Abstract
Increased levels of activated T cells are a hallmark of the chronic stage of human immunodeficiency virus (HIV) infection and are highly correlated with HIV disease progression. We evaluated chloroquine (CQ) as a potential therapy to reduce immune activation during HIV infection. We found that the frequency of CD38+ HLA-DR+ CD8 T cells, as well as Ki-67 expression in CD8 and CD4 T cells, was significantly reduced during CQ treatment. Our data indicate that treatment with CQ reduces systemic T-cell immune activation and, thus, that its use may be beneficial for certain groups of HIV-infected individuals.
Chronic HIV infection is characterized by multifaceted systemic immune activation, including increased frequencies of activated T cells (9, 17) and increased turnover of T cells (5, 12, 18) that correlate directly with disease progression (8, 9). T-cell immune activation is also associated with lower gains in CD4 T-cell count in HIV-infected individuals even while they are on antiretroviral therapy (ART) that appears to suppress viral replication (10). Thus, therapies that reduce immune activation may be of benefit, particularly for such individuals. Three clinical studies have been conducted using hydroxychloroquine monotherapy for patients with HIV infection (6, 21, 22), and the studies showed that hydroxychloroquine-treated patients had decreased viral loads as well as decreased serum interleukin-6 (IL-6) levels, heightened levels of which are correlated with disease progression (13). However, these studies did not examine other parameters of immune activation. Chloroquine (CQ) is known to suppress immune activation by a number of mechanisms, including inhibition of intracellular toll-like receptor (TLR) signaling and inflammatory cytokine secretion (11, 19). In vitro, CQ has been shown to reduce HIV infection-induced T-cell immune activation (14). Here, we report results using samples from a clinical study of HIV-infected individuals treated with CQ monotherapy, where we examine multiple parameters of immune activation during the course of CQ treatment.
Study design and sample collection.
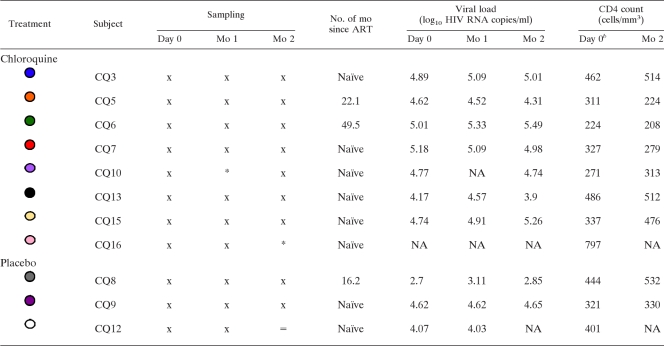
A double-blind, randomized placebo-controlled trial testing the effects of chloroquine in chronically HIV-infected persons was conducted with informed consent and approval by the University of Minnesota Institutional Review Board. Thirteen chronically HIV-infected individuals were randomized, with six receiving 250 mg of chloroquine daily, three receiving 500 mg of chloroquine daily, and four receiving placebo for 2 months. Inclusion criteria included CD4 T-cell counts of >250 cells/ml. All were ART naïve except for four patients who had been off ART for at least 16 months. Peripheral blood was taken at study initiation, month 1 posttreatment, and month 2 posttreatment. Viral RNA loads and CD4 T-cell counts were taken for each patient at each visit.
Cell surface staining for immune activation and intracellular staining for Ki-67.
Peripheral blood mononuclear cells (PBMCs) were isolated with Ficoll-Paque centrifugation and viably cryopreserved. Cells were thawed and washed in RPMI medium supplemented with 10% fetal calf serum (FCS). Aqua (Invitrogen), a viability dye, was first added to 106 cells, and then the following fluorochrome- or quantum dot (QDOT)-conjugated antibodies against cell surface markers (obtained from Becton Dickinson, Pharmingen, or Coulter or provided by Mario Roederer, NIH) were added: CD3-Cy7 phycoerythrin (PE), CD4-Cy5.5PE, CD8-QDOT705, CD27-PE, CD45RO-Texas Red PE (TRPE), HLA-DR-Cy7 allophycocyanin (APC), CD19-Pacific Blue (PacBlue), and CD38-APC. Cell surface staining followed by intracellular staining for Ki-67 was performed as previously described (16). Cells were then resuspended in 1% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) before flow cytometric analysis or cell sorting. Cells were analyzed by 10-parameter flow cytometry with an LSRII instrument (BD), and CD27+ CD45RO+ memory CD4 T cells were sorted for quantitative PCR for viral DNA using a FACS Aria cell sorter (BD). Intracellular cytokine staining of PBMCs for tumor necrosis factor (TNF), gamma interferon (IFN-γ), and IL-2-producing HIV-specific memory T cells was performed as previously described (4). Plasma lipopolysaccharide (LPS) levels were quantified in triplicate using the Limulus amebocyte assay (Lonza) as previously described (3). Plasma was diluted 1:10 in endotoxin-free water and heated to 80°C for 15 min before use in the assay.
