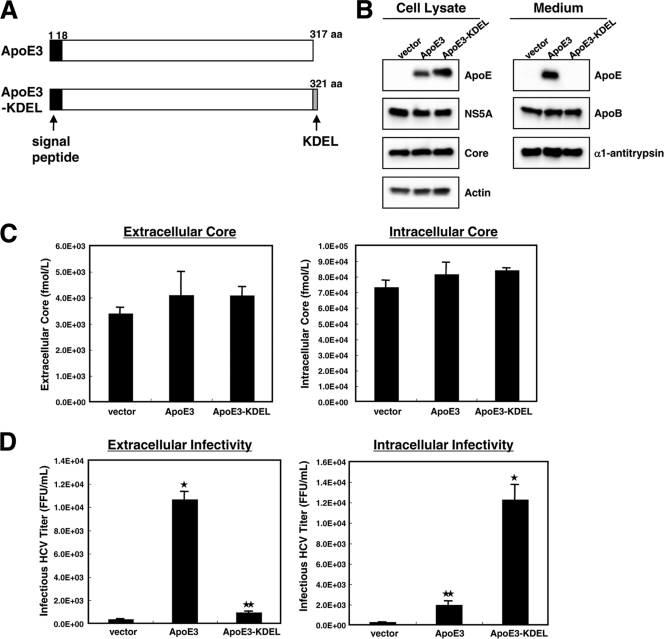
To study the association of ApoE with HCV during the process of infectious virus production, we analyzed the production and infectivity of HCV produced by cells expressing mutant ApoE that is not secreted, due to the addition of Lys-Asp-Glu-Leu (KDEL), an endoplasmic reticulum (ER) retention signal, to its C terminus (Fig. 2A). sh-#3 cells were transfected with an ApoE3 or ApoE3-KDEL expression plasmid followed by HCV infection. Western blot analysis revealed that ApoE3 and ApoE3-KDEL were produced abundantly in transfected cells (Fig. 2B). As expected, ApoE3-KDEL accumulated inside cells, whereas ApoE3 localized both inside and outside cells (Fig. 2B). The expression of actin, NS5A, ApoB, and α1-antitrypsin in the transfected cells was unchanged (Fig. 2B). Additionally, the levels of extracellular and intracellular HCV core protein in the two types of transfected cells were not significantly different (Fig. 2C).
FIG. 2.
The release of infectious HCV into the culture medium depends on the secretion of ApoE. (A) Schematic representation of ApoE3 and the ApoE3-KDEL mutant. (B) Verification of the expression of ectopic ApoE3 and ApoE3-KDEL. The sh-#3 cells were seeded onto 100-mm-diameter dishes. Cells were transfected with either pCAG (vector), pCAG-ApoE3 (ApoE3), or pCAG-ApoE3-KDEL (ApoE3-KDEL). Four hours after transfection, cells were inoculated with JFH1. Forty-eight hours after inoculation, cell lysates and supernatants were analyzed for the production of ApoE and its mutant by Western blotting with anti-ApoE antibodies. The expression of actin, α1-antitrypsin, ApoB, core, and NS5A was also examined. (C) The amounts of core in the culture supernatant and intracellular fractions were determined by a core-specific ELISA. The cells and transfections were the same as for panel B. (D) Analysis of HCV infectivity. The culture medium or cell lysate was inoculated into naïve HuH7.5 cells. The titers of infectious HCV were quantified by focus-forming unit assays. The average values for three independent experiments are shown; error bars, standard deviations of the means. The cells and transfections were the same as for panel B. P values were determined by comparison (by Student's t test) with cells expressing the control vector. *, P < 0.0005; **, P < 0.005.
Next, we analyzed HCV infectivity in the extracellular and intracellular fractions of these cells. Infectious HCV was recovered from cells ectopically expressing ApoE3 (Fig. 2D, left). However, cells producing ApoE3-KDEL released very few infectious virus particles into the culture medium; instead, the infectious virus accumulated in the intracellular fraction (Fig. 2D, right). These results suggest that infectious virus particles constituted with ApoE are produced inside the cells and that the release of these infectious particles depends on the secretion of ApoE.
The ApoE isoform affects the infectivity of HCV.
