Abstract
Rhesus rhadinovirus (RRV) is a gammaherpesvirus closely related to Kaposi's sarcoma-associated herpesvirus (KSHV), an oncogenic virus linked to the development of Kaposi's sarcoma and several other lymphoproliferative diseases, including primary effusion lymphoma and multicentric Castleman's disease. RRV naturally infects rhesus macaques and induces lymphoproliferative diseases under experimental conditions, making it an excellent model for the study of KSHV. Unlike KSHV, which grows poorly in cell culture, RRV replicates efficiently in rhesus fibroblasts (RFs). In this study, we have characterized the entry pathway of RRV in RFs. Using a luciferase-expressing recombinant RRV (RRV-luciferase), we show that the infectivity of RRV is reduced by inhibitors of endosomal acidification. RRV infectivity is also reduced by inhibitors of clathrin-mediated but not caveola-mediated endocytosis, indicating that RRV enters into RFs via clathrin-mediated endocytosis. Using a red fluorescent protein (RFP)-expressing recombinant RRV (RRV-RFP), we show that RRV particles are colocalized with markers of endocytosis (early endosome antigen 1) and clathrin-mediated endocytosis (clathrin heavy chain) during entry into RFs. RRV particles are also colocalized with transferrin, which enters cells by clathrin-mediated endocytosis, but not with cholera toxin B, which enters cells by caveola-mediated endocytosis. Inhibition of clathrin-mediated endocytosis with a dominant-negative construct of EPS15, an essential component of clathrin-coated pits, blocked the entry of RRV into RFs. Together, these results indicate that RRV entry into RFs is mediated by clathrin-mediated endocytosis.
Kaposi's sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV8), is a gammaherpesvirus associated with the development of Kaposi's sarcoma, a malignancy commonly found in AIDS patients (13). KSHV is also associated with the development of multicentric Castleman's disease (MCD) and primary effusion lymphoma (PEL), two rare lymphoproliferative diseases. KSHV has a restricted host range, making it difficult to study KSHV and its related malignances directly in an animal model (25). Rhesus rhadinovirus (RRV) is closely related to KSHV. RRV infects its natural host and induces lymphoproliferative diseases resembling MCD and PEL; thus, it has been proposed as an animal model for the study of KSHV (19, 26, 39). Two isolates of RRV (26-95 and 17577) have been independently isolated and sequenced so far (3, 7, 32).
To establish a successful infection, a virus needs to enter the target cells and release its genome (20). Thus, defining the entry and trafficking pathway of RRV can help us understand its mechanism of infection and replication in vitro and in vivo. Herpesviruses bind to the cell surface through complex interactions between viral glycoproteins and receptor molecules, leading to either plasma membrane fusion or endocytosis (35). Plasma membrane fusion is a pH-independent event between the viral envelope and the host cell plasma membrane (23). Enveloped viruses also take advantage of cellular endocytosis pathways for their internalization (34). Endocytosis leads to fusion between the membrane of the internalized vesicle and the viral envelope at low pHs and to the release of the viral particle into the cytoplasm. Following membrane fusion, the nucleocapsid traffics to the perinuclear space and delivers the viral genome to the nucleus. Thus, endocytosis offers a convenient and fast transit system enabling the virus to enter and traffic across the plasma membrane and cytoplasm of the infected cell.
In mammalian cells, there are several endocytic pathways, including clathrin-mediated endocytosis, caveola-mediated endocytosis, clathrin- and caveola-independent endocytosis, and macropinocytosis (34). These endocytic pathways differ in the nature and size of the cargo. The clathrin-mediated pathway is the most commonly observed uptake pathway for viruses (30). A viral particle is internalized into a clathrin-coated vesicle, which then loses the clathrin-coated subunits before fusing with the early endosome. An activation step occurs in the endosome, leading to the fusion of the viral envelope with the endosomal membrane and the delivery of the viral capsid to the cytosol. The acidic pH in the endosome is thought to play an essential role in triggering the fusion event. Therefore, pH sensitivity is often considered an indication that a virus enters the cell by endocytosis (30).
KSHV has been shown to use clathrin-mediated endocytosis to enter human foreskin fibroblasts, activated primary human B cells, and primary human umbilical vein endothelial cells (1, 12, 29); however, the macropinocytic pathway and plasma membrane fusion pathway have also been implicated (17, 28). The mechanism of RRV entry into cells has not been defined. In this study, using two recombinant RRVs expressing luciferase (RRV-luciferase) and red fluorescent protein (RRV-RFP), respectively, we have characterized the entry pathway of RRV in rhesus fibroblasts (RFs), a cell type that RRV can infect efficiently and in which it can replicate. The results show that RRV entry into RFs occurs primarily via clathrin-mediated endocytosis.
MATERIALS AND METHODS
Cell culture and virus preparation.
The wild-type (WT) RRV isolate 26-95 was kindly provided by Ronald Desrosiers at the New England Primate Research Center. RRV-luciferase and RRV-RFP were generated by replacing open reading frame 49 (Orf49) with a secreted Gaussia luciferase cassette (Targeting Systems, El Cajon, CA) and fusing RFP with Orf65 at the N terminus, respectively, according to the protocol described previously (36, 41). Orf49 is a positional homologue of the Epstein-Barr virus BRRF1 gene (Na), while Orf65 encodes a small capsid protein. RRV-RFP replicates as efficiently as the WT RRV. While RRV-luciferase produced approximately 20-fold fewer infectious virions than the WT RRV (unpublished data), high-titer viral preparations were obtained for this study following concentration of supernatants from infected cultures as described below.