The Wilcoxon matched-pairs test was used to compare different time points within subjects. The analysis included samples from eight CQ- and three placebo-treated individuals (Table 1) instead of the initially enrolled subjects due to two subjects for whom only a baseline time point sample was obtained. Due to the small sample size of the placebo group, the Wilcoxon test was not performed for month 2 of the placebo arm. Graph Pad Prism version 5.0 was used to perform analyses.
TABLE 1.
Study subjects and sample collectiona
Symbols and abbreviation: x, sample was collected; *, sample collection was missed for a reason unrelated to subject health; =, death; NA, sample not available.
b The baseline CD4 cell count was the CD4 cell nadir for each subject at study initiation.
Decreased HLA-DR+ CD38+ CD8 memory T cells in CQ-treated subjects.
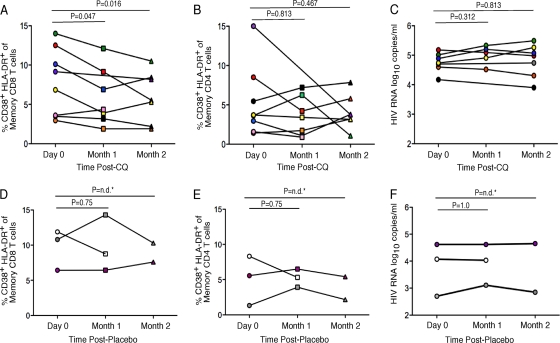
As the percentages of HLA-DR+ CD38+ CD8 memory T cells strongly correlate with HIV disease progression, we chose to evaluate this parameter first during the course of CQ treatment. We found that there was a significant decrease in the percentages of HLA-DR+ CD38+ CD8 memory T cells in the CQ-treated subjects at both month 1 and month 2 after CQ administration relative to baseline (Fig. 1). HLA-DR+ CD38+ cells comprised between 1.9% and 14.3% of total CD8 memory T cells, defined as CD27high CD45RO+, CD27lo CD45RO−, or CD27lo CD45RO+. These ranges were comparable to those previously reported during HIV infection for HLA-DR+ CD38+ CD8 T cells (8). Most of the previous studies of HLA-DR+ CD38+ CD8 T cells during HIV infection have evaluated T-cell activation in the total T-cell population, which included both memory and naïve cells. Here, we were able to discriminate activation specifically within the memory T-cell population by multiparameter flow cytometry. At month 1 after CQ administration, the median percentage of HLA-DR+ CD38+ CD8 memory T cells decreased from 8.0% to 4.35% of total memory CD8 T cells (Fig. 1A) (median change, 3.65%; P = 0.047). At month 2 after CQ administration, the decrease relative to baseline was sustained in the CQ-treated group, with a median of 5.5% of memory CD8 T cells (Fig. 1A) (median change, 2.5%; P = 0.016). Data for the placebo recipients are shown in Fig. 1D. The median change from baseline to month 1 for the placebo controls was 2.05% (P = 0.75), and that from baseline to month 2 was 1.85% (Fig. 1D). There were no significant changes in the frequencies of HLA-DR+ CD38+ CD8 memory T cells in the placebo-treated individuals (Fig. 1D).
FIG. 1.
CD8 T-cell activation is decreased in HIV-infected individuals during chloroquine treatment. Percentages of CD38+ HLA-DR+ CD8 and CD4 memory T cells for chloroquine- (A and B) or placebo-treated (D and E) subjects are shown. Plasma viral RNA loads are also shown for chloroquine- (C) and placebo-treated (F) subjects. Each symbol, as indicated in Table 1, represents an individual study subject at day 0, month 1, or month 2 post-chloroquine or placebo treatment. n.d., not determined; CQ, chloroquine.
We next examined the frequency of HLA-DR+ CD38+ CD4 memory T cells. Previous reports indicate that while the percentage of these cells is increased with HIV infection, this does not serve as a prognostic marker for disease progression, as does the percentage of CD38+ HLA-DR+ CD8 memory T cells (1). We found that CQ treatment did not affect the percentage of HLA-DR CD38-coexpressing CD4 memory T cells (Fig. 1B) (P = 0.813). Similar data were found for placebo controls (Fig. 1E). Furthermore, we found that there were no significant changes in plasma viral RNA for the CQ- (Fig. 1C) or placebo-treated (Fig. 1F) subjects or in cell-associated viral DNA (data not shown) over the 2-month period.