ApoE is a multifunctional protein that plays central roles in lipid metabolism and neurobiology. It has three major isoforms (ApoE2, ApoE3, and ApoE4) that have different effects on lipid and neuronal homeostasis. These isoforms differ by amino acid substitutions at one or two sites (residues 130 and 176). ApoE3 is the most common isoform, and there have been no reports of diseases associated with ApoE3. On the other hand, ApoE2 is the major risk factor for type III hyperlipoproteinemia, and ApoE4 is the major risk factor for Alzheimer's disease (20). ApoE2 has lower affinity for the LDLR than ApoE3 and ApoE4. Since lipoprotein receptors, including the LDLR and scavenger receptor class B, member I (SR-BI), are suspected of acting as receptors for HCV infection (3, 24, 27, 34), we hypothesized that the ApoE isoform may affect HCV infectivity. To clarify this hypothesis, we analyzed the production of infectious HCV from cells expressing different isoforms of ApoE.
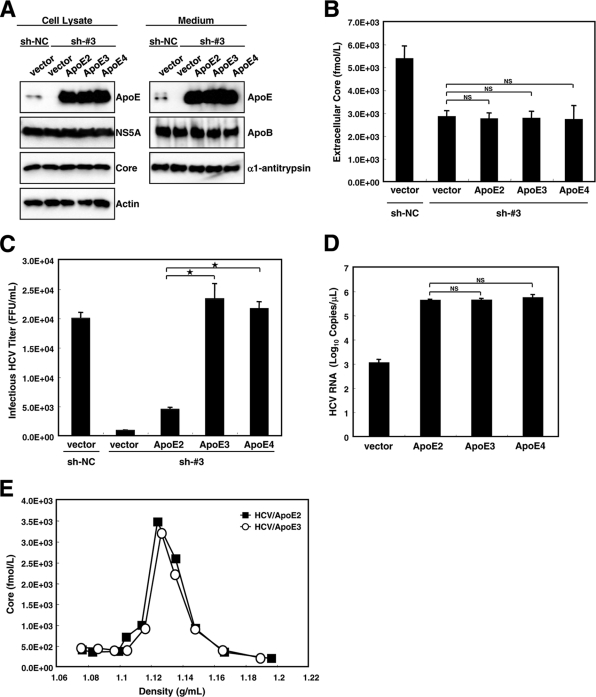
Plasmids expressing ApoE isoforms were transfected into HuH7.5 cells in which endogenous ApoE3 had been knocked down (sh-#3). Although the level of ectopically expressed ApoE was higher than that of endogenous ApoE, the levels of ectopically expressed ApoE and the levels of ApoE secreted into the culture medium were not different for cell groups expressing different ApoE isoforms (Fig. 3A). The replication of the HCV genome, as determined by the amounts of NS5A and core, was unaffected by the expression of different isoforms of ApoE (Fig. 3A). The amount of extracellular core in ApoE isoform-expressing cells was one-half of that in control cells and was not significantly different among cell groups expressing different ApoE isoforms (Fig. 3B, vector versus ApoE2 to ApoE4). We also noticed that the amounts of HCV RNA in extracellular fractions from cells expressing different ApoE isoforms were not significantly different (data not shown). To examine the production of infectious virus, the culture media of these cells were inoculated into naïve HuH7.5 cells, and infectivity was assayed 48 h after infection. Interestingly, ApoE2-expressing cells released substantially less infectious HCV into the culture medium than did ApoE3- or ApoE4-expressing cells (Fig. 3C). We examined the possibility that the affinity of ApoE for HCV differs by isoform. HA-tagged ApoE isoform expression plasmids were transfected into sh-#3 cells, which were then infected with HCV. The culture media from the infected cells were incubated with an anti-HA antibody, and the immunocomplexes were recovered. RNA was extracted from the complexes and analyzed by quantitative RT-PCR. The amounts of HCV RNA in the immune complexes obtained from the culture media of cells expressing different ApoE isoforms were almost the same (Fig. 3D). Furthermore, we examined whether a difference between the densities of ApoE2-containing HCV and ApoE3-containing HCV can be correlated with the difference in infectivity (Fig. 3E). There is no significant density shift between these two viruses to explain the difference in infectivity, suggesting the importance of factors other than particle density for HCV infectivity. These results suggest that the ability of HCV-associated ApoE isoforms to bind to the LDLR seems to be responsible for isoform-based differences in the infectivity of HCV.
FIG. 3.
