Abstract
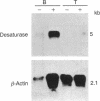
Stearic acid is toxic for T lymphocytes in vitro but has little effect on B lymphocytes. To investigate the molecular basis for this difference, purified murine T and B lymphocytes were compared for their abilities to incorporate and metabolize stearic acid. Unstimulated T and B cells incorporated identical amounts of stearic acid into six different phospholipids and four neutral lipids. After mitogen stimulation, fatty acid uptake was increased in both lymphocyte types, but cell-specific differences were seen in the distribution of stearic acid among the various cellular lipids. Doses of stearic acid that selectively inhibited T-cell proliferation resulted in a 5-fold greater accumulation of distearoylphosphatidylcholine in T cells than in B cells. Whereas T cells did not desaturate the exogenously derived stearic acid, up to 25% of the saturated fatty acid was converted to oleic acid in B cells. These findings suggested a deficiency of stearoyl-CoA desaturase (acyl-CoA, hydrogen-donor:oxygen oxidoreductase, EC 1.14.99.5) activity in T cells, which was confirmed by subsequent studies. Cell-free extracts from B cells displayed nearly 20-fold more stearoyl-CoA desaturase activity than T-cell extracts, and the level of stearoyl-CoA desaturase mRNA was 30-fold higher in B cells. Collectively, our data indicate that murine T cells are deficient in unsaturated fatty acid synthesis. The deficiency of stearoyl-CoA desaturase in T cells may represent the basis for the differing sensitivities of T and B lymphocytes to inhibition by saturated fatty acids.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai S., Yamane I., Tanno Y., Takishima T. Role of bovine serum albumin in blastoid transformation of lymphocytes by phytohemagglutinin. Proc Soc Exp Biol Med. 1977 Mar;154(3):444–448. doi: 10.3181/00379727-154-39690. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bennett M., Uauy R., Grundy S. M. Dietary fatty acid effects on T-cell-mediated immunity in mice infected with mycoplasma pulmonis or given carcinogens by injection. Am J Pathol. 1987 Jan;126(1):103–113. [PMC free article] [PubMed] [Google Scholar]
- Blank M. L., Robinson M., Fitzgerald V., Snyder F. Novel quantitative method for determination of molecular species of phospholipids and diglycerides. J Chromatogr. 1984 Aug 31;298(3):473–482. doi: 10.1016/s0021-9673(01)92744-x. [DOI] [PubMed] [Google Scholar]
- Brown R. E., Steele R. W., Marmer D. J., Hudson J. L., Brewster M. A. Fatty acids and the inhibition of mitogen-induced lymphocyte transformation by leukemic serum. J Immunol. 1983 Aug;131(2):1011–1016. [PubMed] [Google Scholar]
- Buttke T. M., Cuchens M. A. Inhibition of lymphocyte proliferation by free fatty acids. II. Toxicity of stearic acid towards phytohaemagglutinin-activated T cells. Immunology. 1984 Nov;53(3):507–514. [PMC free article] [PubMed] [Google Scholar]
- Buttke T. M., Ingram L. O. Inhibition of unsaturated fatty acid synthesis in escherichia coli by the antibiotic cerulenin. Biochemistry. 1978 Nov 28;17(24):5282–5286. doi: 10.1021/bi00617a031. [DOI] [PubMed] [Google Scholar]
- Buttke T. M. Inhibition of lymphocyte proliferation by free fatty acids. I. Differential effects on mouse B and T lymphocytes. Immunology. 1984 Oct;53(2):235–242. [PMC free article] [PubMed] [Google Scholar]
- Buttke T. M., Mallett G. S., Cuchens M. A. Positive selection of mouse B and T lymphocytes and analysis of isolated populations by flow cytometry. J Immunol Methods. 1983 Mar 11;58(1-2):193–207. doi: 10.1016/0022-1759(83)90275-2. [DOI] [PubMed] [Google Scholar]
- Chang T. Y., Vagelos P. R. Isolation and characterization of an unsaturated fatty acid-requiring mutant of cultured mammalian cells. Proc Natl Acad Sci U S A. 1976 Jan;73(1):24–28. doi: 10.1073/pnas.73.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cuthbert J. A., Lipsky P. E. Promotion of human T lymphocyte activation and proliferation by fatty acids in low density and high density lipoproteins. J Biol Chem. 1986 Mar 15;261(8):3620–3627. [PubMed] [Google Scholar]
- Erickson K. L. Dietary fat modulation of immune response. Int J Immunopharmacol. 1986;8(6):529–543. doi: 10.1016/0192-0561(86)90023-8. [DOI] [PubMed] [Google Scholar]
- Erickson K. L., McNeill C. J., Gershwin M. E., Ossmann J. B. Influence of dietary fat concentration and saturation on immune ontogeny in mice. J Nutr. 1980 Aug;110(8):1555–1572. doi: 10.1093/jn/110.8.1555. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gurr M. I. The role of lipids in the regulation of the immune system. Prog Lipid Res. 1983;22(4):257–287. doi: 10.1016/0163-7827(83)90007-3. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Kleinfeld A. M., Hoover R. L., Karnovsky M. J. Lipid domains in membranes. Evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis. J Biol Chem. 1980 Feb 25;255(4):1286–1295. [PubMed] [Google Scholar]
