Abstract
The 2′-5′ oligoadenylate synthetase (OAS) proteins are traditionally considered intracellular antiviral proteins. However, several studies demonstrate a correlation between the concentration of freely circulating OAS protein in sera from hepatitis C patients and their clinical prognosis. Here we demonstrate that extracellular OAS1 enters into cells and possesses a strong antiviral activity, both in vitro and in vivo, which is independent of RNase L. The OAS protein directly inhibits viral proliferation and does not require the activation of known antiviral signaling pathways. We propose that OAS produced by cells infected with viruses is released to the extracellular space, where it acts as a paracrine antiviral agent. Thus, the OAS protein represents the first direct antiviral compound released by virus-infected cells.
The innate immune system limits viral replication during early stages of infections and primes the development of adaptive immunity. Recently, substantial progress has been made in understanding the mechanism by which viral infections are recognized by the innate immune system (1, 40, 41) and how this recognition leads to initiation of an antiviral immune response. Production of antiviral cytokines, like interferon (IFN), by virus-infected cells is an essential part of the antiviral response. IFN-mediated cellular signaling leads to the induction of several hundred antiviral genes within cells (29). Extensive research has provided insight into the molecular details of IFN signaling. However, our current understanding of the specific mechanism by which the IFN-induced proteins inhibit viral replication is limited.
The 2′-5′ oligoadenylate synthetases (OAS) are a family of antiviral proteins. In humans, this family consists of four genes, OAS1, OAS2, OAS3, and OASL. As is typical for antiviral genes, the transcription of the OAS genes is induced by both virus infection and IFN stimulation (5, 25, 39). None of the OAS genes encode proteins with signal peptides known to mediate secretion to the extracellular environment. Nevertheless, OAS is found in the sera of hepatitis C virus (HCV)-infected individuals. Furthermore, patients undergoing alpha IFN (IFN-α) therapy display elevated levels of OAS activity in serum, and the levels correlate with the success of treating HCV infection by pegylated IFN-α (19, 26, 35).
The OAS1 to -3 genes encode proteins with a characteristic polymerase activity, the 2′-5′ oligoadenylate (2-5A) synthetase activity (16), whereas the OASL protein has significant sequence similarity to the other OAS proteins but is devoid of this enzymatic activity (23). The OAS1 to -3 proteins contain one to three repeats of the basal “OAS unit,” and OASL contains one (4, 13, 15, 22, 30, 31). The OAS1 to -3 proteins are all produced as latent enzymes, which are activated to synthesize 2-5As upon binding to double-stranded RNA (dsRNA) (3). The 2-5As subsequently bind and activate the latent RNase, RNase L (11). Activation of RNase L leads to degradation of cellular as well as viral RNA, resulting in the inhibition of protein synthesis, thus terminating viral replication (7). The generation of small RNAs by RNase L cleavage can further enhance the innate immune response, as they are recognized by the pattern recognition receptors, RIG-I and MDA-5 (21).
The crystal structure of porcine p40 OAS1 revealed an enzyme, which is related to the Pol β family despite being 2′ specific (14). Three aspartic acid residues, D74, D76, and D147, form the catalytic triad of the enzyme. These residues coordinate two Mg2+ ions, which are critical for catalysis. Residues R209 and R212, which are located in a helix opposite the catalytic triad, neutralize the negative charge of the triphosphates of the incoming ATP and facilitates catalysis (14, 37). The RNA binding site of OAS is comprised of a positively charged groove, which interacts with the dsRNA through arginine and lysine residues (R38, K41, K59, R194, R198). A strain containing a simultaneous mutation of these five residues to glutamic acid (referred to as the C5 mutant in this paper) yielded a protein with minimal affinity toward dsRNA and no enzymatic activity (14).
Overexpression of OAS1 in cell culture has demonstrated antiviral activity against encephalomyocarditis virus (EMCV) (8, 33) and mengovirus (6). A recent study used retroviral vectors to overexpress all known isoforms of OAS in human A549 cells and showed that expression of both OAS1 (the p42 and p46 splice isoforms) and OAS3 resulted in inhibition of West Nile virus (WNV) replication in an RNase L-dependent fashion (20). Interestingly, rodents have multiple duplications of the Oas1 gene, and the murine Oas1b gene was shown to be important for resistance toward WNV infection (24, 28). This activity did not appear to require RNase L, as expression of Oas1b in RNase L-negative cells still resulted in antiviral activity (18).
The observation of OAS in the sera of patients suffering from various viral infections, or undergoing IFN treatment, prompted us to investigate whether exogenous OAS could protect cells from infection with virus. We found that exogenous recombinant porcine OAS1 enters into cells and exhibits broad-spectrum antiviral activity. This protection is not mediated through the activation of the IFN system and is independent of RNase L. Moreover, the injection of recombinant OAS1 into mice strongly limited viral replication compared to that in relevant controls, demonstrating this mechanism to be operative in vivo and hence to have a therapeutic potential.
MATERIALS AND METHODS
Cells and viruses.