ApoE isoforms affect HCV infectivity. (A) Verification of expression of ectopically introduced ApoE and the effect of ApoE isoforms on HCV genome replication. sh-NC cells and sh-#3 cells were seeded onto 60-mm-diameter dishes. Cells were transfected with either pCAG (vector), pCAG-ApoE2 (ApoE2), pCAG-ApoE3 (ApoE3), or pCAG-ApoE4 (ApoE4). Four hours after transfection, cells were inoculated with JFH1. Forty-eight hours after inoculation, the expression of ApoE, ApoB, NS5A, core, actin, and α1-antitrypsin in cell lysates and/or supernatants was analyzed by Western blotting using relevant antibodies. (B) The release of HCV core into the culture supernatant by cells expressing different ApoE isoforms was measured by a core-specific ELISA. The cells and transfections in panels B to D were the same as those in panel A. P values were determined by comparison (by Student's t test) with HCVcc from sh-#3 cells expressing the control vector. NS, not significant (P > 0.05). (C) Amount of infectious HCV that egressed from HCV-infected cells. Culture media of the indicated cells were inoculated into naïve HuH7.5 cells. Forty-eight hours after infection, naïve HuH7.5 cells were infected with the supernatant. Forty-eight hours after infection, the titers of infectious HCV were quantified by a focus-forming unit assay. P values were determined by comparison (by Student's t test) with HCVcc from sh-#3 cells expressing ApoE2. *, P < 0.0005. (D) Association of HCV with ApoE isoforms. HCV released into the culture medium from cells bearing each ApoE isoform was incubated with an anti-HA antibody. RNA was extracted from the immunoprecipitant and subjected to quantification by quantitative RT-PCR. P values were determined by comparison (by Student's t test) with HCVcc from sh-#3 cells expressing ApoE2. NS, not significant (P > 0.05). (E) Density gradient analysis of HCVcc containing ApoE2 or ApoE3. Concentrated HCVcc from cells expressing ApoE2 or ApoE3 was fractionated using 14 to 54% iodixanol density gradient centrifugation at 36,000 rpm for 16 h at 4°C. The buoyant density profile is represented by measuring the amount of core protein (in femtomoles per liter). HCV/ApoE3, HCV bearing ApoE3; HCV/ApoE2, HCV bearing ApoE2. Data from a representative of three experiments are shown.
HCV requires the LDLR and SR-BI expression for full infectivity.
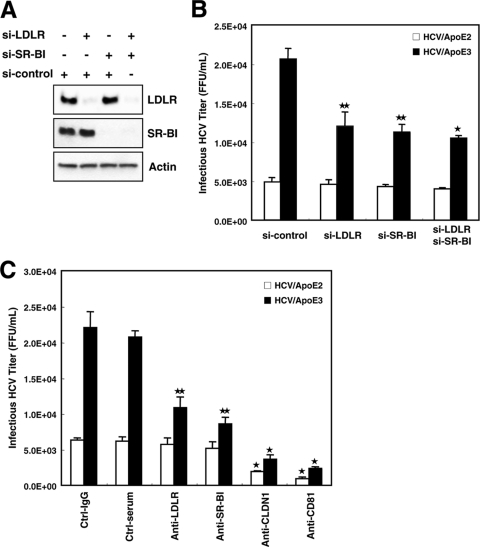
Previously, the LDLR and SR-BI were shown to be involved in HCV infection, possibly through HCV-associating ApoE that functions as a ligand to those molecules. However, there is controversy as to which receptor, together with other receptors, such as CLDN1 and CD81, is involved in more importantly in HCV entry (3, 16, 24, 27, 34). To investigate this point, we conducted an infectivity analysis using ApoE2- and ApoE3-complemented HCV with HuH7.5 cells that were depleted of the LDLR and/or SR-BI by treatment with specific siRNAs. Western blot analysis showed that the levels of the LDLR and SR-BI were substantially reduced by treatment with their specific siRNAs (Fig. 4A). These cells were infected with HCV produced by HuH7.5 cells expressing ectopic ApoE3 (HCV bearing ApoE3 [HCV/ApoE3]) or ApoE2 (HCV bearing ApoE2 [HCV/ApoE2]), and infectivity was analyzed 48 h after infection (Fig. 4B). We observed significant reductions in HCV/ApoE3 infection of cells in which the LDLR or SR-BI was silenced, as expected on the basis of reports from other groups (3, 24, 27, 34). Assuming that LDLR and SR-B1 function independently in the step of HCV entry, a further reduction in infectivity would be expected for cells in which both the LDLR and SR-BI are silenced. However, this was not the case. The reduction in infectivity was almost the same as those for cells in which either the LDLR or SR-BI was silenced (Fig. 4B). The levels of HCV/ApoE2 entry into cells with both or either LDLR or SR-BI knocked down were also nearly the same (Fig. 4B).
FIG. 4.