Recombinant viruses were grown in primary RFs or telomerase-immortalized RFs (TERFs). Primary RFs were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 20% fetal bovine serum (FBS) and 50 μg/ml of gentamicin. TERFs were grown in DMEM with 10% FBS and 5 μg/ml of puromycin. At day 5 postinfection, cells were lysed with three consecutive freeze-thaw cycles, and the supernatant was centrifuged first at 5,000 × g for 30 min to eliminate cell debris and then at 100,000 × g for 2 h using 20% sucrose as a cushion. The final pellet was dissolved in the culture medium overnight and was adjusted to a desired volume. Undissolved debris was eliminated by centrifugation at 5,000 × g for 10 min. All the procedures for virus concentration were carried out at 4°C. Virus preparations used for this study were concentrated 20-fold, and the concentrated virus preparations were aliquoted and stored at −80°C for later experiments.
Virus titration.
Culture supernatants or virus preparations containing infectious virions were subjected to 10-fold serial dilution in DMEM containing 20% FBS. RFs in 96-well plates were inoculated with 100 μl per well of the diluted virus or the culture medium alone as a negative control. Ten replicate wells were used for each dilution. After incubation at 37°C for 2 h, DMEM was added at 100 μl per well, and the cytopathic effect (CPE) was examined daily. The 50% tissue culture infective dose (TCID50) was determined as described previously (40).
Antibodies and chemicals.
A mouse monoclonal antibody to β-actin was obtained from Sigma (St. Louis, MO). A mouse monoclonal antibody to the clathrin heavy chain was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). A fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody to early endosome antigen 1 (EEA1) was purchased from BD Biosciences (San Jose, CA). A rabbit antibody to RFP was a kind gift from Guangming Zhong at the University of Texas Health Science Center at San Antonio. Alexa Fluor 488-phalloidin, Alexa Fluor 488-transferrin, Alexa Fluor 568-transferrin, the Alexa Fluor 488-cholera toxin B (CTB) conjugate, the Alexa Fluor 594-CTB conjugate, and the secondary antibodies, Alexa Fluor 488-conjugated goat anti-mouse IgG and Alexa Fluor 568-conjugated goat anti-rabbit IgG, were from Molecular Probes, Invitrogen (Carlsbad, CA). Heparin and inhibitors of endosomal acidification bafilomycin A1 and monensin were obtained from Sigma, and NH4Cl was purchased from Fisher Scientific (Pittsburgh, PA). The inhibitors of clathrin assembly chlorpromazine and dextrose, and the inhibitors of caveola-mediated endocytosis filipin, nystatin, and methyl-β-cyclodextrin (MβCD), were purchased from Sigma. All inhibitors were prepared according to the manufacturer's directions. 4′,6-Diamidino-2-phenylindole (DAPI) was purchased from Sigma. A propidium iodide (PI) labeling kit was obtained from Roche (Nutley, NJ).
Monitoring of RRV infectivity by a luciferase assay.
RFs pretreated with inhibitors were inoculated with RRV-luciferase at a multiplicity of infection (MOI) of 2 in the presence of the inhibitors for 1 h. Cells were washed three times and were incubated with media containing the inhibitors. Supernatants were collected at different hours postinfection (hpi), and their luciferase activities were measured using a Gaussia luciferase assay kit according to the manufacturer's instructions (Targeting Systems). A parallel infection with no inhibitor was used as a control. All treatments were carried out with 8 replicates in 96-well plates.
Confocal microscopy.
RFs grown on coverslips overnight were inoculated with RRV-RFP at an MOI of 20 in the presence of Alexa Fluor 488-transferrin or -CTB for 1 h. The cells were fixed and processed for immunofluorescence analysis to visualize virus particles. To amplify the signal of virus particles, cells were incubated with a rabbit anti-RFP antibody at a 1:1,000 dilution, and the signal was detected with Alexa Fluor 568-conjugated goat anti-rabbit IgG. To detect actin filaments, cells were costained Alexa Fluor 488-phalloidin. The nuclei were stained by incubation with DAPI for 5 min. Cells were observed with an Olympus FV1000 scanning confocal microscope equipped with a 60× objective (numerical aperture [NA], 1.42) (Olympus Life Science, Center Valley, PA) as described previously (12).
To examine the colocalization of RRV with endosomal markers, RFs grown on coverslips were inoculated with RRV-RFP for 1 h. Following fixation, the cells were costained for virus particles with the rabbit anti-RFP antibody and for the clathrin heavy chain with a mouse anti-clathrin monoclonal antibody. The signals of virus particles and clathrin were revealed with Alexa Fluor 568-conjugated goat anti-rabbit IgG as described above and with Alexa Fluor 488-conjugated goat anti-mouse IgG at a 1:100 dilution, respectively. To detect EEA1, an FITC-conjugated mouse anti-EEA1 antibody was used at a 1:100 dilution.
Cytotoxicity assay.
RFs grown in 24-well plates were treated with the inhibitors at the concentrations and for the lengths of time given in the figures. Cells were labeled with PI 1 h prior to fixation in order to identify nonviable cells. Cells were washed twice with phosphate-buffered saline (PBS), fixed with 2% paraformaldehyde for 30 min, and stained with DAPI. The cells were visualized with a Zeiss Axiovert 200M fluorescence microscope (Carl Zeiss Microimaging Inc., Thornwood, NY).
Inhibition of clathrin-mediated endocytosis with dominant negative constructs (DNs) of EPS15.