- Kuypers F. A., Roelofsen B., Op den Kamp J. A., Van Deenen L. L. The membrane of intact human erythrocytes tolerates only limited changes in the fatty acid composition of its phosphatidylcholine. Biochim Biophys Acta. 1984 Jan 25;769(2):337–347. doi: 10.1016/0005-2736(84)90315-8. [DOI] [PubMed] [Google Scholar]
- Lange L. G., Van Meer G., Op den Kamp J. A., Van Deenen L. L. Hemolysis of rat erythrocytes by replacement of the natural phosphatidylcholine by various phosphatidylcholines. Eur J Biochem. 1980 Sep;110(1):115–121. doi: 10.1111/j.1432-1033.1980.tb04846.x. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Austen K. F. Arachidonic acid metabolism by the 5-lipoxygenase pathway, and the effects of alternative dietary fatty acids. Adv Immunol. 1986;39:145–175. doi: 10.1016/s0065-2776(08)60350-8. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Tolbert N. E., Bieber L. L. Protein determination in membrane and lipoprotein samples: manual and automated procedures. Methods Enzymol. 1981;72:296–303. doi: 10.1016/s0076-6879(81)72018-4. [DOI] [PubMed] [Google Scholar]
- Mavis R. D., Bell R. M., Vagelos P. R. Effect of phospholipase C hydrolysis of membrane phospholipids on membranous enzymes. J Biol Chem. 1972 May 10;247(9):2835–2841. [PubMed] [Google Scholar]
- Meade C. J., Mertin J. Fatty acids and immunity. Adv Lipid Res. 1978;16:127–165. doi: 10.1016/b978-0-12-024916-9.50008-1. [DOI] [PubMed] [Google Scholar]
- Morimoto K., Kanoh H. The role of the de novo synthetic pathway in forming molecular species of phospholipids in resting lymphocytes from human tonsils. Biochim Biophys Acta. 1980 Jan 18;617(1):51–64. doi: 10.1016/0005-2760(80)90223-4. [DOI] [PubMed] [Google Scholar]
- Ntambi J. M., Buhrow S. A., Kaestner K. H., Christy R. J., Sibley E., Kelly T. J., Jr, Lane M. D. Differentiation-induced gene expression in 3T3-L1 preadipocytes. Characterization of a differentially expressed gene encoding stearoyl-CoA desaturase. J Biol Chem. 1988 Nov 25;263(33):17291–17300. [PubMed] [Google Scholar]
- Pourbohloul S., Mallett G. S., Buttke T. M. Inhibition of lymphocyte proliferation by free fatty acids. III. Modulation of thymus-dependent immune responses. Immunology. 1985 Dec;56(4):659–666. [PMC free article] [PubMed] [Google Scholar]
- Resch K., Ferber E. Phospholipid metabolism of stimulated lymphocytes. Effects of phytohemagglutinin, concanavalin A and anti-immunoglobulin serum. Eur J Biochem. 1972 May;27(1):153–161. doi: 10.1111/j.1432-1033.1972.tb01821.x. [DOI] [PubMed] [Google Scholar]
- Shive W., Matthews K. S. Nutritional requirements for growth of human lymphocytes. Annu Rev Nutr. 1988;8:81–97. doi: 10.1146/annurev.nu.08.070188.000501. [DOI] [PubMed] [Google Scholar]
- Smith A. D., Conroy D. M., Belin J. Membrane lipid modification and immune function. Proc Nutr Soc. 1985 Jul;44(2):201–209. doi: 10.1079/pns19850039. [DOI] [PubMed] [Google Scholar]
- Spieker-Polet H., Polet H. Requirement of a combination of a saturated and an unsaturated free fatty acid and a fatty acid carrier protein for in vitro growth of lymphocytes. J Immunol. 1981 Mar;126(3):949–954. [PubMed] [Google Scholar]
- Strittmatter P., Thiede M. A., Hackett C. S., Ozols J. Bacterial synthesis of active rat stearyl-CoA desaturase lacking the 26-residue amino-terminal amino acid sequence. J Biol Chem. 1988 Feb 15;263(5):2532–2535. [PubMed] [Google Scholar]
- Stubbs C. D., Osawa T. The effect of concanavalin A on the incorporation of fatty acids into lymphocyte phospholipids. J Pharmacobiodyn. 1983 Mar;6(3):217–224. doi: 10.1248/bpb1978.6.217. [DOI] [PubMed] [Google Scholar]
- Takamura H., Narita H., Urade R., Kito M. Quantitative analysis of polyenoic phospholipid molecular species by high performance liquid chromatography. Lipids. 1986 May;21(5):356–361. doi: 10.1007/BF02535701. [DOI] [PubMed] [Google Scholar]
- Thiede M. A., Ozols J., Strittmatter P. Construction and sequence of cDNA for rat liver stearyl coenzyme A desaturase. J Biol Chem. 1986 Oct 5;261(28):13230–13235. [PubMed] [Google Scholar]
- Thiede M. A., Strittmatter P. The induction and characterization of rat liver stearyl-CoA desaturase mRNA. J Biol Chem. 1985 Nov 25;260(27):14459–14463. [PubMed] [Google Scholar]
- Traill K. N., Offner F., Winter U., Paltauf F., Wick G. Lipid requirements of human T lymphocytes stimulated with mitogen in serum-free medium. Membrane "fluidity" changes are an artefact of lipid (AL721) uptake by monocytes. Immunobiology. 1988 Mar;176(4-5):450–464. doi: 10.1016/S0171-2985(88)80026-3. [DOI] [PubMed] [Google Scholar]
- Weyman C., Morgan S. J., Belin J., Smith A. D. Phytohaemagglutinin stimulation of human lymphocytes: effect of fatty acids on uridine uptake and phosphoglyceride fatty acid profile. Biochim Biophys Acta. 1977 Jan 24;496(1):155–166. doi: 10.1016/0304-4165(77)90123-4. [DOI] [PubMed] [Google Scholar]