Suppression of LDLR and SR-BI results in reduced HCV infection. (A) Knockdown of the LDLR and SR-BI by siRNA. HuH7.5 cells were seeded onto 24-well plates and transfected with siRNA (si-control, si-LDLR, and/or si-SR-BI). Twenty-four hours after transfection, cell lysates were analyzed for the expression of the LDLR, SR-BI, and actin by Western blotting. (B) Analysis of HCV infectivity for HuH7.5 cells in which either the LDLR, SR-BI, or both were silenced. Cells were transfected with siRNA (si-control, si-LDLR, and/or si-SR-BI). Twenty-four hours after transfection, HCVcc produced from cells bearing ApoE2 (open bars) or ApoE3 (filled bars) was serially diluted and inoculated. Forty-eight hours after infection, infected cells were counted by fluorescence microscopy after staining with an anti-NS5A antibody. HCV/ApoE3, HCV bearing ApoE3; HCV/ApoE2, HCV bearing ApoE2. P values were determined by comparison (by Student's t test) with si-control-treated cells. *, P < 0.0005; **, P < 0.005. (C) HuH7.5 cells were preincubated with control goat IgG (Ctrl-IgG), control rat preimmune serum (Ctrl-serum), or a goat anti-LDLR, rat anti-SR-BI, rat anti-claudin 1 (anti-CLDN1), or mouse anti-CD81 antibody for 1 h at 37°C before infection with serially diluted HCVcc from HuH7.5 cells expressing ApoE2 (open bars) or ApoE3 (filled bars). Forty-eight hours after infection, infected cells were stained with anti-NS5A antibodies and counted by fluorescence microscopy. The average values for three independent experiments are shown; error bars, standard deviations of the means. P values were determined by comparison (by Student's t test) against cells treated with preimmune serum. *, P < 0.0005; **, P < 0.005.
Next, we conducted infectivity assays of HCV/ApoE2 and HCV/ApoE3 after treating them with antibodies against various candidate molecules for the HCV receptor (Fig. 4C). Anti-CLDN1 and anti-CD81 strongly inhibited HCV infection, as shown in Fig. 4C. Anti-LDLR and anti-SR-BI antibodies showed only moderate inhibition of HCV/ApoE3 infection (Fig. 4C, compare with inhibition by anti-CLDN1 and anti CD81 antibodies). Taken together, our results suggest the importance of both the LDLR and SR-BI for HCV infection, possibly through the function of HCV-associating ApoE.
DISCUSSION
Accumulating evidence suggests that HCV is complexed with lipoproteins and that it exhibits both viral and lipoprotein characteristics, leading to the recognition of HCV as an LVP. However, it is not known how the nature of lipoproteins associated with HCV is involved in the cycle of virus proliferation. HCV was found to be secreted in a manner that parallels the formation of VLDL by experiments that used an MTP inhibitor or ApoB knockdown (10, 14). In these studies, the suppression of ApoB significantly impaired the production of both VLDL and HCV. In contrast, another study reported a lesser contribution of ApoB to HCV production (15). On the other hand, ApoE knockdown severely interfered with the production of infectious virus (see below).
We showed here that ApoE is required for HCVcc infectivity for HuH7.5 cells, which is consistent with reports showing that the inhibition of ApoE production leads to reduced HCVcc infectivity (4, 6, 15, 27). Depletion of ApoE resulted in a significant reduction in the infectivity not only of JFH1 but also of TNS2J1, the chimeric HCVcc composed of the structural region of HCV-1b and a nonstructural region derived from JFH1, although the replication efficiencies of the genomes were unchanged (see Fig. S1B in the supplemental material). Thus, the requirement of ApoE for infectious HCV production may be unrelated to the HCV genotype.
ApoE seems to have an additional role in regulating virus assembly/release besides its role in virus entry. Chang et al. report a severe reduction in HCV particle assembly/release following ApoE knockdown (6). We analyzed the amount of HCV by measuring the level of core as well as virus RNA in the culture medium from ApoE-silenced HuH7.5 cells. Under this condition, the production and secretion of ApoE were severely suppressed (see Fig. S1A in the supplemental material). However, we observed only a 50% reduction of both core and HCV RNA levels in the culture medium (see Fig. S1B for core; data for HCV RNA not shown), in strong contrast to the data of Chang et al. Since ApoE knockdown does not affect replicon activity, we established several HuH7.5 clones that stably silenced the production of ApoE. Using some of those clones, sh-#3 and sh-#12, we observed the same result: only a ∼50% reduction of HCV particle release upon HCV infection (see Fig. S1E). At present we cannot explain the difference between our results and those of Chang et al. However, it could be due to a difference in RNA transfection reagents that may affect cell variability or in HuH7.5 cells that might have been genetically modified during a prolonged period of cultivation after distribution from the original supplier.