Decreased Ki-67 expression in CD4 and CD8 memory T cells at month 1 after CQ treatment.
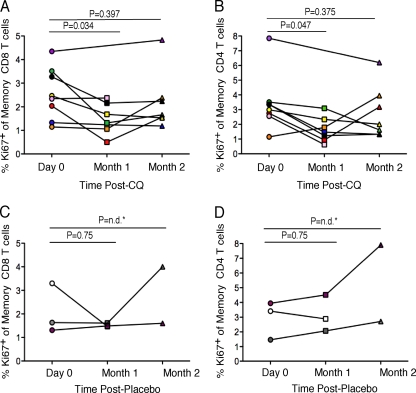
We then evaluated the fraction of T cells in the cell cycle by measuring the percentage of Ki-67+ T cells. At baseline, the median percentage of proliferating CD8 T cells of total CD8 memory T cells was 2.4% for the CQ-treated group, and after 1 month of CQ treatment, there was a significant decrease in the percentage of Ki-67+ memory CD8 T cells, to a median of 1.3% (Fig. 2 A) (median change, 1.1%; P = 0.034). After the second month of CQ treatment, the Ki-67+ memory CD8 T-cell population relative to baseline was not significantly altered (Fig. 2A) 9median change, 0.1%; P = 0.397). The data for the placebo recipients are shown in Fig. 2C. The median decrease from baseline to month 1 was 0.14% (P = 0.75), and there was a median increase of 1.17% at month 2.
FIG. 2.
Ki-67 expression by CD8 and CD4 memory T cells is decreased in HIV-infected individuals during the first but not the second month of chloroquine treatment. The percentages of CD8 or CD4 memory T cells expressing Ki-67 for chloroquine- (A and B) or placebo-treated (C and D) subjects are shown. Each symbol is representative of each subject, as described in Table 1. n.d., not determined; CQ, chloroquine.
The CD4 memory Ki-67+ T-cell population exhibited a trend similar to that observed for the CD8 memory Ki-67+ T-cell population. After 1 month of treatment, a significant decrease in Ki-67 expression was observed, from a median of 3.2% to 1.5% of total memory CD4 T cells (Fig. 2B) (P = 0.047). There was no significant difference from baseline to month 2 (Fig. 2B) (median, 2.0%; P = 0.375). Data for the placebo controls are shown in Fig. 2D. There was a 0.53% decrease in the median at month 1 (P = 0.75) and then a 1.89% increase at month 2 (Fig. 2D). We also examined memory B cells and found a decrease in Ki-67 expression at month 2 post-CQ treatment with a trend toward significance (P = 0.06) (data not shown).
Decreased plasma LPS levels at month 1 after CQ treatment.
We next sought to measure plasma LPS levels during the course of CQ treatment. Plasma LPS levels are elevated during chronic HIV infection and are correlated with immune activation (3). We therefore measured LPS in the plasma and found a significant decrease in LPS levels from a median of 60.2 to 55.2 pg/ml (Fig. 3 A) (median change, 5 pg/ml; P = 0.037) after 1 month of CQ treatment. After a second month of CQ administration, however, the levels were not significantly different from baseline, with a median of 57.9 pg/ml at month 2 (Fig. 3A) (median change, 2.3 pg/ml; P = 0.822). There was a median increase of 10.31 pg/ml in the placebo recipients from baseline to month 1 (Fig. 3B) (P = 0.25). At month 2, there was a median increase in LPS levels in placebo controls of 0.88 pg/ml (Fig. 3B).
FIG. 3.
Plasma LPS levels in HIV-infected individuals are decreased during the first but not the second month of chloroquine treatment. Plasma LPS levels were determined as described in the text. P values were derived using the Wilcoxon matched-pairs test.
Preservation of HIV-specific T-cell responses during CQ treatment.
We measured both CD4 and CD8 T-cell responses to Gag, Pol, Env, and Nef HIV antigens, by using overlapping peptide pools for HIV clade B viruses. In all cases, we found no significant changes in any TNF-, IFN-γ-, or IL-2-producing HIV- or cytomegalovirus (CMV)-specific CD8 or CD4 T cells during the complete course of CQ treatment or in the placebo control group (data not shown).
