Abstract
Gammaherpesviruses are important oncogenic pathogens that transit between lytic and latent life cycles. Silencing the lytic gene expression program enables the establishment of latency and a lifelong chronic infection of the host. In murine gammaherpesvirus 68 (MHV68, γHV68), essential lytic switch gene 50 controls the interchange between lytic and latent gene expression programs. However, negative regulators of gene 50 expression remain largely undefined. We report that the MHV68 lytic cycle is silenced in infected macrophages but not fibroblasts and that histone deacetylases (HDACs) mediate silencing. The HDAC inhibitor trichostatin A (TSA) acts on the gene 50 promoter to induce lytic replication of MHV68. HDAC3, HDAC4, and the nuclear receptor corepressor (NCoR) are required for efficient silencing of gene 50 expression. NCoR is critical for transcriptional repression of cellular genes by unliganded nuclear receptors. Retinoic acid, a known ligand for the NCoR complex, derepresses gene 50 expression and enhances MHV68 lytic replication. Moreover, HDAC3, HDAC4, and NCoR act on the gene 50 promoter and are recruited to this promoter in a retinoic acid-responsive manner. We provide the first example of NCoR-mediated, HDAC-dependent regulation of viral gene expression.
Infection with the gammaherpesviruses Epstein-Barr virus (EBV) or Kaposi's sarcoma-associated herpesvirus (KSHV) is associated with tumorigenesis in immunocompromised patients. Murine gammaherpesvirus 68 (MHV68, γHV68, MuHV4; GenBank accession number U97553) provides a tractable small-animal model of gammaherpesvirus pathogenesis. This virus shares significant genomic homology with EBV and KSHV and is associated with lymphomas and lymphoproliferative disease in immunocompromised mice (26, 47, 50).
Lytic replication of herpesviruses results from sequential immediate-early, early, and late viral gene expression. In contrast, only a limited subset of viral genes is expressed during latency; thus, genes involved in lytic replication must be silenced for latency to occur. In MHV68, essential immediate-early lytic switch gene 50 controls the interchange between lytic and latent gene expression programs. Gene 50 is the only gene necessary and sufficient to initiate MHV68 lytic replication during reactivation and de novo infection (35, 38, 45, 55, 56). Gene 50 encodes the replication and transcription activator (Rta) protein, which stimulates the expression of viral and cellular genes (5, 46). Rta can directly bind to target promoters via Rta-responsive cis elements or interact with cellular transcription factors to indirectly regulate gene expression (5, 46). Silencing gene 50 expression ensures that Rta promoter-responsive genes in the lytic cascade are not expressed and thereby prevents the lytic cycle. The importance of repressing gene 50 is illustrated by MHV68 mutants that constitutively express gene 50. Increased lytic replication occurs in these viruses, and the ability to establish latency in vivo is abolished (32, 40).
Many studies have focused on characterizing positive regulation of the lytic switch gene promoters in latently infected B-cell lines. Only a few negative regulators of lytic switch gene promoters have been identified, including ZEB1 and YY1 in EBV and the viral latency-associated nuclear antigen protein (LANA), recombination signal binding protein for immunoglobulin kappa J region (RBP-Jκ), and NF-κB in KSHV (2, 24, 25, 34, 59, 60). Histone deacetylase (HDAC) inhibitors induce lytic switch gene expression in latently infected B cells, implicating one or more of the 18 known cellular HDACs as additional negative regulators (4, 31, 57). Further, HDAC-associated repressor complexes regulate silencing of alphaherpesvirus gene expression (12-14). HDACs are best characterized for deacetylation of histones, resulting in a condensed chromatin structure and repressed gene transcription (44). Acetylated histones can be found on gammaherpesvirus lytic switch gene promoters upon HDAC inhibitor treatment (4, 31, 57). Furthermore, HDAC3 is reported to bind to the gene 50 promoter in MHV68 and HDACs 1, 5, and 7 are recruited to the gene 50 promoter in KSHV (31, 57). It is not known whether these HDACs are functionally important for MHV68 or KSHV infection. While abundant evidence points to a key role for HDACs in regulation of gammaherpesvirus promoters, the molecular mechanisms that mediate HDAC recruitment to the promoter are poorly understood.
Macrophages are a reservoir for latent MHV68 in vivo (7, 54). The cytokine gamma interferon and the transcription factor Stat1 are the only negative regulators of MHV68 in macrophages described to date (10). Furthermore, despite establishing latency in macrophages in vivo, lytic replication of MHV68 occurs in macrophage cell lines and primary macrophages infected in vitro. Here we report that the MHV68 lytic cycle is silenced in macrophages infected in vitro. HDAC inhibition relieves MHV68 silencing by inducing the gene 50 promoter. Moreover, we identified a novel role for the nuclear receptor corepressor (NCoR) in silencing of MHV68. We propose a model whereby NCoR, in concert with HDAC3 and HDAC4, repress the gene 50 promoter via promoter occupancy to silence lytic replication of MHV68 in macrophages.
MATERIALS AND METHODS
Cells, virus, and viral assays.
Primary bone marrow macrophages were derived as described previously (48). Mouse embryonic fibroblasts (MEFs) and RAW264.7 cells were grown in Dulbecco modified Eagle medium containing 10% fetal calf serum (52). For retinoic acid (RA) studies, serum was treated with dextran-coated charcoal (Sigma). Cells were infected with MHV68 clone WUMS (ATCC VR-1476). Trichostatin A (TSA), sodium butyrate (NaB), MS-275, 12-O-tetradecanoyl phorbol-13-acetate (TPA), ionomycin, or all-trans RA (Sigma) was added at 2 h postinfection (hpi). Viral passage and titer determination were performed on NIH 3T12 fibroblasts, as described previously (52). For immunofluorescence, virus was absorbed at 4°C. Cells were fixed with 1% paraformaldehyde, permeabilized with methanol, and stained with anti-MHV68 polyclonal antibody (1:1,000) (49, 53), followed by Alexa Fluor 488-conjugated antibody (Invitrogen). Coverslips were mounted with 4′,6-diamidino-2-phenylindole (Vectashield; Vector Laboratories).
qRT-PCR.
Total RNA was isolated and cDNA was synthesized as described previously (10). Quantitative reverse transcription-PCR (qRT-PCR) was performed with SYBR green (Invitrogen, Carlsbad, CA) and the following primer sequences: 5′-TGCCCCCATGTTTGTGATG and 5′-TGTGGTCATGAGCCCTTCC for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-AGAAACCCACAGCTCGCACTT and 5′-CAATATGCTGGACAGGCGTATC for gene 50, and 5′-CCAGAAGCTTGTGTACTTGTGGAT and 5′-AAATACCACAGCAGCGTAGAAGGT for gene 73. Viral transcript levels were normalized to the level of transcription of GAPDH within each sample using the ΔΔCT method (where CT is the threshold cycle) (30).