Green fluorescent protein (GFP)-tagged EPS15 constructs (WT, EH29, DIII, and D3Δ2) and the enhanced GFP (EGFP) vector were kind gifts from Alexandre Benmerah at the Institut Cochin, CNRS, Paris, France. Cells were transiently transfected with EPS15 constructs or the EGFP vector by using the Lipofectamine 2000 reagent according to the manufacturer's instructions (Invitrogen). At 24 h posttransfection, cells were inoculated with RRV-luciferase or RRV-RFP for 1 h. RRV-luciferase-infected cells were first washed and then incubated with growth medium in order to measure luciferase activity at different time points. RRV-RFP-infected cells were fixed at 1 hpi and were stained for virus particles with the rabbit anti-RFP antibody. To determine the effectiveness and specificity of the constructs at inhibiting clathrin-mediated endocytosis, cells transfected with the constructs were inoculated with either Alexa Fluor 568-transferrin or Alexa Fluor 594-CTB for 20 min, and their internalization was observed with an Olympus FV1000 scanning confocal microscope equipped with a 60× objective (NA, 1.4).
RESULTS
Tracking of RRV entry with recombinant reporter viruses.
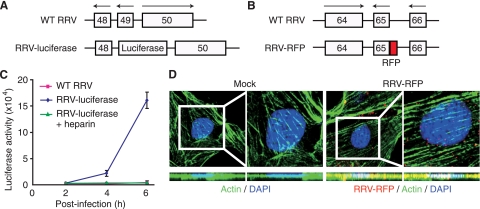
We generated two recombinant viruses, RRV-luciferase and RRV-RFP, to facilitate the tracking of virus entry and trafficking (Fig. 1A and B). RRV-luciferase expresses a secreted Gaussia luciferase. Following infection of RFs with RRV-luciferase, luciferase activity was detected in the supernatants of the infected cultures as early as 2 hpi (Fig. 1C). The luciferase activity increased rapidly in the next 4 h. In contrast, no luciferase activity was detected in cultures infected with WT RRV. The sensitivity of the method, combined with the fast expression of the luciferase reporter and the ability to sample the reporter activity over time in the supernatants following infection, renders RRV-luciferase a useful tool for tracking viral infectivity and examining factors that might regulate virus entry and trafficking. These features are particularly useful considering the possible side effects of the chemical inhibitors commonly used in virus entry studies. To validate the method, RRV-luciferase was incubated with 100 μg/ml heparin (a soluble heparan sulfate-like compound) before inoculation into RF cultures. Treatment with heparin is known to block the entry of herpesviruses, because their attachment to target cells is mediated by heparan sulfate (35). As shown in Fig. 1C, no luciferase activity was detectable in cells infected with RRV-luciferase following treatment with heparin, indicating that RRV-luciferase infection was efficiently blocked by heparin.
FIG. 1.
Tracking of RRV entry and trafficking using recombinant RRVs. (A) RRV-luciferase was generated by replacing Orf49 with a secreted Gaussia luciferase reporter gene. (B) RRV-RFP was generated by fusing an RFP tag at the N terminus of Orf65. (C) Tracking of RRV infectivity by use of RRV-luciferase. Infection of RFs with RRV-luciferase but not with WT RRV resulted in the expression of luciferase activity in the supernatants of infected cultures. Treatment with 100 μg/ml heparin inhibited the infectivity of RRV-luciferase, as indicated by the low luciferase activity. (D) Visualization of RRV particles (red) during entry into RFs at 1 hpi in an XY section and the corresponding YZ cross-section. Actin filaments were stained green, while the nuclei were stained blue with DAPI. Rectangles delineate the regions shown at higher magnifications directly to the right.
To track virus entry more directly, we generated RRV-RFP for the visualization of virus particles (Fig. 1B). We costained for actin filaments and examined both XY and YZ images in order to track the intracellular localization of the virus particles. As shown in Fig. 1D, we easily detected virus particles inside RFs at 1 hpi. A large number of virus particles had reached the perinuclear regions.
Inhibition of endosomal acidification reduces RRV infectivity.
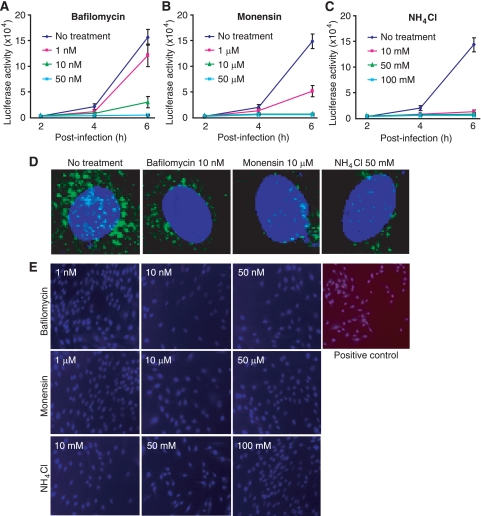
Enveloped viruses enter cells either by direct plasma membrane fusion or by receptor-mediated endocytosis (35). Endocytosis requires a low pH to trigger the fusion between the viral envelope and the endosomal membrane. To determine whether RRV entry into RFs is mediated by endocytosis, we infected RFs with RRV-luciferase in the presence of inhibitors of endosomal acidification. Bafilomycin A1 is a potent and specific inhibitor of vacuolar H+ ATPase, and it specifically inhibits endosomal acidification (6). Monensin, a sodium ionophore, binds monovalent cations, leading to an increase in endosomal pH (16). NH4Cl, an inhibitor of vacuolar acidification, is often used to examine pH sensitivity during virus entry (8). RFs were pretreated with different concentrations of the inhibitors for 1 h and were then infected with RRV-luciferase in the presence of the inhibitors. Luciferase activity was monitored by sampling supernatants from the cultures over time. As shown in Fig. 2A to C, all three inhibitors reduced luciferase activity in a dose-dependent manner, suggesting a pH-sensitive route of RRV entry. These inhibitors also efficiently inhibited the internalization of a fluorescence-labeled transferrin (Alexa Fluor 488-transferrin), a bona fide cargo of clathrin-mediated endocytosis (11), demonstrating their effectiveness (Fig. 2D). To examine the cytotoxicity of the inhibitors, we measured cell viability by PI staining following treatment with the inhibitors. We observed no PI-positive cells at any of the concentrations used for the experiments (Fig. 2E). Taken together, these results indicate that RRV uses the endocytosis pathway to enter RFs.