In summary, we found that administration of CQ during chronic HIV infection resulted in decreased immune activation as measured by a parameter closely correlated with disease progression, the percentage of CD38+ HLA-DR+ CD8 memory T cells. In addition, Ki-67 expression was reduced in both CD4 and CD8 memory T-cell populations during the first phase of CQ treatment. While our study was limited by the small number of placebo controls, we have extended previous studies of CQ therapy to show decreases in T-cell immune activation with CQ treatment.
CD38+ HLA-DR+ CD8 T cells are increased in frequency during chronic viral infections (2) and on stimulation with TLR3 and TLR9 ligands (7). CQ is an inhibitor of signaling by the endosomal TLRs, TLR3, TLR7, TLR8, and TLR9 (11). Thus, it is plausible that suppression of intracellular TLR signaling resulted in the decrease in CD38+ HLA-DR+ CD8 T cells with CQ treatment in these HIV-infected subjects. This could occur by suppression of intracellular TLR signaling in monocytes and dendritic cells (DCs) which, in turn, results in the reduction in CD38+ HLA-DR+ CD8 T cells, as previously shown to occur with DC-T-cell cocultures with CQ in vitro (14). It is unclear why the decrease in CD38 and HLA-DR expression was sustained during the complete course of CQ treatment, whereas Ki-67 and LPS levels were decreased only after the first month of treatment. However, the latter two parameters followed similar patterns of suppression and, therefore, may be more directly causally related.
We have identified parameters of immune activation that are altered by CQ alone, in the absence of ART. It is possible that differences in CQ dosage and regimen and the limited statistical power of our study account for why we did not observe decreases in viral load as reported in other studies. Since our study, another report showed that a daily dosage of 250 mg CQ, as was given to the majority of the participants in our study, was not sufficient to decrease viral load (20). The effects of CQ in combination therapy studies have been reported to be qualitatively different than the antiretroviral effects and not simply additive (15). Taken together, our results suggest that CQ intervention suppresses key aspects of HIV disease pathogenesis that are correlated with disease progression.
Acknowledgments
This work was supported in part by the Intramural Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (5U01-AI1027661-19); the Minnesota Medical Foundation; and the International Center for Antiviral Research and Epidemiology (I CARE).
We thank Martha Nason for assistance with statistical analyses and Christine Fietzer, Kathy Fox, and Max Schmeling for assistance in clinical care and sample preparation.
Footnotes
Published ahead of print on 15 September 2010.
REFERENCES
- 1.Benito, J. M., J. M. Zabay, J. Gil, M. Bermejo, A. Escudero, E. Sanchez, and E. Fernandez-Cruz. 1997. Quantitative alterations of the functionally distinct subsets of CD4 and CD8 T lymphocytes in asymptomatic HIV infection: changes in the expression of CD45RO, CD45RA, CD11b, CD38, HLA-DR, and CD25 antigens. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 14:128-135. [DOI] [PubMed] [Google Scholar]
- 2.Bofill, M., and N. J. Borthwick. 2000. CD38 in health and disease. Chem. Immunol. 75:218-234. [DOI] [PubMed] [Google Scholar]
- 3.Brenchley, J. M., D. A. Price, T. W. Schacker, T. E. Asher, G. Silvestri, S. Rao, Z. Kazzaz, E. Bornstein, O. Lambotte, D. Altmann, B. R. Blazar, B. Rodriguez, L. Teixeira-Johnson, A. Landay, J. N. Martin, F. M. Hecht, L. J. Picker, M. M. Lederman, S. G. Deeks, and D. C. Douek. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12:1365-1371. [DOI] [PubMed] [Google Scholar]
- 4.Brenchley, J. M., L. E. Ruff, J. P. Casazza, R. A. Koup, D. A. Price, and D. C. Douek. 2006. Preferential infection shortens the life span of human immunodeficiency virus-specific CD4+ T cells in vivo. J. Virol. 80:6801-6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catalfamo, M., M. Di Mascio, Z. Hu, S. Srinivasula, V. Thaker, J. Adelsberger, A. Rupert, M. Baseler, Y. Tagaya, G. Roby, C. Rehm, D. Follmann, and H. C. Lane. 2008. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc. Natl. Acad. Sci. U. S. A. 105:19851-19856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang, G., M. Sassaroli, M. Louie, H. Chen, V. J. Stecher, and K. Sperber. 1996. Inhibition of HIV-1 replication by hydroxychloroquine: mechanism of action and comparison with zidovudine. Clin. Ther. 18:1080-1092. [DOI] [PubMed] [Google Scholar]