Parental cell lines used in this study were HepG2 (human carcinoma hepatocytes), HT1080 (human fibrosarcoma cells), Vero cells (African green monkey kidney cells) and mouse embryonic fibroblasts (MEFs) from C57BL/6 mice. The derived cell line used was RNase L−/− MEFs (42). The cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Sigma-Aldrich) with 10% fetal bovine serum (FBS, Sigma-Aldrich) and 100 U/ml of penicillin and 100 μg/ml streptomycin (Invitrogen). Viruses used were EMCV (VR-129B strain), vesicular stomatitis virus (VSV) (New Jersey strain), herpes simplex virus type 2 (HSV-2) (MS strain), and Sendai virus (SeV) (Cantrell strain).
Mice, infection, and in vivo treatment.
C57BL/6 mice were purchased, maintained, and infected as described in reference 2. Briefly, 2 h prior to infection, mice were injected intraperitoneally (i.p.) with 5 mg/kg body weight OAS1 wild-type cells (WT), C5 mutant cells, or phosphate-buffered saline (PBS). Mice were infected i.p. with 1 × 103 PFU EMCV. At 24 h postinfection, treatments were repeated, and at 48 h postinfection the mice were sacrificed and the hearts were harvested and frozen at −70°C for subsequent virus titration. Virus titers were measured as described previously (2). These experiments were done under Danish animal research permit 2009-2014; #2009/516-1613.
MTT assay.
The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay was performed as described in reference 9. The treatments used in this study were recombinant porcine OAS1 (WT or mutants, 200 μg/ml), human IFN-α (Chemicon) (1,000 U/ml), human recombinant IFN-λ3 (400 ng/ml) (9), murine IFN-α (PBL InterferonSource) (100 U/ml), recombinant Schizosaccharomyces pombe Pop2p (200 μg/ml), dialysis buffer (mock, 1:2 dilution), recombinant OAS1-enhanced green fluorescent protein (EGFP) (200 μg/ml), and lipopolysaccharide (LPS) (Escherichia coli; Sigma-Aldrich) (50 ng/ml). Cells were seeded at a concentration of 1 × 104 cells/well in 96 wells and incubated with treatments. The treatments were subjected to a 2-fold dilution series (or 0.67-fold for MEFs and Vero cells). Twenty-four hours posttreatment, cells were infected with EMCV at a multiplicity of infection (MOI) of 0.04 (HepG2 and Vero) or an MOI of 0.004 (MEFs) and incubated for 20 h; MTT (Sigma-Aldrich) was added, and cells were incubated for an additional 4 h. Cells were dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich). MTT conversion was determined by measurement of the A595 with background at 655 nm subtracted. Data were treated as described in reference 9.
Virus yield assay.
Vero cells were seeded at 4 × 105 cells/well in 12 wells, incubated for 4 to 6 h, and treated with either recombinant OAS1 (100 μg/ml), C5 mutant (100 μg/ml), or human IFN-α (500 U/ml) or left untreated for 24 h. Supernatants were removed, and cells were washed with PBS. Cells were infected with the indicated virus at an MOI of 0.001 in DMEM with antibiotics and 2% FBS for 1 h; media were exchanged for DMEM with 10% FBS, and the mixtures were incubated for 24 h (VSV and EMCV) or 48 h (HSV). Virus supernatants were harvested by freeze-thawing and centrifuged for 10 min at 1,000 rpm. Virus titers were measured by a 50% tissue culture infective dose (TCID50) assay.
Cell stimulations, RNA extraction, cDNA synthesis, and real-time quantitative PCR.
HT1080 cells were seeded at 1 × 106 cells/well 24 h prior to treatment with OAS1 (100 μg/ml), Pop2p (100 μg/ml), or human IFN-α (1,000 U/ml) or infection with SeV (MOI, 1) or left untreated. Cells were incubated for 4 h (protein treatments) or 6 h (SeV) and collected using Trizol reagent (Invitrogen). RNA extraction, cDNA synthesis, and analysis by real-time quantitative PCR were performed as previously described (25). Primer sequences are listed in Table S4 in the supplemental material.
Cloning and mutagenesis.
The porcine OAS1 cDNA was cloned and mutants were generated as described previously (14, 37). EGFP from pcDNA3.1/V5-HisA-EGFP was cloned into the BamHI and XbaI sites of the pET9d expression vector encoding the C-terminal 6×His-tagged OAS1 WT. Pop2p was cloned as described previously (17).
Protein expression and purification.
OAS1 WT, mutants, and OAS1-EGFP were expressed in E. coli BL21(DE3) (Novagen) and Pop2p in E. coli Rosetta (DE3) (Novagen). Purifications and protein activity assays were performed as described previously (37).
Imaging.