ApoE associates with NS5A (4, 9, 15). Since NS5A is suggested to be involved in virus particle assembly (2, 21, 31), it is possible that ApoE participates, at least in part, in virus particle assembly by interacting with NS5A, as suggested by others (4, 15). However, the facts that the release of virus particles into the culture medium from cells in which ApoE is silenced is not completely suppressed and that HCV retained inside cells expressing the ApoE3-KDEL mutant and not released into culture medium indicate that ApoE is not an essential factor for assembly and release.
As for the effect of ApoE on HCV infectivity, we observed a dramatic reduction following ApoE knockdown (see Fig. S1B and S1E in the supplemental material). Thus, we think that ApoE affects HCV infectivity severely but affects virus assembly and/or release only slightly. We tried to find a difference between HCV derived from HuH7.5 cells and HCV from ApoE-depleted HuH7.5 cells by density gradient centrifugation, because a correlation between low HCV particle density and infectivity has been known. However, we could not see any difference in buoyant density to explain the difference in infectivity (Fig. 1A). We think that association with ApoE does not drastically change the physical nature of HCV. It will be important, however, to look for differences in the physical and biochemical nature of HCV in detail, including lipid contents, the status of glycoproteins such as E1 and E2, and so on, in the future.
An association between ApoE and secreted HCV was observed (see Fig. S3A in the supplemental material) (6, 15, 27). To obtain insight into the function of ApoE in the virus life cycle, and particularly in the step of infectious virus secretion, we analyzed the infectious virus released into the culture medium from cells expressing the ApoE mutant ApoE3-KDEL, which is not secreted, because it contains the ER retention signal peptide KDEL. We confirmed that ApoE3-KDEL was not secreted into the culture medium (Fig. 2B). Indirect immunofluorescence showed that the majority of ApoE3-KDEL was retained on the ER, whereas ApoE3 was localized on the ER and the Golgi apparatus (data not shown). The amount of virus released into the culture medium from cells expressing ApoE3-KDEL was almost the same as that from cells expressing ApoE3 (Fig. 2C). However, the production of infectious virus in the culture medium was severely suppressed, since the infectious HCV accumulated in the cell lysate (Fig. 2D). The accelerated level of intracellular accumulation of infectious virus, which was higher than that in cells expressing ApoE3, suggests that the secretion of “infectious” virus depends on ApoE production and secretion. The amounts of HCV released into the culture medium from cells expressing ApoE3-KDEL and ApoE3 were almost the same as that released by cells lacking endogenous ApoE expression (Fig. 2C and D), indicating that ApoE affects the assembly and release of noninfectious virus into the culture medium only slightly but mainly affects the release of infectious virus. Our result also suggests that the association of ApoE and HCV occurs prior to the secretion of the virus from cells, which is required for the virus to gain infectivity. Incubation of recombinant ApoE with HCV produced from ApoE knockdown cells did not show a significant increase in HCV infectivity (Fig. 1B). Moreover, no interaction of ApoE with HCV in vitro was observed (data not shown); this observation confirms the establishment of the association before virus secretion.
The physical structure of HCV as an LVP is uncertain. It is not known if the association of ApoE with infectious virus depends on a coassociation with or integration of lipoprotein. We observed the importance of a substrate of lipoprotein lipase (LPL), which associates with HCV, for infectivity (29). LPL hydrolyzes triglycerides in VLDL and converts them to intermediate-density lipoproteins (IDL). When HCV produced from HuH7.5 cells was treated with LPL followed by hepatic lipase, the density of the virus was shifted higher than the density prior to treatment, and infectivity was simultaneously lost. Importantly, the amount of ApoE associated with HCV was reduced (29). This observation suggests that the interaction of ApoE with HCV depends on the presence of a virus-associated triglycerol ester, most likely a lipid component of lipoprotein. This observation also suggests the importance of an association of lipoprotein with HCV in order to maintain the function of ApoE for HCV infection. However, more study is needed to clarify how ApoE interacts with HCV to increase infectivity.