- 7.Funderburg, N., A. A. Luciano, W. Jiang, B. Rodriguez, S. F. Sieg, and M. M. Lederman. 2008. Toll-like receptor ligands induce human T cell activation and death, a model for HIV pathogenesis. PLoS One 3:e1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giorgi, J. V., H. N. Ho, K. Hirji, C. C. Chou, L. E. Hultin, S. O'Rourke, L. Park, J. B. Margolick, J. Ferbas, and J. P. Phair. 1994. CD8+ lymphocyte activation at human immunodeficiency virus type 1 seroconversion: development of HLA-DR+ CD38- CD8+ cells is associated with subsequent stable CD4+ cell levels. The Multicenter AIDS Cohort Study Group. J. Infect. Dis. 170:775-781. [DOI] [PubMed] [Google Scholar]
- 9.Giorgi, J. V., L. E. Hultin, J. A. McKeating, T. D. Johnson, B. Owens, L. P. Jacobson, R. Shih, J. Lewis, D. J. Wiley, J. P. Phair, S. M. Wolinsky, and R. Detels. 1999. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 179:859-870. [DOI] [PubMed] [Google Scholar]
- 10.Hunt, P. W., J. N. Martin, E. Sinclair, B. Bredt, E. Hagos, H. Lampiris, and S. G. Deeks. 2003. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J. Infect. Dis. 187:1534-1543. [DOI] [PubMed] [Google Scholar]
- 11.Kalia, S., and J. P. Dutz. 2007. New concepts in antimalarial use and mode of action in dermatology. Dermatol. Ther. 20:160-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacs, J. A., R. A. Lempicki, I. A. Sidorov, J. W. Adelsberger, B. Herpin, J. A. Metcalf, I. Sereti, M. A. Polis, R. T. Davey, J. Tavel, J. Falloon, R. Stevens, L. Lambert, R. Dewar, D. J. Schwartzentruber, M. R. Anver, M. W. Baseler, H. Masur, D. S. Dimitrov, and H. C. Lane. 2001. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J. Exp. Med. 194:1731-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuller, L. H., R. Tracy, W. Belloso, S. De Wit, F. Drummond, H. C. Lane, B. Ledergerber, J. Lundgren, J. Neuhaus, D. Nixon, N. I. Paton, and J. D. Neaton. 2008. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinson, J. A., C. J. Montoya, X. Usuga, R. Ronquillo, A. L. Landay, and S. N. Desai. 2010. Chloroquine modulates HIV-1-induced plasmacytoid dendritic cell alpha interferon: implication for T-cell activation. Antimicrob. Agents Chemother. 54:871-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paton, N. I., J. Aboulhab, and F. Karim. 2002. Hydroxychloroquine, hydroxycarbamide, and didanosine as economic treatment for HIV-1. Lancet 359:1667-1668. [DOI] [PubMed] [Google Scholar]
- 16.Pitcher, C. J., S. I. Hagen, J. M. Walker, R. Lum, B. L. Mitchell, V. C. Maino, M. K. Axthelm, and L. J. Picker. 2002. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 168:29-43. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez, B., A. K. Sethi, V. K. Cheruvu, W. Mackay, R. J. Bosch, M. Kitahata, S. L. Boswell, W. C. Mathews, D. R. Bangsberg, J. Martin, C. C. Whalen, S. Sieg, S. Yadavalli, S. G. Deeks, and M. M. Lederman. 2006. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA 296:1498-1506. [DOI] [PubMed] [Google Scholar]
- 18.Sachsenberg, N., A. S. Perelson, S. Yerly, G. A. Schockmel, D. Leduc, B. Hirschel, and L. Perrin. 1998. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. J. Exp. Med. 187:1295-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savarino, A., J. R. Boelaert, A. Cassone, G. Majori, and R. Cauda. 2003. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect. Dis. 3:722-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savarino, A., L. Di Trani, I. Donatelli, R. Cauda, and A. Cassone. 2006. New insights into the antiviral effects of chloroquine. Lancet Infect. Dis. 6:67-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sperber, K., G. Chiang, H. Chen, W. Ross, E. Chusid, M. Gonchar, R. Chow, and O. Liriano. 1997. Comparison of hydroxychloroquine with zidovudine in asymptomatic patients infected with human immunodeficiency virus type 1. Clin. Ther. 19:913-923. [DOI] [PubMed] [Google Scholar]
- 22.Sperber, K., M. Louie, T. Kraus, J. Proner, E. Sapira, S. Lin, V. Stecher, and L. Mayer. 1995. Hydroxychloroquine treatment of patients with human immunodeficiency virus type 1. Clin. Ther. 17:622-636. [DOI] [PubMed] [Google Scholar]