Frequency of cells that support lytic replication.
After viral absorption, cells were washed in fresh medium with vortexing. Serial 2-fold dilutions of cells were plated with dimethyl sulfoxide (DMSO) or TSA onto confluent monolayers of primary MEFs in 96-well plates. The virus-induced cytopathic effect (CPE) was scored 8 to 9 days later. Mechanical disruption of parallel samples was performed as described previously (52). Prism software (GraphPad) was used to analyze the data by nonlinear regression (sigmoidal dose curve with a nonvariable slope), and frequencies were determined using the Poisson distribution (52).
siRNA.
HiPerFect transfection reagent (Qiagen) was used to transfect cells with 750 ng of On-Targetplus siControl nontargeting pool or On-Targetplus Smartpool small interfering RNA (siRNA) targeting mouse HDAC1, HDAC3, HDAC4, NCoR, or the nuclear receptor corepressor SMRT (Dharmacon). Samples were analyzed by Western blotting using anti-HDAC1, anti-HDAC3, and anti-SMRT (Santa Cruz), anti-HDAC4 and anti-NCoR (Millipore), and antiactin (AC-74) (Sigma). A Storm phosphorimager and ImageQuant software (GE Healthcare) were used to visualize and quantify the protein signals. Protein signals were normalized to the signal for the actin loading control. To confirm that siRNA transfection did not induce an interferon response, all siRNAs were assessed for induction of Stat1, interferon-stimulated gene 15 (ISG15), and ISG56 expression by qRT-PCR.
Plasmid constructs and transfection.
pGL2-410bp and pGL2-ORF57 are described elsewhere (28). Rta from the pcDNA3-Rta vector (56) was cloned into the pUB/V5-His expression vector (Invitrogen) to construct pUB-Rta. HDAC3 and HDAC4 were amplified from cDNA prepared from primary macrophages using the Expand long-template PCR system (Roche Applied Science) and the following primers: 5′-GGTACCATGGCCAAGACCGTGG and 5′-TCTAGACTAAATCTCCACATCACTTTCCTTG for HDAC3 and 5′-GGTACCATGAGCTCCCAAAGCCA and 5′-TCTAGACTACAGTGGTGGTTCCTCCTCCA for HDAC4. PCR products were cloned into pCR2.1-TOPO (Invitrogen) and subcloned into pUB/V5-His. Cesium chloride preparations of plasmids were transfected by an Amaxa nucleofector kit (Lonza).
Luciferase assay.
Four micrograms of pGL2-410bp or pGL2-ORF57 and 3 μg of pUB/V5-His/lacZ (Invitrogen) were transfected into primary macrophages (2 × 106 cells) prior to DMSO or TSA treatment. Luciferase activity was measured with the luciferase assay system kit (Promega), and the level of expression was normalized to that of β-galactosidase. The value from cells transfected with empty pGL2 vector was subtracted for each condition.
ChIP.
DNA was prepared for chromatin immunoprecipitation (ChIP) as described previously (37). qPCR was performed with the following primers: 5′-CTCTGTCAGATGTGACCATGAG and 5′-CTCTCTCCTCAGCCTTTGAAGG for the 410-bp promoter and 5′-GGATTGCCTGAGAGACTGCTAAAAT and 5′-GGCCACAAGGTGGTGTCTATCCA for the gene 57 promoter. Copy number was calculated from the sample CT value using a standard curve for each primer set generated by dilutions of a MHV68-BAC plasmid. The background signal from a no-antibody control was subtracted from the sample signal, and the percentage of ChIP DNA relative to the amount of input DNA was calculated. Antibodies used for ChIP include an isotype anti-His control (Santa Cruz) and antibodies listed in the methods described in “siRNA” above.
Statistical analysis.
The statistical tests used are listed in the figure legends. All experimental conditions were compared to those for their corresponding controls, and only significant differences are noted.
RESULTS
HDACs silence MHV68 lytic replication in macrophages but not fibroblasts.
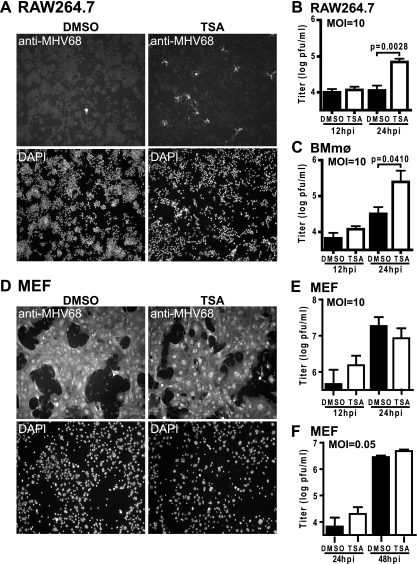
Chemical agents that induce lytic replication in latently infected B cells were tested for induction of MHV68 lytic replication in macrophages. Infected cells were treated with TSA (HDAC inhibitor), TPA (phorbol ester), or ionomycin (calcium ionophore) at 2 hpi; and lytic protein expression was assessed at 24 hpi using the anti-MHV68 polyclonal antibody (49, 53). Chemical agents were added after infection to eliminate the possibility of interfering with virus binding to the cell or entry. Despite a high multiplicity of infection (MOI) (MOI = 10), very few cells expressed lytic proteins in infected RAW264.7 cultures treated with the DMSO vehicle control or medium alone (Fig. 1 A and data not shown). Compared to DMSO treatment, cultures treated with TSA had significantly more cells expressing lytic proteins (Fig. 1A). In a dose-dependent manner, TSA increased the MHV68 titer in RAW264.7 cells by 24 hpi (Fig. 1B and data not shown). TSA also increased the viral titer in primary macrophage cultures (Fig. 1C). TPA and ionomycin did not alter MHV68 infection (data not shown). These data suggest that HDAC activity is required to suppress lytic replication of MHV68 in macrophages.
FIG. 1.
TSA increases the number of cells expressing MHV68 lytic proteins and increases MHV68 replication in macrophage cultures. (A) Infected RAW264.7 cells (MOI = 10) treated with DMSO or 130 nM TSA and examined by immunofluorescence for MHV68 lytic protein expression at 24 hpi (one of three experiments shown); (B and C) viral titer from RAW264.7 cells (mean ± standard error of the mean [SEM], 3 to 4 experiments) (B) and primary macrophages (BMmφ) (mean ± SEM, 3 to 5 experiments) (C) treated as described for panel A; (D) immunofluorescence of MEFs treated as described for panel A (the results of one of three experiments are shown); (E and F) viral titer from MEFs infected at an MOI of 10 (E) or an MOI of 0.05 (F) and treated as described for panel A (panel E, mean ± SEM, 5 experiments; panel F, mean ± SEM, 4 experiments). Statistical analyses were done by Student's t test.