Vero cells were seeded at 5 × 105 cells/well in 6-well multidishes and incubated for 2 h prior to treatment with OAS1-EGFP (75 μg/ml) for 2 to 3 hours. Cells were then trypsinated and reseeded at 1 × 104 cells/well on coverslips in 24-well multidishes and allowed to settle (a total of 4 h prior to further staining). Cell culture medium was removed, and cells were washed three to six times with PBS. Cells were fixed using 4% paraformaldehyde, pH 7.4, in PBS for 20 min and stained with DAPI (4′,6-diamidino-2-phenylindole) (30 μM) (Invitrogen). Cells were mounted on coverslips using ProlongGold mounting medium (Invitrogen). Cells were visualized using confocal microscopy (LSM710 Zeiss microscope). Images were recorded and processed using Zen 2008 software.
RESULTS
Addition of exogenous recombinant OAS1 protects cells against EMCV-induced cytopathology independent of a functional type I interferon system.
We have adapted the classical antiviral assay described by Rubenstein et al. (32), originally developed to evaluate the antiviral activity of IFN, to measure the activity of recombinant OAS1. The assay evaluates the ability of an antiviral compound, when added to the cell culture, to protect against the cytotoxicity induced by infection with virus. In our experiments, cells were treated with increasing doses of recombinant porcine OAS1 and subsequently infected with EMCV.
Figure 1A shows the antiviral protection of recombinant OAS1 in the hepatocyte cell line HepG2. The cells were treated with both OAS1 and the S. pombe protein Pop2p. OAS1 induced a clear, dose-dependent protection of the cells against the cytopathic effect of EMCV. The 50% effective concentration (EC50) was 24.5 μg/ml, and the maximal cell survival was 94% of the noninfected cell control. Data and statistical analysis obtained from the OAS1 antiviral activity assays are presented in Fig. S1 and Table S1 in the supplemental material. Pop2p, a yeast RNA metabolic protein without any known antiviral activity (17), was purified using a protocol identical to that for OAS1 and served as a negative-control protein. Extracellular Pop2p induced no measurable antiviral activity, even at high concentrations (Fig. 1A). To assess the requirement for type I IFN in the antiviral activity of OAS1, we repeated the antiviral assay with Vero cells (Fig. 1B), which are incapable of producing type I IFN (10). Similar to the situation with HepG2 cells, recombinant OAS1 exhibited antiviral activity in Vero cells, with an EC50 equal to 18.5 μg/ml and maximum cell survival of 78%. Thus, a functional type I IFN system is not required. Furthermore, heat-denatured OAS1 or LPS stimulation of Vero cells did not induce protection against virus-induced cytotoxicity (Fig. 1B and C), but the positive control IFN-α did (Fig. 1D).
FIG. 1.
Antiviral protection of exogenous OAS in vitro and in vivo. (A to D) OAS1 in vitro. HepG2 (A) and Vero (B to D) cells were treated with recombinant porcine OAS1, Pop2p, heat-denatured OAS1, LPS, or IFN-α for 24 h and subsequently infected with EMCV. Cell survival was analyzed using the conversion of MTT to formazan in living cells, measuring the A595. Data are presented as a percentage of a noninfected cell control. Error bars depict standard deviations of results of four independent experiments. Data represent results of multiple experiments with similar results. (E) OAS1 in vivo. On day 1, C57BL/6 mice (n = 6) were treated with OAS1, C5 mutant (R38E/K41E/K59E/R194E/R198E), or PBS (mock) 2 h prior to i.p. infection with 1 × 103 PFU EMCV. Treatments were repeated on day 2, hearts were harvested on day 3, and viral titers were determined. Data were analyzed by a Mann-Whitney non-Gaussian one-tailed test with a 99% confidence interval. See also Fig. S1 and Table S1 in the supplemental material. EID50, 50% egg infective dose.
In conclusion, these data demonstrate a novel antiviral activity of OAS1 added exogenously to cell cultures. This protection cannot be attributed to contaminating LPS or dsRNA, nor is it mediated through the production of type I IFN.
In vivo antiviral activity of exogenous recombinant OAS1.
Next, we asked if OAS1 was able to exert the antiviral activity in vivo. We did this by testing the ability of the OAS1 protein to protect mice against EMCV infection. Mice were treated intraperitoneally (i.p.) with 5 mg/kg body weight of OAS1 or the C5 mutant, incubated for 2 h, and infected i.p. with 1 × 103 PFU EMCV. The protein treatments were repeated 24 h postinfection; mice were sacrificed 24 h later, and the viral load in their hearts was determined. As seen in Fig. 1E, OAS1 decreased the viral load in hearts 10-fold compared to that of mock-treated mice (P = 0.0022). The C5 mutant did not exhibit any protection. Thus, extracellular OAS1 protein possesses an antiviral activity in vivo as well as in vitro.
OAS1 inhibits replication of widely different classes of viruses.
We tested the antiviral activity of recombinant OAS1 against vesicular stomatitis virus (VSV), herpes simplex virus type 2 (HSV-2), and EMCV in a virus yield assay (Fig. 2). We treated Vero cells with OAS1, IFN-α, or the C5 mutant prior to virus infection. IFN-α was included as a positive control. After infection, the supernatants were titrated and the TCID50/ml was calculated. As shown in Fig. 2 and in Table S2 in the supplemental material, recombinant OAS1 reduced the virus titers of VSV 12-fold, HSV-2 6-fold, and EMCV 30-fold. The C5 mutant did not reduce the viral output, and as expected, IFN-α lowered the yield of EMCV 1,000-fold and that of VSV 100-fold but had no apparent effect on HSV-2 replication.