The ApoE gene is polymorphic, with three common alleles, apoE2, apoE3, and apoE4, which produce 3 isoforms of ApoE. Because the ApoE isoforms have different affinities for the LDLR (ApoE2 has low affinity, while ApoE3 and ApoE4 have high affinity), we analyzed the effect of ApoE isoforms on HCV infectivity. HCV/ApoE3 and HCV/ApoE4 showed almost the same infectivity as the control virus produced from HCV-infected HuH7.5 cells expressing endogenous ApoE3 (Fig. 3C). However, the infectivity of HCV/ApoE2 was about one-fifth that of HCV/ApoE3, even though the level of virus particles was almost the same as that of HCV/ApoE3 (Fig. 3B and C). Assuming that the LDLR plays a role as a receptor of HCV (24, 27, 33), this observation is in agreement with the fact that the binding affinity of ApoE for the LDLR is well correlated with the difference in HCV infectivity by ApoE isoforms.
HCV/ApoE2 showed reduced infectivity for LDLR-silenced HuH7.5 cells (Fig. 4B). Since the level of infectivity was almost the same as that for control cells, entry through SR-BI on HuH7.5 cells was not utilized by HCV/ApoE2. This is also suggested by analysis of the infectivity of HCV/ApoE2 for doubly silenced HuH7.5 cells (Fig. 4B). Because the possibility remains that ApoE2 interacts weakly with both the LDLR and SR-BI, although the binding regions for the LDLR and SR-BI on the ApoE molecule do not overlap (17), we analyzed the infectivity of HCV/ApoE3 for cells in which either the LDLR, SR-BI, or both were silenced (Fig. 4B). As expected on the basis of reports from other groups, suppression of infectivity for LDLR- or SR-BI-silenced HuH7.5 cells was observed. However, to our surprise, infectivity was not further reduced for doubly silenced cells (Fig. 4B). This result suggests that both the LDLR and SR-BI are required for virus entry. The absence of either of these proteins would result in a reduction in infectivity. It is important to determine whether these receptor molecules function independently or cooperatively for HCV entry.
When infectivity was analyzed by treating HCV with an anti-SR-BI or anti-LDLR antibody, the infectivity of HCV remained at a level higher than that of HCV treated with an anti-CLDN1 or anti-CD81 antibody (Fig. 4C). Further, a significant level of HCV/ApoE3 infectivity for LDLR- and SR-BI-silenced HuH7.5 cells was observed (Fig. 4B). These data suggest the presence of another receptor molecule(s) that is relevant to the LDLR and SR-BI regarding the ability to interact with ApoE.
The functional importance of ApoE for HCV infectivity is not limited to the HCVcc used in the present study. An epidemiological study of Caucasians with persistent chronic hepatitis indicates a notable absence of the ApoE2/ApoE2 genotype in HCV antibody-positive individuals (28), which is in agreement with the lower infectivity of ApoE2-bearing HCVcc. The authors did not describe any role for ApoE2 on HCV infectivity. However, our results strongly suggest that HCV produced from ApoE2-bearing individuals is eliminated quickly because it is less infectious. It will be important to conduct a virological study of HCV obtained from individuals carrying different isoforms of ApoE in the future.
Supplementary Material
Acknowledgments
We are grateful to C. Rice (Rockefeller University) for HuH7.5 cells. We thank H. Yamamoto, R. Shiina, and H. Kato for technical assistance. We also thank H. Okamoto for helpful discussions.
This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Health, Labor, and Welfare of Japan and from the Ministry of Education, Culture, Sports, Science, and Technology.