To assess whether HDACs regulate MHV68 in a cell type that efficiently supports lytic replication in vitro, we examined lytic protein expression and viral titer in primary MEFs. In contrast to macrophages, infection of MEFs (MOI = 10) induced lytic protein expression in the majority of cells by 24 hpi (Fig. 1D). TSA did not change the number of cells infected or the viral titer (Fig. 1D and E). Furthermore, TSA did not alter the viral titer in MEFs when a small number of cells were infected by using a lower viral inoculum (MOI = 0.05) (Fig. 1F). Thus, in contrast to macrophages, lytic replication of MHV68 in MEF cells is unresponsive to TSA.
TSA increases the frequency of macrophage cells that support MHV68 replication.
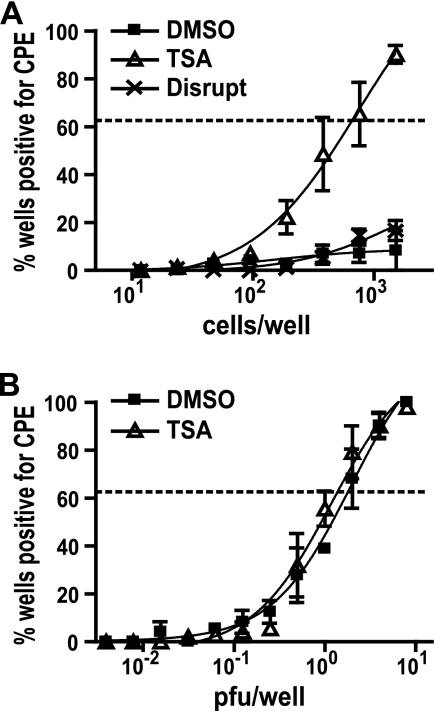
Given that a high MOI was used to infect macrophages yet few cells expressed lytic proteins (Fig. 1), we hypothesized that there were more infected cells than cells that expressed lytic proteins. This notion is supported by the finding that TSA treatment after infection increased the number of cells expressing lytic proteins, suggesting that HDAC activity contributes to silencing of the MHV68 lytic cycle in macrophages. We therefore used a limiting-dilution assay to directly measure the number of cells undergoing productive MHV68 infection. Cells were infected, incubated for 1 h to allow the virus to enter, washed extensively, and then plated in limiting dilutions onto MEF monolayers, which serve as indicators for release of infectious virus from productively infected cells. Negligible amounts of infectious virus remained after viral absorption, as determined by mechanical disruption of the cells before they were plated (Fig. 2 A). The effect of TSA on the number of productively infected cells was measured by adding the drug at the time that the cells were plated onto MEF monolayers. TSA increased the frequency of RAW264.7 cells that support lytic replication of MHV68 compared to that achieved with DMSO (Fig. 2A). Further, TSA did not alter the sensitivity of MEF monolayers for detection of released virus (Fig. 2B). Taken together, these data indicate that the MHV68 lytic cycle is silenced in a subset of infected cells and that HDAC activity contributes to this silencing.
FIG. 2.
TSA increases the frequency of macrophage cells that support MHV68 lytic replication. (A) Limiting dilutions of infected RAW264.7 cells (MOI = 10) plated onto MEF monolayers in the presence of DMSO or 130 nM TSA. Parallel samples were mechanically disrupted to kill the cells before they were plated. The statistical difference between TSA and DMSO is P < 0.05 (mean ± SEM, 3 experiments). (B) Limiting dilutions of MHV68 plated onto MEFs in the presence of DMSO or 130 nM TSA (mean ± SEM, 3 experiments). Dashed lines indicate the 63.2% Poisson distribution line used to calculate frequencies. Statistical analysis was done by paired t test.
TSA induces gene 50 expression in macrophages but not fibroblasts.
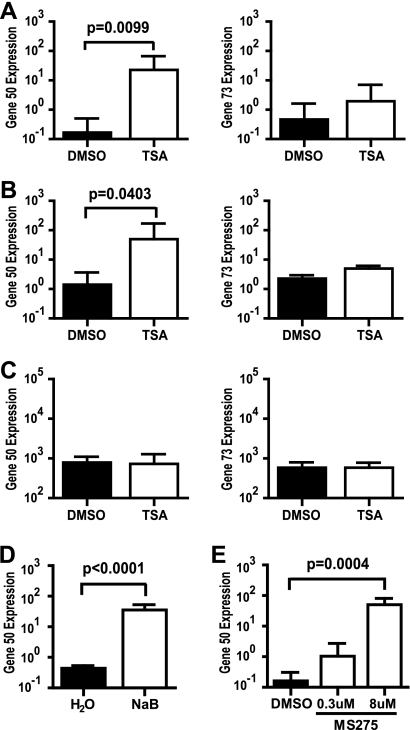
Since gene 50 is necessary and sufficient to trigger the lytic replication cycle, we examined whether TSA induced expression of gene 50. Given that an antibody specific to MHV68 Rta is not currently available, we assessed the effect of TSA on gene 50 transcript levels instead of Rta protein levels. Infected macrophages were treated at 2 hpi with DMSO or TSA, and RNA was harvested 12 hpi. A 12-hpi time point was selected, as no difference in viral titer was observed between DMSO and TSA at this time (Fig. 1B and C). TSA significantly increased gene 50 transcript levels ∼135-fold and ∼35-fold in RAW264.7 cells and primary macrophages, respectively (Fig. 3 A and B). In contrast, TSA did not alter transcript levels for immediate-early latency-associated gene 73 (Fig. 3A and B). Furthermore, TSA did not alter gene 50 expression in infected MEFs (Fig. 3C), likely accounting for the inability of TSA to alter MHV68 lytic replication in these cells (Fig. 1D to F). Taken together, these data suggest that HDACs silence the MHV68 lytic cycle in macrophages by specific regulation of viral genes, including gene 50.
FIG. 3.
HDAC inhibitors stimulate expression of gene 50 in macrophages. Infected cells (MOI = 10) were treated with HDAC inhibitor, and viral transcript levels were examined at 12 hpi. (A to C) Gene 50 and gene 73 transcript levels in RAW264.7 cells (mean ± SEM, 6 experiments) (A), primary macrophages (mean ± SEM, 5 to 7 experiments) (B), and MEF cells (mean ± SEM, 3 experiments) (C) treated with 130 nM TSA; (D) gene 50 transcript levels in RAW264.7 cells treated with 3 mM NaB (mean ± SEM, 3 experiments); (E) gene 50 transcript levels in RAW264.7 cells treated with 0.3 μM or 8 μM MS-275 (mean ± SEM, 4 experiments). Statistical analyses were done by Student's t test.