FIG. 2.
Antiviral protection of OAS1 against EMCV, VSV, and HSV-2. Vero cells were treated with OAS1 WT, the OAS1 RNA binding site mutant, C5 (R38E/K41E/K59E/R194E/R198E), or IFN-α or left untreated (UT) as a control (n = 3). Cells were incubated for 24 h, washed extensively to remove access or unbound protein, and infected with the indicated virus at an MOI of 0.001. The infection was allowed to occur until substantial cytopathic effect was observed in the UT samples, and supernatants were collected by freeze-thawing and subsequent centrifugation. Supernatants were titrated on Vero cells in 96 wells to determine TCID50/ml. Data were analyzed using a paired, one-tailed t test. The data are paired, as the individual replicates were titrated together. See also Table S2 in the supplemental material.
OAS1 treatment does not induce transcriptional activation of typical antiviral genes.
Contamination of the recombinant OAS1 with E. coli-derived pathogen-associated molecular patterns (PAMPs), such as dsRNA or LPS, would lead to transcriptional induction of innate immunity genes and thus could give rise to the observed antiviral activity. In addition, we could not beforehand exclude a possible activation of innate immunity signaling pathways by the OAS1 protein. Therefore, we analyzed the mRNA levels of four genes that are central to the antiviral response, IFNB, ISG56, OASL, and OAS1, following treatment with OAS1, Pop2p, or IFN-α or infection with Sendai virus (SeV). We used both HepG2 cells and HT1080 cells, which are known to be highly sensitive to both LPS and dsRNA (25, 36) and therefore should respond to even low levels of contamination in the OAS1 protein preparations. SeV infection induced IFNB, ISG56, and OASL transcription: IFNB more than 1,000-fold and ISG56 and OASL more than 100-fold. As expected, IFN-α treatment did not induce IFNB, but ISG56, OASL, and OAS1 transcript levels were induced 100-fold compared to those of the untreated sample. Neither the negative control Pop2p nor OAS1 treatment resulted in any significant change of mRNA levels (Fig. 3). Similar results were obtained with HepG2 cells (data not shown). Thus, treatment with recombinant OAS1 did not result in the induction of any of the typical antiviral genes, and therefore it is highly unlikely that contamination of our protein preparations is the source of the observed antiviral protection.
FIG. 3.
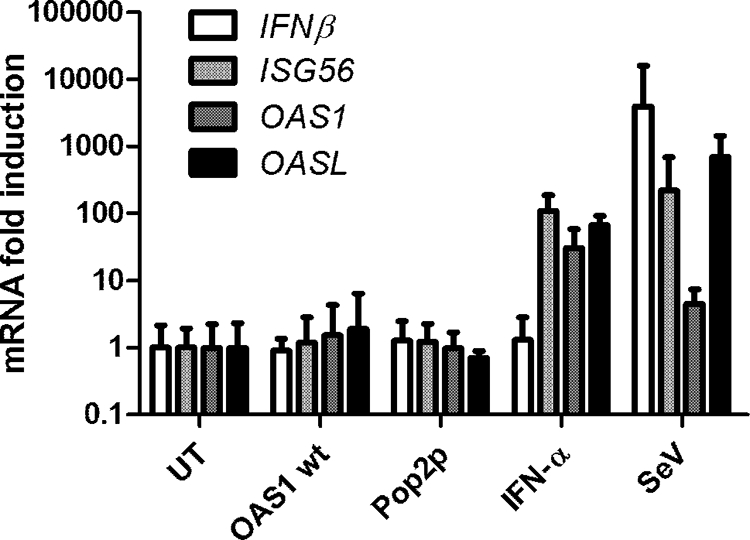
Antiviral protection of exogenous OAS1 is not mediated through IFN signaling. HT1080 cells were treated with Pop2p, OAS1WT, or IFN-α or left untreated as a control (UT) for 4 h or infected with SeV for 6 h. Cells were harvested, RNA purified, cDNA synthesized, and subjected to quantitative PCR using gene-specific primers for IFNB, ISG56, OASL, OAS1, and GAPDH as a reference. Data are presented relative to results for the untreated sample, and error bars depict standard deviations (n = 3).
Uptake of an OAS1-EGFP fusion protein by cells.