FIG. 2.
Inhibitors of endosomal acidification block the entry of RRV into RFs. (A to C) RFs were infected with RRV-luciferase in the presence of different concentrations of bafilomycin A1 (A), monensin (B), or NH4Cl (C). Luciferase activities in the culture supernatants were monitored at the indicated time points and compared with those for control cells infected with RRV-luciferase in parallel without inhibitor treatment. (D) Inhibitors of endosomal acidification blocked the entry of Alexa Fluor 488-transferrin into RFs. (E) Inhibitors of endosomal acidification had no effect on cell viability. RFs were treated with inhibitors of endosomal acidification under the conditions used for the virus infection assay and were evaluated for viability by staining with PI and DAPI. Cells left without medium and CO2 for 1 h were used as positive controls.
Inhibition of clathrin-mediated endocytosis reduces RRV infectivity.
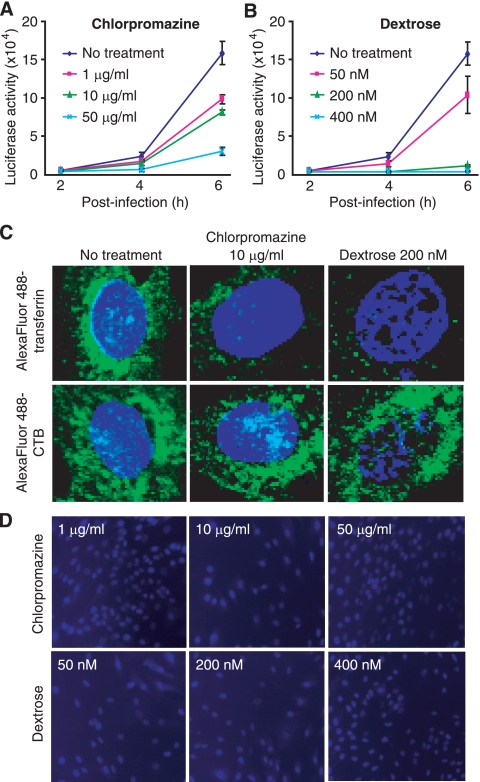
The endocytosis pathway can be clathrin dependent or clathrin independent (34). We examined the effects of inhibitors of clathrin-mediated endocytosis on RRV entry. The cationic amphiphilic agent chlorpromazine inhibits the recycling of the AP-2 adaptor protein complex between membranes and thus causes the misassembly of clathrin-coated pits at the cell surface (38). The hypertonic reagent dextrose inhibits the formation of clathrin-coated pits at the plasma membrane (14). RFs pretreated with different concentrations of chlorpromazine or dextrose for 1 h were infected with RRV-luciferase in the presence of the inhibitors. As shown in Fig. 3A and B, both inhibitors of clathrin assembly reduced luciferase activity in a dose-dependent manner. Both inhibitors also effectively inhibited the internalization of Alexa Fluor 488-transferrin but had no effect on the internalization of fluorescence-labeled CTB (Alexa Fluor 488-CTB) (Fig. 3C), which enters cells through the caveola-mediated endocytosis pathway (27). Furthermore, no cytotoxicity was observed with the inhibitors (Fig. 3D). Together, these results indicate that RRV is likely to enter RFs through clathrin-mediated endocytosis.
FIG. 3.
Inhibitors of clathrin-mediated endocytosis block the entry of RRV into RFs. (A and B) RFs were infected with RRV-luciferase in the presence of different concentrations of chlorpromazine (A) or dextrose (B). Luciferase activities in the culture supernatants were monitored at the indicated time points and were compared with those of control cells infected with RRV-luciferase in parallel without inhibitor treatment. (C) Inhibitors of clathrin-mediated endocytosis blocked the entry of Alexa Fluor 488-transferrin, but not that of Alexa Fluor 488-CTB, into RFs. (D) Inhibitors of clathrin-mediated endocytosis had no effect on cell viability. RFs were treated with inhibitors of clathrin-mediated endocytosis under the conditions used for the virus infection assay and were evaluated for viability by staining with PI and DAPI.
Inhibition of caveola-mediated endocytosis has no effect on RRV infectivity.