TSA is a broad-spectrum inhibitor targeting class I (HDACs 1, 2, 3, and 8), class IIa (HDACs 4, 5, 7, and 9), class IIb (HDACs 6 and 10), and class IV (HDAC11) HDACs (1). To narrow the list of candidate HDACs required to silence gene 50, we next examined whether gene 50 expression increased in RAW264.7 cells treated 2 hpi with NaB or MS-275. NaB is an inhibitor of class I and class IIa HDACs, whereas MS-275 is a class I-selective inhibitor (1). NaB significantly increased gene 50 transcript levels ∼67-fold (Fig. 3D). Furthermore, gene 50 transcript levels significantly increased ∼309-fold in response to 8 μM MS-275 treatment (the predicted 50% inhibitory concentration [IC50] for HDAC3) (Fig. 3E) (16). In contrast, gene 50 expression was not significantly altered by 0.3 μM MS-275 (the predicted IC50 for HDAC1) (Fig. 3E) (16). Taken together, these drug studies suggest that HDAC3 and, possibly, class IIa HDACs are important for silencing gene 50 expression.
HDAC3 and HDAC4 mediate silencing of the MHV68 lytic cycle in macrophages.
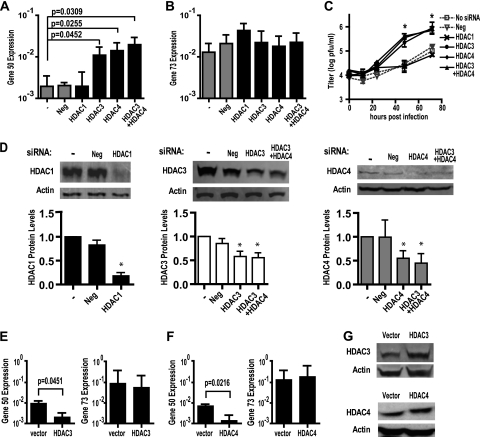
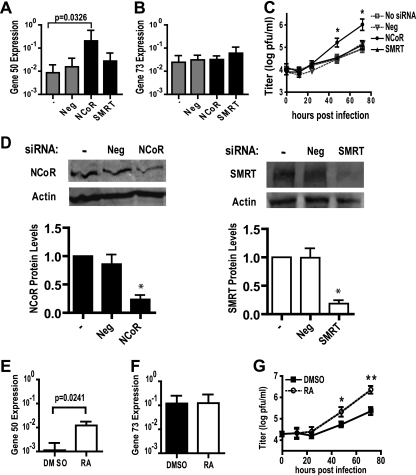
To provide insight into the mechanism of HDAC-mediated silencing of MHV68, we sought to further identify the specific HDAC(s) which repressed gene 50 expression and viral replication. Given the dose-dependent effect of MS-275 on gene 50 expression (Fig. 3E), we reasoned that HDAC3, but not HDAC1, may be required to silence gene 50. Given that HDAC4 is a class IIa HDAC found complexed with HDAC3 as part of a multiprotein corepressor complex (6, 27), we wondered whether HDAC4 was also important for repressing gene 50. We therefore transfected RAW264.7 cells with siRNA to target HDAC 1, 3, or 4 and then infected cells 24 h later. At 12 hpi, gene 50 transcript levels were significantly higher in cells transfected with siRNA targeting HDAC3 or HDAC4 than in cells transfected with no siRNA (Fig. 4 A). Gene 50 transcript levels were not significantly changed by siRNA targeting HDAC1 or a negative-control siRNA with no predicted targets (Fig. 4A). Gene 73 transcript levels were not significantly altered by the siRNAs tested (Fig. 4B). The viral titer was also significantly increased in cells transfected with siRNA targeting HDAC3 or HDAC4 but not HDAC1 or the negative control (Fig. 4C). A significant ∼5- to 8-fold increase in gene 50 levels and an ∼10- to 17-fold increase in viral replication occurred with only an ∼50% knockdown of HDAC3 or HDAC4 expression (Fig. 4D).
FIG. 4.
HDAC3 and HDAC4 silence gene 50 expression and MHV68 replication in macrophages. RAW264.7 cells were transfected with the indicated siRNA 24 h before they were infected with MHV68 (MOI = 10). (A and B) Gene 50 (A) and gene 73 (B) transcript levels at 12 hpi (mean ± SEM, 3 to 5 experiments); (C) viral titer (mean ± SEM, 2 to 4 experiments); (D) representative Western blots of siRNA knockdown and corresponding quantification of protein levels normalized to those for actin (mean ± SEM, 2 to 4 experiments); (E and F) gene 50 and gene 73 transcript levels in infected RAW264.7 cells (MOI = 10) transfected with 5 μg of pUB-HDAC3 (E; mean ± SEM, 3 experiments) or 4 μg of pUB-HDAC4 (F; mean ± SEM, 4 experiments); (G) representative Western blots of HDAC3 and HDAC4 expression in RAW264.7 treated as described for panels E and F. Statistical analyses were done by Student's t test. *, P < 0.05.
To confirm that HDAC3 and HDAC4 silenced the MHV68 lytic cycle, RAW264.7 cells were transfected with a vector expressing HDAC3 or HDAC4. Cells were infected at 24 h posttransfection and RNA was collected 12 hpi. Compared to the results for the empty vector control, overexpression of HDAC3 or HDAC4 significantly decreased gene 50 transcript levels (Fig. 4E to G). In contrast, gene 73 transcript levels were not significantly changed, indicating that HDAC3 and HDAC4 overexpression did not markedly interfere with early events during infection (Fig. 4E to G).
HDAC3 and HDAC4 are found together as part of a multiprotein nuclear receptor corepressor complex (6, 27). The observation that gene 50 expression and MHV68 replication were increased by siRNA knockdown of HDAC3 or HDAC4, but not HDAC1, suggested that a complex containing HDAC3 and HDAC4 might regulate MHV68. Interestingly, no difference in gene 50 transcript levels or MHV68 replication was observed after knockdown of both HDAC3 and HDAC4 compared to the findings observed after knockdown of HDAC3 or HDAC4 alone (Fig. 4A and C). These data are consistent with HDAC3 and HDAC4 working in a single pathway to repress the MHV68 lytic cycle.
NCoR mediates silencing of MHV68 in macrophages.
HDAC3 and HDAC4 can associate in a complex with the nuclear receptor corepressors NCoR and SMRT to mediate transcriptional repression by nuclear receptors (9, 22). Therefore, we tested whether NCoR and SMRT silence the MHV68 lytic cycle in macrophages. RAW264.7 cells were transfected with siRNA targeting NCoR or SMRT and infected 24 h later. At 12 hpi, siRNA knockdown of NCoR significantly increased gene 50 transcript levels compared to those for the control transfected cells (Fig. 5 A). Knockdown of NCoR did not significantly change gene 73 transcript levels (Fig. 5B). The viral titer was also significantly increased in cells transfected with siRNA targeting NCoR (Fig. 5C). In contrast, gene 50 transcript levels and the viral titer were not significantly altered by siRNA targeting SMRT (Fig. 5A to D). Taken together, these data indicate that NCoR silences gene 50 expression and viral replication in macrophages.