In order to elucidate the fate of exogenously added OAS1, we constructed an OAS1-EGFP fusion protein. The EGFP protein was fused to the C terminus of the OAS1 protein. As shown in Table S3 in the supplemental material, the fusion protein displayed enzymatic activity, although at a lower level than that of the untagged enzyme. In addition, the fusion protein displayed antiviral activity against EMCV, with an EC50 of 67.1 μg/ml and maximal cell survival of 85%. Vero cells were incubated with OAS1-EGFP for 4 h; the cells were washed thoroughly to remove excess protein, trypsinated, and reseeded. The cells were then fixed and the cell nucleus was stained using DAPI. The cells were visualized using confocal microscopy. Figure 4 shows the presence of OAS1-EGFP within the cell. We also tested a fusion protein of the OAS1 mutant C5 and EGFP (C5-EGFP); this protein is not taken up by cells. Prior studies of the HIV TAT protein have demonstrated that this protein can cross the cellular membrane and that a stretch of arginine and lysine residues is required for TAT to cross the cell membrane (38). As the C5 mutant has five arginine/lysine residues replaced by glutamic acid, it appears that the basic nature of the OAS1 protein is required in order for the protein to be able to cross the cell membrane.
FIG. 4.
Uptake of an OAS-EGFP fusion protein. Vero cells were treated with OAS1-EGFP (green) (upper panels) or C5-EGFP (middle panels) or left untreated (UT) (lower panels) for 4 h, trypsinated, and reseeded on coverslips. Supernatants were removed, cells were washed thoroughly with PBS and fixed, and the nuclei were stained with DAPI (blue). Blue, nucleus; green, OAS1-EGFP. Merge indicates merged results. Cells were visualized using confocal microscopy.
The antiviral activity of OAS1 does not require its 2-5A enzymatic activity.
The active site and the RNA binding site of OAS1 have been characterized in detail (14, 37). In order to elucidate the molecular mechanism by which OAS1 exerts protection, we selected a number of well-described OAS1 mutants, all of which exhibit little or no 2-5A synthetase activity. We expressed and purified these mutants, tested the enzymatic activity, and subsequently analyzed the antiviral activity against the cytopathology induced by EMCV, as described in Fig. 1. The data are presented in Fig. 5A and in Table S3 in the supplemental material.
FIG. 5.

Antiviral activity of exogenous OAS1 is independent of the 2-5A activity and RNase L. HepG2 (A) and MEF (B) cells were treated with recombinant OAS1 or mutants for 24 h and subsequently infected with EMCV. Cell survival was analyzed using the conversion of MTT to formazan in living cells, measuring the A595. Data are presented as a percentage of results for a noninfected cell control. Error bars depict standard deviations (n = 4). See also Table S3 in the supplemental material.
Figure 5A demonstrates the antiviral activity of recombinant OAS1 and two selected mutants, D74A/D76A and C5 (R38E/K41E/K59E/R194E/R198E). The D74A/D76A mutant is enzymatically inactive (see Table S3 in the supplemental material) (34). However, in our antiviral assay it displayed protection almost identical to that of the wild-type protein. The C5 mutant exhibits no significant protection. Table S3 lists the tested mutants, with a description of the mutated residues, their 2-5A activity (as a percentage of the WT protein), and the EC50 and efficacy obtained in the antiviral assay. We did not observe any correlation between the antiviral activity and the 2-5A synthetase activity.
The RNase L RNA degradation pathway is not required for the antiviral protection of exogenous OAS.
To confirm the results found in the mutational study, we tested the antiviral activity of recombinant OAS1 in wild-type and RNase L-deficient (RNase L−/−) mouse fibroblasts (42) (Fig. 5B). OAS1 induced clear protection in both the WT and RNase L−/− cells, with efficacies of 67% (WT) and 78% (RNase L−/−) and EC50s of 44.5 μg/ml and 74.5 μg/ml, respectively. Thus, the results presented in Fig. 5 and in Table S3 in the supplemental material demonstrate the existence of a novel alternative antiviral pathway of the OAS1 protein, independent of RNase L. Until we have further molecular details on this pathway, we will simply refer to it as the alternative pathway.
DISCUSSION
Our data demonstrate that exogenously added recombinant OAS1 is internalized into cultured cells and exerts a potent antiviral effect both in vivo and in vitro. In vitro, the OAS1 protein protected cells from the cytopathology induced by EMCV in a dose-dependent manner, and it clearly reduced the production of infectious virions from both EMCV- and VSV-infected cells. HSV-2 was reduced to a lower extent (6-fold), but the effect was still significant (P < 0.035). Using a murine model for EMCV infection, we demonstrated a potent in vivo antiviral activity of the OAS1 protein. This experiment validates our in vitro findings and raises the question of possible therapeutic applications of OAS. However, this needs further investigation.