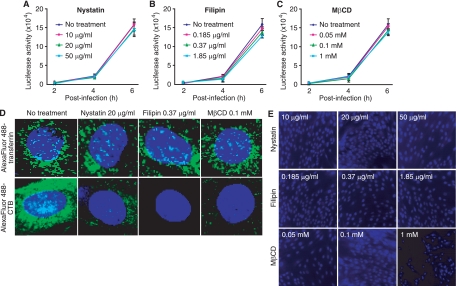
Caveola-mediated endocytosis is another highly efficient pathway for the internalization of cargos and is often exploited by viruses for entry into cells (34). Several inhibitors can target caveola-mediated endocytosis. Both nystatin and filipin disrupt lipid rafts, while MβCD sequesters cholesterol from the plasma membrane (18, 31). As shown in Fig. 4A to C, all three inhibitors had no effect on luciferase activity following RRV-luciferase infection, indicating that they do not inhibit RRV infectivity. To confirm that these inhibitors do indeed inhibit caveola-mediated endocytosis, we determined their effects on the internalization of Alexa Fluor 488-CTB. As shown in Fig. 4D, nystatin, filipin, and MβCD effectively inhibited the internalization of Alexa Fluor 488-CTB but had no effect on the internalization of Alexa Fluor 488-transferrin, thus demonstrating the specificity of these inhibitors for caveola-mediated endocytosis. Furthermore, PI staining did not detect any cytotoxicity of these inhibitors (Fig. 4E). Together, these results indicate that RRV is unlikely to enter RFs through caveola-mediated endocytosis.
FIG. 4.
Inhibitors of caveola-mediated endocytosis fail to block the entry of RRV into RFs. (A to C) RFs were infected with RRV-luciferase in the presence of different concentrations of nystatin (A), filipin (B), or MβCD (C). Luciferase activities in the culture supernatants were monitored at the indicated time points and were compared with those for control cells infected with RRV-luciferase in parallel without inhibitor treatment. (D) Inhibitors of caveola-mediated endocytosis blocked the entry of Alexa Fluor 488-CTB, but not that of Alexa Fluor 488-transferrin, into RFs. (E) Inhibitors of caveola-mediated endocytosis had no effect on cell viability. RFs were treated with inhibitors of caveola-mediated endocytosis under the conditions used for the virus infection assay and were evaluated for viability by staining with PI and DAPI.
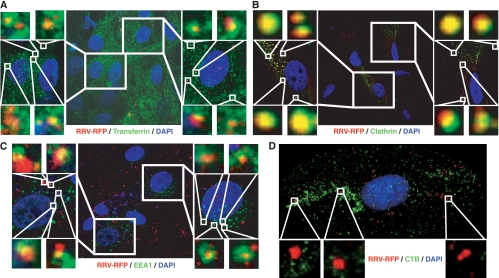
RRV particles are colocalized with transferrin and markers of clathrin-mediated endocytosis during entry into RFs.
Our results so far indicate that the entry of RRV into RFs is mediated by clathrin-mediated endocytosis. To further confirm these observations, we examined the colocalization of RRV particles with different endocytic markers. As shown in Fig. 5, colocalization of RRV particles with Alexa Fluor 488-transferrin, clathrin, and EEA1, but not with Alexa Fluor 488-CTB, was observed. These results confirmed that RRV enters RFs primarily through clathrin-mediated endocytosis.
FIG. 5.
RRV particles are colocalized with markers of clathrin-mediated endocytosis during entry into RFs. (A to D) Detection of colocalization of RRV-RFP particles with Alexa Fluor 488-transferrin (A), clathrin (B), and EEA1 (C), but not with Alexa Fluor 488-CTB (D), at 1 hpi. Regions delineated by rectangles are shown at higher magnifications in adjacent panels.
The entry of RRV into RFs is inhibited by an EPS15 DN.
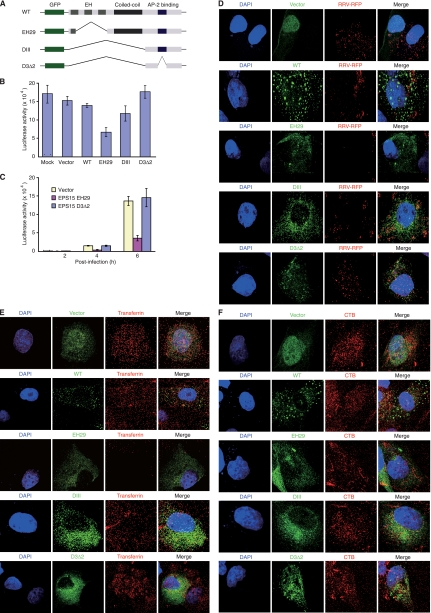
To provide additional evidence to support the results with chemical inhibitors, we used DNs of EPS15 to inhibit clathrin-mediated endocytosis and assayed their effects on RRV entry (Fig. 6A). EPS15 is a component of clathrin-coated pits and interacts with AP-2, an adaptor protein of clathrin-mediated endocytosis (4, 5). Mutant EH29 lacks part of the EPS15 homology (EH) domains but retains the AP-2 binding site. Mutant DIII lacks the entire EH domains and coiled-coil domains but also retains the AP-2 binding site. In human cells, both mutants are capable of blocking the efficient targeting of AP-2 and clathrin to clathrin-coated pits without affecting other endocytic pathways, and thus, they are often used as DNs for clathrin-mediated endocytosis (Fig. 6A) (4, 5). In contrast, mutant D3Δ2, which lacks the AP-2 binding site in addition to lacking the EH and coiled-coil domains, has no effect on the formation of clathrin-coated pits and thus is often used as negative control for the inhibition of clathrin-mediated endocytosis (4, 5). We used a set of EPS15-GFP fusion constructs to examine their effects on RRV infectivity in RFs following transient overexpression (Fig. 6A). As shown in Fig. 6B, when transfection rates reached 50 to 60% based on the percentage of GFP-positive cells, overexpression of the GFP vector, WT EPS15, or D3Δ2 had no obvious effect on luciferase activity following RRV-luciferase infection compared to that for cells that were not transfected with any of the constructs (mock). In contrast, transfection with EH29 inhibited 51% of luciferase activity compared to that for cells transfected with GFP vector. In a kinetic experiment in which the transfection rates reached 80 to 90%, EH29 inhibited ∼70% of the luciferase activity while D3Δ2 had no obvious effect compared to activity for the GFP vector (Fig. 6C). By direct tracking of the internalization of RRV-RFP, it was observed that EH29 but not GFP vector, WT EPS15, or D3Δ2 inhibited the internalization of RRV particles (Fig. 6D). As expected, EH29, but not the GFP vector, WT EPS15, or D3Δ2, inhibited the internalization of Alexa Fluor 568-transferrin (Fig. 6E). In contrast, none of the constructs blocked the internalization of Alexa Fluor 594-CTB (Fig. 6F). These results demonstrated the specificity of EH29 in inhibiting clathrin-mediated endocytosis, and further confirmed this pathway as the entry pathway of RRV into RFs.