FIG. 5.
NCoR represses gene 50 expression and MHV68 replication in macrophages. (A and B) Gene 50 and gene 73 transcript levels measured at 12 hpi (mean ± SEM, 5 to 7 experiments); (C) viral titer (mean ± SEM, 3 to 4 experiments) from RAW264.7 cells transfected with the indicated siRNA 24 h before infection with MHV68 (MOI = 10); (D) representative Western blots of siRNA knockdown and corresponding quantification of protein levels normalized to those of actin (mean ± SEM, 3 to 5 experiments); (E and F) gene 50 and gene 73 transcript levels measured at 12 hpi (mean ± SEM, 4 experiments); (G) viral titer (mean ± SEM, 3 to 6 experiments) from RAW264.7 cells infected with MHV68 (MOI = 10) and treated with DMSO or 1 μM RA. Statistical analyses were done by Student's t test. *, P < 0.05; **, P < 0.01.
Retinoic acid induces lytic cycle of MHV68 in macrophages.
NCoR can bind to and serve as a corepressor for retinoic acid receptors (RARs). Upon ligand binding, this interaction is disrupted, allowing derepression of gene expression (41). Therefore, we hypothesized that derepression of gene 50 might occur in macrophages treated with a ligand of the NCoR complex. We observed significantly higher gene 50 but not gene 73 transcript levels at 12 hpi in RAW264.7 cells treated at 2 hpi with RA than in those treated with DMSO (Fig. 5E and F). The viral titer was also significantly enhanced by RA (Fig. 5G). These data indicate that the MHV68 lytic cycle is retinoic acid responsive in macrophages and suggested the hypothesis that NCoR-HDAC activity is responsible for this responsiveness.
Lytic switch gene 50 promoter activity is regulated by HDACs in primary macrophages.
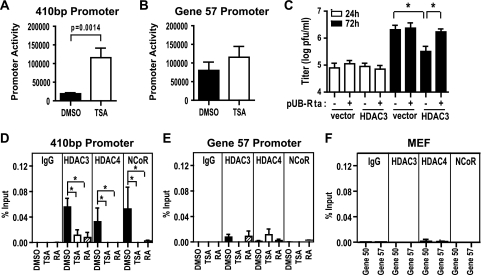
Previous studies show that the lytic switch gene promoters in EBV and KSHV are responsive to HDAC inhibitors in latently infected B-cell lines (4, 31, 58). We therefore tested whether TSA acted on the gene 50 promoter in primary macrophages. Cells were transfected with a luciferase reporter containing the proximal 410-bp gene 50 promoter (11, 28). Transfected cells were treated with TSA or DMSO, and promoter activity was measured 12 h later. TSA increased promoter activity ∼7-fold (Fig. 6 A). In contrast, TSA did not significantly change promoter activity for the previously described immediate-early gene 57 promoter (28), suggesting that HDAC activity regulates specific viral promoters (Fig. 6B).
FIG. 6.
The gene 50 promoter is TSA responsive and NCoR, HDAC3, and HDAC4 are recruited to the promoter in an RA-dependent manner. (A and B) Uninfected primary macrophages were transfected with pGL2-410bp (A; mean ± SEM, 8 experiments) or pGL2-ORF57 (B; mean ± SEM, 5 experiments) and treated with DMSO or 130 nM TSA. (C) Viral titer from RAW264.7 cells cotransfected with 0.5 μg of pUB-Rta and 4 μg pUB-HDAC3 or empty vector (mean ± SEM, 3 to 5 experiments). (D and E) ChIP analysis of HDAC3, HDAC4, and NCoR recruitment to the 410-bp promoter and gene 57 promoter in infected primary macrophages (MOI = 10) at 12 hpi. Cells were treated at 2 hpi with DMSO, 130 nM TSA, or 1 μM RA (mean ± SEM, 3 to 4 experiments). (F) ChIP analysis of the 410-bp promoter and gene 57 promoter in infected MEF cells (MOI = 10) at 12 hpi using antibodies to HDAC3, HDAC4, and NCoR (mean ± SEM, 2 experiments). Statistical analyses were done by Student's t test (A to C) or the Mann-Whitney test (D to F). *, P < 0.05.
Given that the gene 50 promoter could be regulated by HDACs, we tested whether the ability of HDAC3 to silence the MHV68 lytic cycle was associated with regulation of the gene 50 promoter. We examined whether HDAC3 overexpression suppressed MHV68 replication and whether such inhibition could be relieved by supplying the gene 50 product, Rta, in trans. RAW264.7 cells were transfected with HDAC3 or empty vector and infected 24 h later. At 72 hpi, overexpression of HDAC3 significantly reduced the viral titer (Fig. 6C). The HDAC3-mediated decrease in viral titer could be rescued by cotransfecting gene 50 driven by the ubiquitin promoter (pUB-Rta) (Fig. 6C). These data suggest that HDAC3 repression of the gene 50 promoter is important for the observed HDAC3-mediated decrease in gene 50 transcripts and silencing of lytic replication (Fig. 4).
NCoR, HDAC3, and HDAC4 are recruited to lytic switch gene 50 promoter in a retinoic acid-responsive manner.
To determine whether NCoR, HDAC3, and HDAC4 exert their effects on gene 50 by binding to the gene 50 promoter, ChIP assays were performed on infected primary macrophages at 12 hpi. NCoR, HDAC3, and HDAC4 were detected on the 410-bp promoter in DMSO-treated samples (Fig. 6D). Recruitment of HDAC3, HDAC4, and NCoR to the gene 50 promoter was significantly reduced by either TSA or RA (Fig. 6D). The removal of NCoR, HDAC3, and HDAC4 from the gene 50 promoter likely contributes to the observed derepression of gene 50 in response to TSA and RA (Fig. 3B and 5E). Importantly, NCoR, HDAC3, and HDAC4 were not associated with the gene 57 promoter, and no significant changes in their association occurred with TSA or RA (Fig. 6E). Furthermore, NCoR, HDAC3, and HDAC4 were not recruited to the gene 50 or gene 57 promoter in infected MEF cells (Fig. 6F). This finding demonstrates that NCoR, HDAC3, and HDAC4 bind to the promoters of specific viral genes in macrophages and is consistent with these proteins silencing gene 50 by repressing the promoter in a cell-type-specific manner.