Our first and primary concerns were the possibility of contaminating PAMPs such as LPS or dsRNA originating from the E. coli expression system. However, we were able to eliminate contaminating E. coli PAMPs as the cause of the antiviral activity observed by several independent means. We included three classical negative controls: heat-denaturized OAS1 protein, the Pop2p protein purified in a manner identical to that for OAS1, and the C5 mutant, which was devoid of antiviral activity. All of the controls were negative, indicating a direct antiviral effect of the OAS1 protein. We also addressed the possibility that the OAS1 protein, or contaminants, could activate the transcription of antiviral genes by analyzing the mRNA levels of four genes (IFNB, ISG56, OASL, and OAS1) following treatment with OAS1. All four genes are known to be strongly induced by virus infection or treatment with dsRNA or IFN-α (12, 27). We performed this experiment with both HepG2 cells, which we used for determining the antiviral activity, and the cell line HT1080. HT1080 cells are highly sensitive to treatment with various PAMPs. We did get highly similar results for the two cell lines. OAS1 treatment did not lead to an increase of the mRNA levels of any of the tested genes. These data argue for a direct effect of the OAS1 protein on the viral growth and against contamination with E. coli-derived PAMPs. Therefore, we believe that our observations are best explained by an effect of the OAS1 protein on specific steps in the viral replication cycle. The broad effect observed across the different virus models tested here (EMCV, VSV, and HSV-2) suggests that OAS1 targets steps in the replication shared by most (if not all) viruses.
The OAS family of proteins mediates antiviral activity via the synthesis of 2-5A and subsequent activation of RNase L. This pathway leads to the inhibition of protein synthesis within the infected cell and thus can potentially affect all viruses. However, in our case this would require uptake of the OAS1 protein by cells and its release into the cytoplasm. By using a fusion protein of OAS1-EGFP, we demonstrated that the protein was internalized into cells, but whether it remained in endosomes or was released to the cytoplasm of the cell is not clear. We tested the importance of the RNase L pathway by using mutants of OAS1, which is devoid of enzymatic activity, and by using RNase L-negative cells. Both experiments demonstrated an RNase L-independent effect of the exogenously added OAS1 protein and thus the existence of a novel RNase L-independent mechanism.
Our observations challenge the traditional view of the function of OAS1, and we carefully considered the in vivo physiological relevance of the findings of this study. Several studies report the presence of OAS activity in the sera of patients with viral infections and following treatment with IFN (19, 26, 35). Furthermore, a clear correlation was found between the levels of OAS activity in the sera of patients undergoing IFN therapy and the success of the therapy. This correlation is interesting, and it suggests a biological relevance of extracellular OAS. It is difficult to relate the reported OAS activity in serum directly to protein levels, but it appears that serum levels of OAS in IFN-treated HCV patients are substantially lower than the EC50s we measured (10- to 1,000-fold lower). However, we find it quite plausible that the serum concentration of free OAS in hepatitis C patients is substantially lower than what is found in the infected liver. We suggest a model where infection leads to high intracellular levels of OAS protein, which is then released to the extracellular environment and will help protect neighboring cells from infection. The release of OAS could act as a specific signal, indicating viral lysis of cells and distinguishing viral lysis from tissue damage of other origin. We believe that this mechanism works locally at the site of infection, not globally.
Supplementary Material
Acknowledgments
R.H. is supported by grants from the Danish Cancer Society, the Danish Medical Research Council (grant 49 63 04), Novo Nordisk Foundation, and the Carlsberg Foundation.
The RNase L-negative cells were generously donated by Robert H. Silverman.
Author contributions: H.K., S.R.P., and R.H. designed the research, with critical suggestions from C.A.S., M.M., S.P.I., and F.W. H.K., S.V., K.T., T.B.S., T.K., K.A.H., and S.R.P. performed the experiments. H.K. and R.H. wrote the manuscript, with critical comments from the remaining authors.
Footnotes