FIG. 6.
Inhibition of clathrin-mediated endocytosis with a DN of EPS15 blocks the entry of RRV into RFs. (A) Schematic illustration of different EPS15 constructs. (B) EH29, but not the GFP vector, WT EPS15, or D3Δ2, efficiently inhibited the entry of RRV into RFs, while DIII inhibited 20% of RRV entry. For this experiment, transfection rates were estimated at 50 to 60% based on percentages of GFP-positive cells. (C) Inhibition of RRV entry into RFs by EH29, but not D3Δ2, at different time points postinfection. The transfection rates were estimated at 80 to 90% for this experiment. (D) EH29, but not the GFP vector, WT EPS15, D3Δ2, or DIII, efficiently inhibited the entry of RRV into RFs, as monitored by direct staining of RRV particles at 1 hpi. (E) EH29, but not the GFP vector, WT EPS15, D3Δ2, or DIII, efficiently inhibited the entry of Alexa Fluor 568-transferrin into RFs. (F) None of the EPS15 constructs had any effect on the entry of Alexa Fluor 594-CTB into RFs.
In contrast to those for EH29, the results obtained with DIII are intriguing. Transfection with DIII inhibited only 20% of the luciferase activity following RRV-luciferase infection compared to that for cells transfected with the GFP vector (Fig. 6B), indicating that inhibition of clathrin-mediated endocytosis in RFs by DIII was not efficient. Indeed, by tracking the RRV-RFP particles, we were able to observe the internalization of RRV particles in cells transfected with DIII, albeit at a slightly lower rate than that for cells transfected with the GFP vector, WT EPS15, or D3Δ2 (Fig. 6D). DIII also did not inhibit the internalization of Alexa Fluor 488-transferrin or Alexa Fluor 488-CTB, as expected (Fig. 6E and F). Compared to EH29, DIII is a weaker inhibitor of clathrin-mediated endocytosis in human cells (4). Our results confirm the previous observation and indicate that DIII is likely not functional in RFs.
DISCUSSION
Enveloped viruses enter cells either by fusion of the viral envelope with the plasma membrane or by using endocytic pathways (23). Endocytosis, an essential biological process mediating cellular internalization events, is often exploited by many enveloped and nonenveloped viruses for their entry into target cells (34). Endocytosis pathways allow viruses to pass quickly and in an orderly manner through the plasma membrane barrier and the crowded cytoplasm so that viral nucleocapsids can gain access to the perinuclear space and deliver their genomes to the nucleus (30, 34). In this study, we used two recombinant RRVs, RRV-luciferase and RRV-RFP, to examine the entry pathway of RRV in RFs. RRV-luciferase expresses a secreted Gaussia luciferase reporter, allowing consecutive sampling of supernatants from the infected cultures. The high sensitivity and simplicity of the luciferase assay, as well as the early expression of the luciferase gene, also make this reporter virus attractive for the study of virus entry. On the other hand, RRV-RFP provides a convenient way to directly track the entry of a single virus particle into cells. While it has not been shown in this study, it is also possible to track the entry of RRV-RFP particles in real time.
In this study, by using several complementary approaches, we have shown that RRV enters RFs through the clathrin-mediated endocytosis pathway. First, we used chemical inhibitors to block different virus entry pathways. The entry of many viruses by endocytosis is thought to be pH dependent (34). RRV entry is blocked by inhibitors of endosomal acidification, indicating the requirement for an acidification step during RRV entry into cells, and thus the involvement of a receptor-mediated endocytosis pathway rather than a plasma membrane fusion pathway. To identify the endocytosis pathway involved in RRV entry, we further used a variety of chemical reagents to inhibit clathrin- and caveola-mediated endocytosis, in turn. The results have shown that RRV entry is sensitive to inhibitors of clathrin-mediated endocytosis but not to inhibitors of caveola-mediated endocytosis.
Second, we have demonstrated the colocalization of RRV particles with transferrin but not with CTB. Transferrin enters cells though clathrin-mediated endocytosis, while CTB enters cells through caveola-mediated endocytosis (11, 27). During clathrin-mediated endocytosis, the clathrin subunit mediates the formation of clathrin-coated pits following the interaction of the ligand with its cell surface receptor, resulting in the internalization of the ligand into the clathrin-coated vesicle and the subsequent formation of the early endosome (30). Different markers can be used to identify these early vesicles during clathrin-mediated endocytosis. For example, the clathrin heavy chain and EEA1 have been widely used for the identification of clathrin-containing vesicles and early endosomes, respectively (12, 30). By staining for clathrin and EEA1, we have observed the colocalization of RRV particles with both clathrin and EEA1, indicating that RRV indeed enters clathrin-coated vesicles and early endosomes during internalization.