DISCUSSION
In this report, we show that TSA reverses silencing of the MHV68 lytic cycle in macrophages by directly stimulating gene 50 promoter activity and gene 50 expression, thereby increasing the frequency of cells permissive for lytic replication. Although direct binding of histones on the transfected 410-bp promoter was not assessed in this study, work by Lu et al. demonstrates that nucleosomes associate with a transfected gene 50 promoter reporter and are positioned similarly to the endogenous gene 50 promoter in KSHV (31). We show a novel role for NCoR, HDAC3, and HDAC4 in silencing the MHV68 lytic cycle and demonstrate binding of NCoR, HDAC3, and HDAC4 to the endogenous gene 50 promoter during MHV68 infection. Given that gene 50 is necessary and sufficient to trigger the lytic cycle, recruitment of NCoR, HDAC3, and HDAC4 to the gene 50 promoter likely mediates repression of gene 50 expression and viral replication. This idea is supported by our finding that HDAC3-mediated inhibition of lytic replication is rescued by expressing gene 50 in trans (Fig. 6C).
Finally, we demonstrate that RA derepresses gene 50 expression in macrophages likely by regulating recruitment of NCoR, HDAC3, and HDAC4 to the gene 50 promoter. Interestingly, the EBV lytic switch gene BZLF1 induces cellular DHRS9 expression to convert retinol into RA and thereby is predicted to promote lytic replication (21). In contrast, 9-cis-retinoic acid and synthesized retinoid compounds appear to restrict KSHV replication in HUVEC and 293T cells (3). Taken together with data presented here, these studies suggest a possible general role of the RA pathway for gammaherpesviruses; however, depending on the cell type and the specific retinoid, there may be interesting differences between MHV68 and KSHV.
HDAC-mediated silencing of other herpesviruses.
HDAC-mediated silencing of the lytic cycle is not unique to gammaherpesviruses. In a fibroblast model of quiescent herpes simplex virus type 1 infection, deletion of the ICP0 protein results in silencing of early and late gene expression. Interestingly, ICP0 harbors a domain that mediates direct binding to the cellular corepressor CoREST (13). In cells infected with wild-type virus, the CoREST-HDAC1/2 complex is disrupted and components are independently translocated to the cytoplasm (12, 14). Furthermore, silencing of the ICP0 mutant virus is partially rescued in a recombinant mutant virus that expresses a CoREST peptide to displace HDAC1 (13).
In latent-like human cytomegalovirus (HCMV) infection of undifferentiated NT2 or THP-1 cells, immediate-early viral gene expression and lytic replication are silenced by establishing a repressive chromatin structure on the major immediate-early promoter (36). This silencing is mediated by the corepressor Daxx and requires HDAC activity (36, 43). Silencing is abolished in differentiated NT2 or THP-1 cells by the degradation of Daxx, a process mediated by HCMV protein pp71 (43). HDAC-mediated silencing does not occur in fibroblasts infected with wild-type HCMV because pp71 is able to degrade Daxx in these cells (42). Together with the data presented here, these findings suggest a general role for HDACs in herpesvirus silencing that is mediated by virus and cell-type-specific molecular mechanisms.
Implications of NCoR-mediated silencing of MHV68 in macrophages.
It is intriguing that MHV68 maintains an NCoR-responsive lytic switch gene, given that NCoR serves as a corepressor for numerous nuclear receptors. The NCoR complex can also interact with other transcription factors, some of which are reported to regulate the lytic cycle of gammaherpesviruses (e.g., NF-κB, AP-1, and MEF2) (2, 23, 29, 33, 39, 51). This raises the possibility that NCoR-mediated regulation of gene 50 may provide many ligands with cell-extrinsic control over the interchange between viral life cycles. Our finding that RA dissociates NCoR from the gene 50 promoter and derepresses gene 50 expression suggests that at least one regulator of NCoR can mediate cell-extrinsic control over the MHV68 lytic cycle. Determining whether NCoR represses gene 50 to silence lytic replication and facilitate latency in vivo will be important to understanding the molecular mechanisms of latency and chronic infection.
We did not detect a role for SMRT in silencing of MHV68, a finding potentially due to insufficient depletion of SMRT expression. HDAC3 and HDAC4 interact with both NCoR and SMRT, and binding to NCoR or SMRT is required to activate HDAC3 enzymatic activity (6, 15, 17, 27). However, NCoR and SMRT have independent roles in cellular gene expression, in addition to functioning together. While deletion of either protein is embryonic lethal, the phenotypes of NCoR−/− and SMRT−/− embryos suggest that they do not have redundant functions (18-20). Interestingly, recent studies in primary macrophages indicate that a broad set of inflammatory genes can be regulated in an NCoR-specific, SMRT-specific, or NCoR/SMRT-specific manner (8). Thus, it is possible that NCoR repression of gene 50 represents an NCoR-specific activity.
It is intriguing to postulate that NCoR-specific regulation may be cell type dependent, as this could contribute to the differences observed between the MHV68 lytic cycle in macrophages and MEFs. Studies of acute promyelocytic leukemia highlight the critical nature of NCoR-mediated repression by RARs in myeloid cells (22); however, the relevance of NCoR-specific gene regulation in other cell types is not well defined. Alternatively, histone acetylases (HATs) are a diverse set of enzymes, and the HATs that regulate gene 50 or whether differences in HAT activity contribute to the cell-type-specific effects observed here remains unknown.
We have identified a novel role for HDAC3, HDAC4, and NCoR in repression of the MHV68 lytic cycle. It is intriguing that distinct HDAC complexes are utilized to silence different herpesviruses. Perhaps some of the variation in herpesvirus biology, including cell-type- and tissue-specific sites of latency or virus-induced pathologies, is a reflection of distinct HDAC complex usage.
Acknowledgments
We thank Erik S. Barton and Douglas W. White for expert advice contributing to this study.
M.M.G. was supported by a National Science Foundation Graduate Research Fellowship, M.A.E.H. was supported by the NCIC and Krembil Foundation, R.B. was supported by the Canadian Cancer Society Research Institute, and H.W.V. was supported by NIH grant RO1 CA 96511.
Footnotes
Published ahead of print on 18 August 2010.