Published ahead of print on 15 September 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 2.Ank, N., H. West, C. Bartholdy, K. Eriksson, A. R. Thomsen, and S. R. Paludan. 2006. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J. Virol. 80:4501-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baglioni, C., M. A. Minks, and E. De Clercq. 1981. Structural requirements of polynucleotides for the activation of (2′ - 5′)An polymerase and protein kinase. Nucleic Acids Res. 9:4939-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benech, P., Y. Mory, M. Revel, and J. Chebath. 1985. Structure of two forms of the interferon-induced (2′-5′) oligo A synthetase of human cells based on cDNAs and gene sequences. EMBO J. 4:2249-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benech, P., M. Vigneron, D. Peretz, M. Revel, and J. Chebath. 1987. Interferon-responsive regulatory elements in the promoter of the human 2′,5′-oligo(A) synthetase gene. Mol. Cell. Biol. 7:4498-4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chebath, J., P. Benech, M. Revel, and M. Vigneron. 1987. Constitutive expression of (2′-5′) oligo A synthetase confers resistance to picornavirus infection. Nature 330:587-588. [DOI] [PubMed] [Google Scholar]
- 7.Clemens, M. J., and B. R. Williams. 1978. Inhibition of cell-free protein synthesis by pppA2′p5′A2′p5′A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell 13:565-572. [DOI] [PubMed] [Google Scholar]
- 8.Coccia, E. M., G. Romeo, A. Nissim, G. Marziali, R. Albertini, E. Affabris, A. Battistini, G. Fiorucci, R. Orsatti, G. B. Rossi, et al. 1990. A full-length murine 2-5A synthetase cDNA transfected in NIH-3T3 cells impairs EMCV but not VSV replication. Virology 179:228-233. [DOI] [PubMed] [Google Scholar]
- 9.Dellgren, C., H. H. Gad, O. J. Hamming, J. Melchjorsen, and R. Hartmann. 2009. Human interferon-lambda3 is a potent member of the type III interferon family. Genes Immun. 10:125-131. [DOI] [PubMed] [Google Scholar]
- 10.Desmyter, J., J. L. Melnick, and W. E. Rawls. 1968. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero). J. Virol. 2:955-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong, B., and R. H. Silverman. 1997. A bipartite model of 2-5A-dependent RNase L. J. Biol. Chem. 272:22236-22242. [DOI] [PubMed] [Google Scholar]
- 12.Elco, C. P., J. M. Guenther, B. R. Williams, and G. C. Sen. 2005. Analysis of genes induced by Sendai virus infection of mutant cell lines reveals essential roles of interferon regulatory factor 3, NF-kappaB, and interferon but not toll-like receptor 3. J. Virol. 79:3920-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh, S. K., J. Kusari, S. K. Bandyopadhyay, H. Samanta, R. Kumar, and G. C. Sen. 1991. Cloning, sequencing, and expression of two murine 2′-5′-oligoadenylate synthetases. Structure-function relationships. J. Biol. Chem. 266:15293-15299. [PubMed] [Google Scholar]
- 14.Hartmann, R., J. Justesen, S. N. Sarkar, G. C. Sen, and V. C. Yee. 2003. Crystal structure of the 2′-specific and double-stranded RNA-activated interferon-induced antiviral protein 2′-5′-oligoadenylate synthetase. Mol. Cell 12:1173-1185. [DOI] [PubMed] [Google Scholar]
- 15.Hartmann, R., H. S. Olsen, S. Widder, R. Jorgensen, and J. Justesen. 1998. p59OASL, a 2′-5′ oligoadenylate synthetase like protein: a novel human gene related to the 2′-5′ oligoadenylate synthetase family. Nucleic Acids Res. 26:4121-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hovanessian, A. G., and I. M. Kerr. 1978. Synthesis of an oligonucleotide inhibitor of protein synthesis in rabbit reticulocyte lysates analogous to that formed in extracts from interferon-treated cells. Eur. J. Biochem. 84:149-159. [DOI] [PubMed] [Google Scholar]
- 17.Jonstrup, A. T., K. R. Andersen, L. B. Van, and D. E. Brodersen. 2007. The 1.4-A crystal structure of the S. pombe Pop2p deadenylase subunit unveils the configuration of an active enzyme. Nucleic Acids Res. 35:3153-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kajaste-Rudnitski, A., T. Mashimo, M. P. Frenkiel, J. L. Guenet, M. Lucas, and P. Despres. 2006. The 2′,5′-oligoadenylate synthetase 1b is a potent inhibitor of West Nile virus replication inside infected cells. J. Biol. Chem. 281:4624-4637. [DOI] [PubMed] [Google Scholar]
- 19.Kim, K. I., S. R. Kim, N. Sasase, M. Taniguchi, S. Harada, K. Kinoshita, S. H. Kim, Y. Akimoto, M. Shikata, N. Kimura, S. Izawa, A. Ohtani, K. Nakao, M. Motojima, M. Kinoshita, M. Hirai, M. Ohzu, T. Hirooka, S. Nabeshima, F. Ishii, K. Tanaka, and H. Hotta. 2006. 2′-,5′-Oligoadenylate synthetase response ratio predicting virological response to PEG-interferon-alpha2b plus ribavirin therapy in patients with chronic hepatitis C. J. Clin. Pharm. Ther. 31:441-446. [DOI] [PubMed] [Google Scholar]
- 20.Lin, R. J., H. P. Yu, B. L. Chang, W. C. Tang, C. L. Liao, and Y. L. Lin. 2009. Distinct antiviral roles for human 2′,5′-oligoadenylate synthetase family members against dengue virus infection. J. Immunol. 183:8035-8043. [DOI] [PubMed] [Google Scholar]
- 21.Malathi, K., B. Dong, M. Gale, Jr., and R. H. Silverman. 2007. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature 448:816-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marie, I., and A. G. Hovanessian. 1992. The 69-kDa 2-5A synthetase is composed of two homologous and adjacent functional domains. J. Biol. Chem. 267:9933-9939. [PubMed] [Google Scholar]