Finally, we have shown, either by monitoring luciferase activity following RRV-luciferase infection or by tracking RRV particles following RRV-RFP infection, that inhibition of clathrin-mediated endocytosis with a DN of EPS15, EH29, blocks the entry of RRV into RFs, thus further confirming the role of clathrin-mediated endocytosis in RRV entry. EH29 also inhibits the internalization of transferrin but not that of CTB, demonstrating the specificity of this construct in inhibiting clathrin-mediated endocytosis. EPS15 DNs have been widely used for studying endocytosis in human cells (4, 5). However, limited information is available regarding the functions of these constructs in rhesus cells. Our results show that EH29 is fully functional while DIII is a weak inhibitor of clathrin endocytosis in rhesus cells; these findings are consistent with those reported for human cells (4).
It is well accepted that viruses may use more than one pathway to enter target cells and that the entry pathway could be cell type dependent. For example, human immunodeficiency virus type 1 (HIV-1) is commonly known to enter cells through plasma membrane fusion at a neutral pH (37); however, HIV-1 also enters cells by endocytosis (22). Herpesviruses encode multiple glycoproteins that interact with different receptors during entry (35). The entry of herpesviruses is a complex process, and both plasma membrane fusion and endocytosis have been reported. Herpes simplex virus type 1 infects some cell types through plasma membrane fusion but enters others through endocytosis (9, 21, 24). Epstein-Barr virus enters epithelial cells through plasma membrane fusion but infects B cells via endocytosis (15). KSHV entry is also complex, involving multiple receptors and entry pathways, including both plasma membrane fusion and endocytosis, depending on the cell type (1, 2, 10, 12, 17, 28, 29). However, KSHV enters human foreskin fibroblasts and primary human umbilical vein endothelial cells through the clathrin-mediated pathway (1, 13). Since RRV is closely related to KSHV and is often used as an animal model for KSHV infection, it would be interesting to determine whether RRV entry into RFs involves viral glycoproteins and cellular receptors similar to those reported in KSHV entry. While we have provided evidence that clathrin-mediated endocytosis is the major pathway mediating RRV entry into RFs, it is entirely possible that other pathways might also be involved, particularly in other cell types. This is even more likely considering the recent discovery of sequence variation in RRV glycoproteins (33).
Acknowledgments
We thank Ronald C. Desrosiers at the New England Primate Research Center for providing the RRV strain RRV26-95; Alexandre Benmerah at Institut Cochin, CNRS, Paris, France, for all the EPS15 constructs; Roger Tsien at the University of California at San Diego for the pRSETB-mRFD1 plasmid; and Guangming Zhong at the University of Texas Health Science Center at San Antonio for the RFP antibody.
This work was supported by grants from the National Institutes of Health (CA096512, CA124332, and CA119889) to S.-J. Gao.
Footnotes
Published ahead of print on 8 September 2010.
REFERENCES
- 1.Akula, S. M., P. P. Naranatt, N. S. Walia, F. Z. Wang, B. Fegley, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J. Virol. 77:7978-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. [DOI] [PubMed] [Google Scholar]
- 3.Alexander, L., L. Denekamp, A. Knapp, M. R. Auerbach, B. Damania, and R. C. Desrosiers. 2000. The primary sequence of rhesus monkey rhadinovirus isolate 26-95: sequence similarities to Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J. Virol. 74:3388-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112(Pt. 9):1303-1311. [DOI] [PubMed] [Google Scholar]
- 5.Benmerah, A., C. Lamaze, B. Begue, S. L. Schmid, A. Dautry-Varsat, and N. Cerf-Bensussan. 1998. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 140:1055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowman, E. J., A. Siebers, and K. Altendorf. 1988. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. U. S. A. 85:7972-7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desrosiers, R. C., V. G. Sasseville, S. C. Czajak, X. Zhang, K. G. Mansfield, A. Kaur, R. P. Johnson, A. A. Lackner, and J. U. Jung. 1997. A herpesvirus of rhesus monkeys related to the human Kaposi's sarcoma-associated herpesvirus. J. Virol. 71:9764-9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira, D. F., M. P. Santo, M. A. Rebello, and M. C. Rebello. 2000. Weak bases affect late stages of Mayaro virus replication cycle in vertebrate cells. J. Med. Microbiol. 49:313-318. [DOI] [PubMed] [Google Scholar]
- 9.Fuller, A. O., and P. G. Spear. 1987. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc. Natl. Acad. Sci. U. S. A. 84:5454-5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrigues, H. J., Y. E. Rubinchikova, C. M. Dipersio, and T. M. Rose. 2008. Integrin alphaVbeta3 binds to the RGD motif of glycoprotein B of Kaposi's sarcoma-associated herpesvirus and functions as an RGD-dependent entry receptor. J. Virol. 82:1570-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant, B. D., and J. G. Donaldson. 2009. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 10:597-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene, W., and S. J. Gao. 2009. Actin dynamics regulate multiple endosomal steps during Kaposi's sarcoma-associated herpesvirus entry and trafficking in endothelial cells. PLoS Pathog. 5:e1000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene, W., K. Kuhne, F. Ye, J. Chen, F. Zhou, X. Lei, and S. J. Gao. 2007. Molecular biology of KSHV in relation to AIDS-associated oncogenesis. Cancer Treat. Res. 133:69-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heuser, J. E., and R. G. Anderson. 1989. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 108:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutt-Fletcher, L. M. 2007. Epstein-Barr virus entry. J. Virol. 81:7825-7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inabayashi, M., S. Miyauchi, N. Kamo, and T. Jin. 1995. Conductance change in phospholipid bilayer membrane by an electroneutral ionophore, monensin. Biochemistry 34:3455-3460. [DOI] [PubMed] [Google Scholar]