Footnotes
Published ahead of print on 8 September 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.André, P., F. Komurian-Pradel, S. Deforges, M. Perret, J. L. Berland, M. Sodoyer, S. Pol, C. Brechot, G. Paranhos-Baccala, and V. Lotteau. 2002. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J. Virol. 76:6919-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appel, N., M. Zayas, S. Miller, J. Krijnse-Locker, T. Schaller, P. Friebe, S. Kallis, U. Engel, and R. Bartenschlager. 2008. Essential role of domain III of nonstructural protein 5A for hepatitis C virus infectious particle assembly. PLoS Pathog. 4:e1000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartosch, B., A. Vitelli, C. Granier, C. Goujon, J. Dubuisson, S. Pascale, E. Scarselli, R. Cortese, A. Nicosia, and F. L. Cosset. 2003. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J. Biol. Chem. 278:41624-41630. [DOI] [PubMed] [Google Scholar]
- 4.Benga, W. J., S. E. Krieger, M. Dimitrova, M. B. Zeisel, M. Parnot, J. Lupberger, E. Hildt, G. Luo, J. McLauchlan, T. F. Baumert, and C. Schuster. 2010. Apolipoprotein E interacts with hepatitis C virus nonstructural protein 5A and determines assembly of infectious particles. Hepatology 51:43-53. [DOI] [PubMed] [Google Scholar]
- 5.Blasiole, D. A., R. A. Davis, and A. D. Attie. 2007. The physiological and molecular regulation of lipoprotein assembly and secretion. Mol. Biosyst. 3:608-619. [DOI] [PubMed] [Google Scholar]
- 6.Chang, K. S., J. Jiang, Z. Cai, and G. Luo. 2007. Human apolipoprotein E is required for infectivity and production of hepatitis C virus in cell culture. J. Virol. 81:13783-13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davignon, J., R. E. Gregg, and C. F. Sing. 1988. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis 8:1-21. [DOI] [PubMed] [Google Scholar]
- 8.Diaz, O., F. Delers, M. Maynard, S. Demignot, F. Zoulim, J. Chambaz, C. Trepo, V. Lotteau, and P. André. 2006. Preferential association of hepatitis C virus with apolipoprotein B48-containing lipoproteins. J. Gen. Virol. 87:2983-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans, M. J., C. M. Rice, and S. P. Goff. 2004. Phosphorylation of hepatitis C virus nonstructural protein 5A modulates its protein interactions and viral RNA replication. Proc. Natl. Acad. Sci. U. S. A. 101:13038-13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gastaminza, P., G. Cheng, S. Wieland, J. Zhong, W. Liao, and F. V. Chisari. 2008. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J. Virol. 82:2120-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbons, G. F., K. Islam, and R. J. Pease. 2000. Mobilisation of triacylglycerol stores. Biochim. Biophys. Acta 1483:37-57. [DOI] [PubMed] [Google Scholar]
- 12.Grakoui, A., C. Wychowski, C. Lin, S. M. Feinstone, and C. M. Rice. 1993. Expression and identification of hepatitis C virus polyprotein cleavage products. J. Virol. 67:1385-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hijikata, M., H. Mizushima, T. Akagi, S. Mori, N. Kakiuchi, N. Kato, T. Tanaka, K. Kimura, and K. Shimotohno. 1993. Two distinct proteinase activities required for the processing of a putative nonstructural precursor protein of hepatitis C virus. J. Virol. 67:4665-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang, H., F. Sun, D. M. Owen, W. Li, Y. Chen, M. Gale, Jr., and J. Ye. 2007. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc. Natl. Acad. Sci. U. S. A. 104:5848-5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang, J., and G. Luo. 2009. Apolipoprotein E but not B is required for the formation of infectious hepatitis C virus particles. J. Virol. 83:12680-12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieger, S. E., M. B. Zeisel, C. Davis, C. Thumann, H. J. Harris, E. K. Schnober, C. Mee, E. Soulier, C. Royer, M. Lambotin, F. Grunert, V. L. Dao Thi, M. Dreux, F. L. Cosset, J. A. McKeating, C. Schuster, and T. F. Baumert. 2010. Inhibition of hepatitis C virus infection by anti-claudin-1 antibodies is mediated by neutralization of E2-CD81-claudin-1 associations. Hepatology 51:1144-1157. [DOI] [PubMed] [Google Scholar]
- 17.Li, X., H. Y. Kan, S. Lavrentiadou, M. Krieger, and V. Zannis. 2002. Reconstituted discoidal ApoE-phospholipid particles are ligands for the scavenger receptor BI. The amino-terminal 1-165 domain of ApoE suffices for receptor binding. J. Biol. Chem. 277:21149-21157. [DOI] [PubMed] [Google Scholar]
- 18.Liang, T. J., L. J. Jeffers, K. R. Reddy, M. De Medina, I. T. Parker, H. Cheinquer, V. Idrovo, A. Rabassa, and E. R. Schiff. 1993. Viral pathogenesis of hepatocellular carcinoma in the United States. Hepatology 18:1326-1333. [PubMed] [Google Scholar]