REFERENCES
- 1.Bolden, J. E., M. J. Peart, and R. W. Johnstone. 2006. Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov. 5:769-784. [DOI] [PubMed] [Google Scholar]
- 2.Brown, H. J., M. J. Song, H. Y. Deng, T. T. Wu, G. H. Cheng, and R. Sun. 2003. NF-kappa B inhibits gammaherpesvirus lytic replication. J. Virol. 77:8532-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caselli, E., M. Galvan, F. Santoni, S. Alvarez, A. R. de Lera, D. Ivanova, H. Gronemeyer, A. Caruso, M. Guidoboni, E. Cassai, R. Dolcetti, and L. D. Di. 2008. Retinoic acid analogues inhibit human herpesvirus 8 replication. Antivir. Ther. 13:199-209. [PubMed] [Google Scholar]
- 4.Chang, L. K., and S. T. Liu. 2000. Activation of the BRLF1 promoter and lytic cycle of Epstein-Barr virus by histone acetylation. Nucleic Acids Res. 28:3918-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng, H., Y. Liang, and R. Sun. 2007. Regulation of KSHV lytic gene expression. Curr. Top. Microbiol. Immunol. 312:157-183. [DOI] [PubMed] [Google Scholar]
- 6.Fischle, W., F. Dequiedt, M. J. Hendzel, M. G. Guenther, M. A. Lazar, W. Voelter, and E. Verdin. 2002. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol. Cell 9:45-57. [DOI] [PubMed] [Google Scholar]
- 7.Flano, E., S. M. Husain, J. T. Sample, D. L. Woodland, and M. A. Blackman. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 165:1074-1081. [DOI] [PubMed] [Google Scholar]
- 8.Ghisletti, S., W. Huang, K. Jepsen, C. Benner, G. Hardiman, M. G. Rosenfeld, and C. K. Glass. 2009. Cooperative NCoR/SMRT interactions establish a corepressor-based strategy for integration of inflammatory and anti-inflammatory signaling pathways. Genes Dev. 23:681-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glass, C. K., and M. G. Rosenfeld. 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14:121-141. [PubMed] [Google Scholar]
- 10.Goodwin, M. M., S. Canny, A. Steed, and H. W. Virgin. 2010. Murine gammaherpesvirus 68 has evolved gamma interferon and Stat1-repressible promoters for the lytic switch gene 50. J. Virol. 84:3711-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray, K. S., R. D. Allen III, M. L. Farrell, J. C. Forrest, and S. H. Speck. 2009. Alternatively initiated gene 50/RTA transcripts expressed during murine and human gammaherpesvirus reactivation from latency. J. Virol. 83:314-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu, H., Y. Liang, G. Mandel, and B. Roizman. 2005. Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells. Proc. Natl. Acad. Sci. U. S. A. 102:7571-7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu, H., and B. Roizman. 2007. Herpes simplex virus-infected cell protein 0 blocks the silencing of viral DNA by dissociating histone deacetylases from the CoREST-REST complex. Proc. Natl. Acad. Sci. U. S. A. 104:17134-17139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu, H., and B. Roizman. 2009. Engagement of the lysine-specific demethylase/HDAC1/CoREST/REST complex by herpes simplex virus 1. J. Virol. 83:4376-4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guenther, M. G., O. Barak, and M. A. Lazar. 2001. The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol. Cell. Biol. 21:6091-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu, E., E. Dul, C. M. Sung, Z. Chen, R. Kirkpatrick, G. F. Zhang, K. Johanson, R. Liu, A. Lago, G. Hofmann, R. Macarron, M. de los Frailes, P. Perez, J. Krawiec, J. Winkler, and M. Jaye. 2003. Identification of novel isoform-selective inhibitors within class I histone deacetylases. J. Pharmacol. Exp. Ther. 307:720-728. [DOI] [PubMed] [Google Scholar]
- 17.Huang, E. Y., J. Zhang, E. A. Miska, M. G. Guenther, T. Kouzarides, and M. A. Lazar. 2000. Nuclear receptor corepressors partner with class II histone deacetylases in a Sin3-independent repression pathway. Genes Dev. 14:45-54. [PMC free article] [PubMed] [Google Scholar]
- 18.Jepsen, K., A. S. Gleiberman, C. Shi, D. I. Simon, and M. G. Rosenfeld. 2008. Cooperative regulation in development by SMRT and FOXP1. Genes Dev. 22:740-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jepsen, K., O. Hermanson, T. M. Onami, A. S. Gleiberman, V. Lunyak, R. J. McEvilly, R. Kurokawa, V. Kumar, F. Liu, E. Seto, S. M. Hedrick, G. Mandel, C. K. Glass, D. W. Rose, and M. G. Rosenfeld. 2000. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell 102:753-763. [DOI] [PubMed] [Google Scholar]
- 20.Jepsen, K., D. Solum, T. Zhou, R. J. McEvilly, H. J. Kim, C. K. Glass, O. Hermanson, and M. G. Rosenfeld. 2007. SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature 450:415-419. [DOI] [PubMed] [Google Scholar]
- 21.Jones, R. J., S. Dickerson, P. M. Bhende, H. J. Delecluse, and S. C. Kenney. 2007. Epstein-Barr virus lytic infection induces retinoic acid-responsive genes through induction of a retinol-metabolizing enzyme, DHRS9. J. Biol. Chem. 282:8317-8324. [DOI] [PubMed] [Google Scholar]
- 22.Karagianni, P., and J. Wong. 2007. HDAC3: taking the SMRT-N-CoRrect road to repression. Oncogene 26:5439-5449. [DOI] [PubMed] [Google Scholar]
- 23.Krug, L. T., C. M. Collins, L. M. Gargano, and S. H. Speck. 2009. NF-kappaB p50 plays distinct roles in the establishment and control of murine gammaherpesvirus 68 latency. J. Virol. 83:4732-4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lan, K., D. A. Kuppers, and E. S. Robertson. 2005. Kaposi's sarcoma-associated herpesvirus reactivation is regulated by interaction of latency-associated nuclear antigen with recombination signal sequence-binding protein Jkappa, the major downstream effector of the Notch signaling pathway. J. Virol. 79:3468-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lan, K., D. A. Kuppers, S. C. Verma, and E. S. Robertson. 2004. Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen inhibits lytic replication by targeting Rta: a potential mechanism for virus-mediated control of latency. J. Virol. 78:6585-6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, K. S., S. D. Groshong, C. D. Cool, B. K. Kleinschmidt-DeMasters, and L. F. Van Dyk. 2009. Murine gammaherpesvirus 68 infection of IFNgamma unresponsive mice: a small animal model for gammaherpesvirus-associated B-cell lymphoproliferative disease. Cancer Res. 69:5481-5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, J., J. Wang, J. Wang, Z. Nawaz, J. M. Liu, J. Qin, and J. Wong. 2000. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J. 19:4342-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, S., I. V. Pavlova, H. W. Virgin, and S. H. Speck. 2000. Characterization of gammaherpesvirus 68 gene 50 transcription. J. Virol. 74:2029-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, S. F., P. F. Liu, A. Borras, T. Chatila, and S. H. Speck. 1997. Cyclosporin A-sensitive induction of the Epstein-Barr virus lytic switch is mediated via a novel pathway involving a MEF2 family member. EMBO J. 16:143-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 31.Lu, F., J. Zhou, A. Wiedmer, K. Madden, Y. Yuan, and P. M. Lieberman. 2003. Chromatin remodeling of the Kaposi's sarcoma-associated herpesvirus ORF50 promoter correlates with reactivation from latency. J. Virol. 77:11425-11435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.May, J. S., H. M. Coleman, B. Smillie, S. Efstathiou, and P. G. Stevenson. 2004. Forced lytic replication impairs host colonization by a latency-deficient mutant of murine gammaherpesvirus-68. J. Gen. Virol. 85:137-146. [DOI] [PubMed] [Google Scholar]