- 17.Kaleeba, J. A., and E. A. Berger. 2006. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 311:1921-1924. [DOI] [PubMed] [Google Scholar]
- 18.Kranenburg, O., I. Verlaan, and W. H. Moolenaar. 2001. Regulating c-Ras function. cholesterol depletion affects caveolin association, GTP loading, and signaling. Curr. Biol. 11:1880-1884. [DOI] [PubMed] [Google Scholar]
- 19.Mansfield, K. G., S. V. Westmoreland, C. D. DeBakker, S. Czajak, A. A. Lackner, and R. C. Desrosiers. 1999. Experimental infection of rhesus and pig-tailed macaques with macaque rhadinoviruses. J. Virol. 73:10320-10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsh, M., and A. Helenius. 2006. Virus entry: open sesame. Cell 124:729-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milne, R. S., A. V. Nicola, J. C. Whitbeck, R. J. Eisenberg, and G. H. Cohen. 2005. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J. Virol. 79:6655-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyauchi, K., Y. Kim, O. Latinovic, V. Morozov, and G. B. Melikyan. 2009. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell 137:433-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mudhakir, D., and H. Harashima. 2009. Learning from the viral journey: how to enter cells and how to overcome intracellular barriers to reach the nucleus. AAPS J. 11:65-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicola, A. V., A. M. McEvoy, and S. E. Straus. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 77:5324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Connor, C. M., and D. H. Kedes. 2007. Rhesus monkey rhadinovirus: a model for the study of KSHV. Curr. Top. Microbiol. Immunol. 312:43-69. [DOI] [PubMed] [Google Scholar]
- 26.Orzechowska, B. U., M. F. Powers, J. Sprague, H. Li, B. Yen, R. P. Searles, M. K. Axthelm, and S. W. Wong. 2008. Rhesus macaque rhadinovirus-associated non-Hodgkin lymphoma: animal model for KSHV-associated malignancies. Blood 112:4227-4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pang, H., P. U. Le, and I. R. Nabi. 2004. Ganglioside GM1 levels are a determinant of the extent of caveolae/raft-dependent endocytosis of cholera toxin to the Golgi apparatus. J. Cell Sci. 117:1421-1430. [DOI] [PubMed] [Google Scholar]
- 28.Raghu, H., N. Sharma-Walia, M. V. Veettil, S. Sadagopan, and B. Chandran. 2009. Kaposi's sarcoma-associated herpesvirus utilizes an actin polymerization-dependent macropinocytic pathway to enter human dermal microvascular endothelial and human umbilical vein endothelial cells. J. Virol. 83:4895-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rappocciolo, G., H. R. Hensler, M. Jais, T. A. Reinhart, A. Pegu, F. J. Jenkins, and C. R. Rinaldo. 2008. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J. Virol. 82:4793-4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rappoport, J. Z. 2008. Focusing on clathrin-mediated endocytosis. Biochem. J. 412:415-423. [DOI] [PubMed] [Google Scholar]
- 31.Rothberg, K. G., J. E. Heuser, W. C. Donzell, Y. S. Ying, J. R. Glenney, and R. G. Anderson. 1992. Caveolin, a protein component of caveolae membrane coats. Cell 68:673-682. [DOI] [PubMed] [Google Scholar]
- 32.Searles, R. P., E. P. Bergquam, M. K. Axthelm, and S. W. Wong. 1999. Sequence and genomic analysis of a rhesus macaque rhadinovirus with similarity to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 73:3040-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin, Y. C., L. R. Jones, J. Manrique, W. Lauer, A. Carville, K. G. Mansfield, and R. C. Desrosiers. 2010. Glycoprotein gene sequence variation in rhesus monkey rhadinovirus. Virology 400:175-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sieczkarski, S. B., and G. R. Whittaker. 2002. Dissecting virus entry via endocytosis. J. Gen. Virol. 83:1535-1545. [DOI] [PubMed] [Google Scholar]
- 35.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tischer, B. K., J. von Einem, B. Kaufer, and N. Osterrieder. 2006. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques 40:191-197. [DOI] [PubMed] [Google Scholar]
- 37.Uchil, P. D., and W. Mothes. 2009. HIV entry revisited. Cell 137:402-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong, S. W., E. P. Bergquam, R. M. Swanson, F. W. Lee, S. M. Shiigi, N. A. Avery, J. W. Fanton, and M. K. Axthelm. 1999. Induction of B cell hyperplasia in simian immunodeficiency virus-infected rhesus macaques with the simian homologue of Kaposi's sarcoma-associated herpesvirus. J. Exp. Med. 190:827-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou, F., Q. Li, S. W. Wong, and S. J. Gao. 2010. Autoexcision of bacterial artificial chromosome facilitated by terminal repeat-mediated homologous recombination: a novel approach for generating traceless genetic mutants of herpesviruses. J. Virol. 84:2871-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou, F. C., Y. J. Zhang, J. H. Deng, X. P. Wang, H. Y. Pan, E. Hettler, and S. J. Gao. 2002. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J. Virol. 76:6185-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]