- 19.Lindenbach, B. D., P. Meuleman, A. Ploss, T. Vanwolleghem, A. J. Syder, J. A. McKeating, R. E. Lanford, S. M. Feinstone, M. E. Major, G. Leroux-Roels, and C. M. Rice. 2006. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc. Natl. Acad. Sci. U. S. A. 103:3805-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahley, R. W., and S. C. Rall, Jr. 2000. Apolipoprotein E: far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 1:507-537. [DOI] [PubMed] [Google Scholar]
- 21.Masaki, T., R. Suzuki, K. Murakami, H. Aizaki, K. Ishii, A. Murayama, T. Date, Y. Matsuura, T. Miyamura, T. Wakita, and T. Suzuki. 2008. Interaction of hepatitis C virus nonstructural protein 5A with core protein is critical for the production of infectious virus particles. J. Virol. 82:7964-7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meunier, J. C., R. S. Russell, R. E. Engle, K. N. Faulk, R. H. Purcell, and S. U. Emerson. 2008. Apolipoprotein C1 association with hepatitis C virus. J. Virol. 82:9647-9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyanari, Y., K. Atsuzawa, N. Usuda, K. Watashi, T. Hishiki, M. Zayas, R. Bartenschlager, T. Wakita, M. Hijikata, and K. Shimotohno. 2007. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 9:1089-1097. [DOI] [PubMed] [Google Scholar]
- 24.Molina, S., V. Castet, C. Fournier-Wirth, L. Pichard-Garcia, R. Avner, D. Harats, J. Roitelman, R. Barbaras, P. Graber, P. Ghersa, M. Smolarsky, A. Funaro, F. Malavasi, D. Larrey, J. Coste, J. M. Fabre, A. Sa-Cunha, and P. Maurel. 2007. The low-density lipoprotein receptor plays a role in the infection of primary human hepatocytes by hepatitis C virus. J. Hepatol. 46:411-419. [DOI] [PubMed] [Google Scholar]
- 25.Nahmias, Y., J. Goldwasser, M. Casali, D. van Poll, T. Wakita, R. T. Chung, and M. L. Yarmush. 2008. Apolipoprotein B-dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology 47:1437-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen, S. U., M. F. Bassendine, A. D. Burt, C. Martin, W. Pumeechockchai, and G. L. Toms. 2006. Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. J. Virol. 80:2418-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owen, D. M., H. Huang, J. Ye, and M. Gale, Jr. 2009. Apolipoprotein E on hepatitis C virion facilitates infection through interaction with low-density lipoprotein receptor. Virology 394:99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price, D. A., M. F. Bassendine, S. M. Norris, C. Golding, G. L. Toms, M. L. Schmid, C. M. Morris, A. D. Burt, and P. T. Donaldson. 2006. Apolipoprotein ɛ3 allele is associated with persistent hepatitis C virus infection. Gut 55:715-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu, Y., T. Hishiki, K. Sugiyama, K. Ogawa, K. Funami, A. Kato, Y. Ohsaki, T. Fujimoto, H. Takaku, and K. Shimotohno. 3 September 2010. Lipoprotein lipase and hepatic triglyceride lipase reduce the infectivity of hepatitis C virus (HCV) through their catalytic activities on HCV-associated lipoproteins. Virology. doi: 10.1016/j.virol.2010.08.011. [DOI] [PubMed]
- 30.Sugiyama, K., K. Suzuki, T. Nakazawa, K. Funami, T. Hishiki, K. Ogawa, S. Saito, K. W. Shimotohno, T. Suzuki, Y. Shimizu, R. Tobita, M. Hijikata, H. Takaku, and K. Shimotohno. 2009. Genetic analysis of hepatitis C virus with defective genome and its infectivity in vitro. J. Virol. 83:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tellinghuisen, T. L., K. L. Foss, and J. Treadaway. 2008. Regulation of hepatitis C virion production via phosphorylation of the NS5A protein. PLoS Pathog. 4:e1000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakita, T., T. Pietschmann, T. Kato, T. Date, M. Miyamoto, Z. Zhao, K. Murthy, A. Habermann, H. G. Krausslich, M. Mizokami, R. Bartenschlager, and T. J. Liang. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11:791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wünschmann, S., J. D. Medh, D. Klinzmann, W. N. Schmidt, and J. T. Stapleton. 2000. Characterization of hepatitis C virus (HCV) and HCV E2 interactions with CD81 and the low-density lipoprotein receptor. J. Virol. 74:10055-10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeisel, M. B., G. Koutsoudakis, E. K. Schnober, A. Haberstroh, H. E. Blum, F. L. Cosset, T. Wakita, D. Jaeck, M. Doffoel, C. Royer, E. Soulier, E. Schvoerer, C. Schuster, F. Stoll-Keller, R. Bartenschlager, T. Pietschmann, H. Barth, and T. F. Baumert. 2007. Scavenger receptor class B type I is a key host factor for hepatitis C virus infection required for an entry step closely linked to CD81. Hepatology 46:1722-1731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.