- 23.Marques, J., J. Anwar, S. Eskildsen-Larsen, D. Rebouillat, S. R. Paludan, G. Sen, B. R. Williams, and R. Hartmann. 2008. The p59 oligoadenylate synthetase-like protein possesses antiviral activity that requires the C-terminal ubiquitin-like domain. J. Gen. Virol. 89:2767-2772. [DOI] [PubMed] [Google Scholar]
- 24.Mashimo, T., M. Lucas, D. Simon-Chazottes, M. P. Frenkiel, X. Montagutelli, P. E. Ceccaldi, V. Deubel, J. L. Guenet, and P. Despres. 2002. A nonsense mutation in the gene encoding 2′-5′-oligoadenylate synthetase/L1 isoform is associated with West Nile virus susceptibility in laboratory mice. Proc. Natl. Acad. Sci. U. S. A. 99:11311-11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melchjorsen, J., H. Kristiansen, R. Christiansen, J. Rintahaka, S. Matikainen, S. R. Paludan, and R. Hartmann. 2009. Differential regulation of the OASL and OAS1 genes in response to viral infections. J. Interferon Cytokine Res. 29:199-208. [DOI] [PubMed] [Google Scholar]
- 26.Mihm, U., O. Ackermann, C. Welsch, E. Herrmann, W. P. Hofmann, N. Grigorian, M. W. Welker, T. Lengauer, S. Zeuzem, and C. Sarrazin. 2009. Clinical relevance of the 2′-5′-oligoadenylate synthetase/RNase L system for treatment response in chronic hepatitis C. J. Hepatol. 50:49-58. [DOI] [PubMed] [Google Scholar]
- 27.Nakaya, T., M. Sato, N. Hata, M. Asagiri, H. Suemori, S. Noguchi, N. Tanaka, and T. Taniguchi. 2001. Gene induction pathways mediated by distinct IRFs during viral infection. Biochem. Biophys. Res. Commun. 283:1150-1156. [DOI] [PubMed] [Google Scholar]
- 28.Perelygin, A. A., S. V. Scherbik, I. B. Zhulin, B. M. Stockman, Y. Li, and M. A. Brinton. 2002. Positional cloning of the murine flavivirus resistance gene. Proc. Natl. Acad. Sci. U. S. A. 99:9322-9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Randall, R. E., and S. Goodbourn. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89:1-47. [DOI] [PubMed] [Google Scholar]
- 30.Rebouillat, D., A. Hovnanian, I. Marie, and A. G. Hovanessian. 1999. The 100-kDa 2′,5′-oligoadenylate synthetase catalyzing preferentially the synthesis of dimeric pppA2′p5′A molecules is composed of three homologous domains. J. Biol. Chem. 274:1557-1565. [DOI] [PubMed] [Google Scholar]
- 31.Rebouillat, D., I. Marie, and A. G. Hovanessian. 1998. Molecular cloning and characterization of two related and interferon-induced 56-kDa and 30-kDa proteins highly similar to 2′-5′ oligoadenylate synthetase. Eur. J. Biochem. 257:319-330. [DOI] [PubMed] [Google Scholar]
- 32.Rubinstein, S., P. C. Familletti, and S. Pestka. 1981. Convenient assay for interferons. J. Virol. 37:755-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rysiecki, G., D. R. Gewert, and B. R. Williams. 1989. Constitutive expression of a 2′,5′-oligoadenylate synthetase cDNA results in increased antiviral activity and growth suppression. J. Interferon Res. 9:649-657. [DOI] [PubMed] [Google Scholar]
- 34.Sarkar, S. N., A. Ghosh, H. W. Wang, S. S. Sung, and G. C. Sen. 1999. The nature of the catalytic domain of 2′-5′-oligoadenylate synthetases. J. Biol. Chem. 274:25535-25542. [DOI] [PubMed] [Google Scholar]
- 35.Shindo, M., K. Hamada, T. Morikawa, Y. Harano, T. Nakajima, and T. Okuno. 2008. In vivo interferon system assessed by 2′-5′ oligoadenylate synthetase activity in chronic hepatitis C virus patients treated with pegylated interferon and ribavirin. Hepatol. Res. 38:1213-1220. [DOI] [PubMed] [Google Scholar]
- 36.Sun, Y., and D. W. Leaman. 2004. Ectopic expression of toll-like receptor-3 (TLR-3) overcomes the double-stranded RNA (dsRNA) signaling defects of P2.1 cells. J. Interferon Cytokine Res. 24:350-361. [DOI] [PubMed] [Google Scholar]
- 37.Torralba, S., J. Sojat, and R. Hartmann. 2008. 2′-5′ oligoadenylate synthetase shares active site architecture with the archaeal CCA-adding enzyme. Cell. Mol. Life Sci. 65:2613-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vives, E., P. Brodin, and B. Lebleu. 1997. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 272:16010-16017. [DOI] [PubMed] [Google Scholar]
- 39.Williams, B. R., R. R. Golgher, R. E. Brown, C. S. Gilbert, and I. M. Kerr. 1979. Natural occurrence of 2-5A in interferon-treated EMC virus-infected L cells. Nature 282:582-586. [DOI] [PubMed] [Google Scholar]
- 40.Yoneyama, M., and T. Fujita. 2010. Recognition of viral nucleic acids in innate immunity. Rev. Med. Virol. 20:4-22. [DOI] [PubMed] [Google Scholar]
- 41.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 42.Zhou, A., J. Paranjape, T. L. Brown, H. Nie, S. Naik, B. Dong, A. Chang, B. Trapp, R. Fairchild, C. Colmenares, and R. H. Silverman. 1997. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. EMBO J. 16:6355-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