- 33.Miska, E. A., C. Karlsson, E. Langley, S. J. Nielsen, J. Pines, and T. Kouzarides. 1999. HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J. 18:5099-5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montalvo, E. A., M. Cottam, S. Hill, and Y. J. Wang. 1995. YY1 binds to and regulates cis-acting negative elements in the Epstein-Barr virus BZLF1 promoter. J. Virol. 69:4158-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moorman, N. J., C. Y. Lin, and S. H. Speck. 2004. Identification of candidate gammaherpesvirus 68 genes required for virus replication by signature-tagged transposon mutagenesis. J. Virol. 78:10282-10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy, J. C., W. Fischle, E. Verdin, and J. H. Sinclair. 2002. Control of cytomegalovirus lytic gene expression by histone acetylation. EMBO J. 21:1112-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ni, Z., and R. Bremner. 2007. Brahma-related gene 1-dependent STAT3 recruitment at IL-6-inducible genes. J. Immunol. 178:345-351. [DOI] [PubMed] [Google Scholar]
- 38.Pavlova, L. V., H. W. Virgin, and S. H. Speck. 2003. Disruption of gammaherpesvirus 68 gene 50 demonstrates that Rta is essential for virus replication. J. Virol. 77:5731-5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perissi, V., K. Jepsen, C. K. Glass, and M. G. Rosenfeld. 2010. Deconstructing repression: evolving models of co-repressor action. Nat. Rev. Genet. 11:109-123. [DOI] [PubMed] [Google Scholar]
- 40.Rickabaugh, T. M., H. J. Brown, D. A. Martinez-Guzman, T. T. Wu, L. M. Tong, F. Q. Yu, S. Cole, and R. Sun. 2004. Generation of a latency-deficient gammaherpesvirus that is protective against secondary infection. J. Virol. 78:9215-9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rochette-Egly, C., and P. Germain. 2009. Dynamic and combinatorial control of gene expression by nuclear retinoic acid receptors (RARs). Nucl. Recept. Signal. 7:e005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saffert, R. T., and R. F. Kalejta. 2006. Inactivating a cellular intrinsic immune defense mediated by Daxx is the mechanism through which the human cytomegalovirus pp71 protein stimulates viral immediate-early gene expression. J. Virol. 80:3863-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saffert, R. T., and R. F. Kalejta. 2007. Human cytomegalovirus gene expression is silenced by Daxx-mediated intrinsic immune defense in model latent infections established in vitro. J. Virol. 81:9109-9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shahbazian, M. D., and M. Grunstein. 2007. Functions of site-specific histone acetylation and deacetylation. Annu. Rev. Biochem. 76:75-100. [DOI] [PubMed] [Google Scholar]
- 45.Song, M. J., S. M. Hwang, W. H. Wong, T. T. Wu, S. M. Lee, H. I. Liao, and R. Sun. 2005. Identification of viral genes essential for replication of murine gamma-herpesvirus 68 using signature-tagged mutagenesis. Proc. Natl. Acad. Sci. U. S. A. 102:3805-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staudt, M. R., and D. P. Dittmer. 2006. The Rta/Orf50 transactivator proteins of the Gamma-Herpesviridae. Curr. Top. Microbiol. Immunol. 312:71-100. [DOI] [PubMed] [Google Scholar]
- 47.Tarakanova, V. L., F. Kreisel, D. W. White, and H. W. Virgin IV. 2008. Murine gammaherpesvirus-68 genes both induce and suppress lymphoproliferative disease. J. Virol. 82:1034-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tarakanova, V. L., V. Leung-Pineda, S. Hwang, C. W. Yang, K. Matatall, M. Basson, R. Sun, H. Piwnica-Worms, B. P. Sleckman, and H. W. Virgin. 2007. Gamma-herpesvirus kinase actively initiates a DNA damage response by inducing phosphorylation of H2AX to foster viral replication. Cell Host Microbe 1:275-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tarakanova, V. L., J. M. Molleston, M. Goodwin, and H. W. Virgin. 2010. MHV68 complement regulatory protein facilitates MHV68 replication in primary macrophages in a complement independent manner. Virology 396:323-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarakanova, V. L., F. Suarez, S. A. Tibbetts, M. A. Jacoby, K. E. Weck, J. L. Hess, S. H. Speck, and H. W. Virgin. 2005. Murine gammaherpesvirus 68 infection is associated with lymphoproliferative disease and lymphoma in BALB beta2 microglobulin-deficient mice. J. Virol. 79:14668-14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, S. E., F. Y. Wu, H. Chen, M. Shamay, Q. Zheng, and G. S. Hayward. 2004. Early activation of the Kaposi's sarcoma-associated herpesvirus RTA, RAP, and MTA promoters by the tetradecanoyl phorbol acetate-induced AP1 pathway. J. Virol. 78:4248-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weck, K. E., M. L. Barkon, L. I. Yoo, S. H. Speck, and H. W. Virgin. 1996. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J. Virol. 70:6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weck, K. E., A. J. Dal Canto, J. D. Gould, A. K. O'Guin, K. A. Roth, J. E. Saffitz, S. H. Speck, and H. W. Virgin. 1997. Murine gammaherpesvirus 68 causes severe large vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus induced vascular disease. Nat. Med. 3:1346-1353. [DOI] [PubMed] [Google Scholar]
- 54.Weck, K. E., S. S. Kim, H. W. Virgin, and S. H. Speck. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 73:3273-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu, T. T., L. Tong, T. Rickabaugh, S. Speck, and R. Sun. 2001. Function of Rta is essential for lytic replication of murine gammaherpesvirus 68. J. Virol. 75:9262-9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu, T. T., E. J. Usherwood, J. P. Stewart, A. A. Nash, and R. Sun. 2000. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J. Virol. 74:3659-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang, Z., H. Tang, H. Huang, and H. Deng. 2009. RTA promoter demethylation and histone acetylation regulation of murine gammaherpesvirus 68 reactivation. PLoS One 4:e4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ye, J., D. Shedd, and G. Miller. 2005. An Sp1 response element in the Kaposi's sarcoma-associated herpesvirus open reading frame 50 promoter mediates lytic cycle induction by butyrate. J. Virol. 79:1397-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu, X., Z. Wang, and J. E. Mertz. 2007. ZEB1 regulates the latent-lytic switch in infection by Epstein-Barr virus. PLoS Pathog. 3:e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zalani, S., A. Coppage, E. Holley-Guthrie, and S. Kenney. 1997. The cellular YY1 transcription factor binds a cis-acting, negatively regulating element in the Epstein-Barr virus BRLF1 promoter. J. Virol. 71:3268-3274. [DOI] [PMC free article] [PubMed] [Google Scholar]