Abstract
Rapid and accurate assessment of allograft function in the early postoperative period is critical for successful liver transplantation. This study evaluated the efficacy of lecithin:cholesterol acyltransferase (LCAT) activity as an indicator of early allograft function in human orthotopic liver transplantation (OLTx). During a three-month period between September and November 1987, 9 of 11 adult OLTx recipients whose graft exhibited poor function were studied. Poor graft function was defined as primary nonfunction, need for retransplantation within a week after OLTx, or elevation of the prothrombin time over 20 sec early after OLTx. Plasma LCAT activities (measured pretransplant and at 0, 6, 12, 18, and 24 hr, as well as 3 days, after OLTx) and pretransplant clinical variables were compared with those of 15 control patients whose graft exhibited good function. A significant correlation was found between mean LCAT activities during the first 24-hr after OLTx and early allograft function (P<0.05, χ2=5.23). When pretransplant histological findings of the 6 grafts with poor function and the 15 controls together were correlated with the mean LCAT activity within 24 hr following OLTx, a significant association was demonstrated (P<0.05). This study suggests that plasma LCAT activity is an effective and practical method for assessing early allograft function following OLTx.
Since the first human orthotopic liver transplant (OLTx) performed in 1963, enormous progress has been made in the overall management and survival of patients with end-stage liver diseases (1–3). Factors responsible for this improved outcome include the introduction of cyclosporine (4), the use of venovenous bypass (5), and a standardization of the technique of biliary tract reconstruction (6). The recent introduction of University of Wisconsin (UW) solution by Belzer et al. (7, 8) for preservation of the liver has improved the results even further, and has allowed the procedure to be performed more widely. However, graft nonfunction—either primary, or secondary due to technical failure—remains a major complication of OLTx, and one that carries a high morbidity, mortality, and need for retransplantation (9). In an earlier studies (10), we have demonstrated that the most commonly used donor characteristics did not distinguish between grafts that functioned and those that failed to function. Moreover, only a few studies have been reported on allograft viability in the early postoperative period (11–16).
Lecithin:cholesterol acyltransferase (LCAT), an enzyme that esterifies free cholesterol (17) and functions in the transportation of cholesterol and the metabolism of lipoproteins (18), has been known to be useful in assessing liver function following hepatic resection or other surgical procedures such as operations for esophageal varices (19). LCAT activity also has been shown to be a valuable indicator of prognosis in fulminant liver failure (20). Reduced LCAT activity in patients with liver diseases is considered to be due to impaired protein synthesis occurring secondary to hepatic dysfunction. Based upon the data obtained in various types of hepatic diseases, the present study was performed to determine if serial quantitation of the plasma LCAT activity could be utilized as a predictor of allograft function early after OLTx.
MATERIALS AND METHODS
During a three-month period between September 1 and November 30, 1987, 76 OLTx were performed in 68 adult patients at the Presbyterian University Hospital, University of Pittsburgh. The first 42 grafts were preserved in Euro-Collins solution (4°C), while the other 34 were stored in University of Wisconsin (UW) solution (4°C). Eleven of these grafts (14.5%) exhibited poor function, as manifested by death due to primary nonfunction (two grafts), need for retransplantation within the first week after OLTx (4 grafts), and poor synthetic function with a peak prothrombin time of over 20 sec (control 11.8 sec) during the first 5 posttransplant days (5 grafts).
From the recipients of 9 of the 11 grafts with poor function, blood samples were obtained at predetermined time points: immediately prior to OLTx, immediately after OLTx; at 6, 12, 18, and 24 hr after OLTx; and at 3 days posttransplant. Plasma was separated from the blood by centrifugation at 2000 RPM for 5 min at 37°C and stored at −60°C. The selection was random and was based primarily on the availability of pretransplant blood samples. Plasma LCAT activity and pretransplant clinical variables of these recipients were compared with those of 15 recipients with adequate immediate posttransplant allograft function (control), as manifested by a peak SGOT, SGPT, and prothrombin time of less than 2000 JU/L, 2000 JU/L, and 20 sec, respectively.
Plasma lecithim:cholesterol acyltransferase activity was determined using a LCAT test kit-S obtained from Nippon Shoji Co., Ltd., Osaka, loan using a modification of the method of Nagasaki and Akanuma (21). The modification consists of the addition of ascorbic acid oxidase and sodium azide (NaN3) to the mixture to minimize interference by plasma levels of ascorbic acid and hemoglobin, and also the use of DAOS* (Sodium N-ethyl-N [2-hydroxy-3-sulfopropyl]-3, 5-dimethoxyaniline) instead of phenol for the color reaction such that absorbance is read at 600 nm, thereby preventing the influence of the presence of bilirubin. Plasma LCAT activity was determined by its ability to esterify cholesterol at 37°C. A unit of LCAT activity was expressed as the reduction in free cholesterol (μg/ml/hr) in the assay mixture.
In order to assess the association between plasma LCAT activity and the quality of the allograft, histological findings of the grafts with poor function and the controls together were correlated with the LCAT activities. Allograft biopsy was obtained by a Trucut biopsy needle (Travenol Laboratories) immediately prior to implantation in 6 of the 11 grafts with poor function and all 15 control livers. The biopsy specimens were fixed with 10% buffered formalin and stained with hematoxylin and eosin. The histological findings were graded as follows: Grade I (12 grafts)—minimal or no evidence of preservation damage; Grade II (9 grafts)—changes consistent with ischemic injury, as manifested by diffuse hepatocellular swelling, steatosis, or acidophilic necrosis.
For statistical analysis, the Mann-Whitney U and chi-square tests were used. Results of the plasma LCAT activity were expressed as means ± SD.
RESULTS
Table 1 demonstrates the relationship between LCAT activity during the first 24 hr after OLTx and graft function. A mean LCAT activity of less than 5 U correlated with poor graft function (P<0.05, χ2=5.23). Table 2 lists the pre- and intraoperative clinical variables of the patients in each group. No significant difference was identified among the clinical parameters between the two groups. Similarly, preengraftment LCAT activity for both groups was not different. Table 3 lists the clinical data of the 9 patients who exhibited poor graft function. Three of these 9 patients (cases 6, 7, and 8) required retransplantation and one (case 1) died within a week.
Table 1.
Correlation between allograft function and mean plasma LCAT activity during the first 24 hours following OLTx
| Mean LCATa activity (U) | Allograft function |
|
|---|---|---|
| Adequate | Poor | |
| <5b | 3 (20.0%) | 6 (66.7%) |
| ≥5b | 12 (80.0%) | 3 (33.3%) |
LCAT, lecithin:cholesterol acyltransferase; OLTx, orthotopic liver transplantation.
P<0.05 (χ2=5.23).
Table 2.
Relationship between pre- or intraoperative clinical variables and early allograft function
| Variables | Allograft function |
|
|---|---|---|
| Adequate | Poor | |
| Preoperative: | ||
| Age (years) | 42.2±12.2 | 43.1±17.8 |
| Sex (M:F) | 6:4 | 2:4 |
| T. bil (mg/dl)a | 7.1±8.3 | 12.7±13.2 |
| PT (sec) | 15.5±2.7 | 16.0±3.5 |
| Albumin (g/dl) | 2.8±0.6 | 2.6±0.7 |
| ICG (20′) (%) | 37.7±19.6 | 28.5±24.0 |
| LCAT (U) | 6.6±5.6 | 4.1±3.9 |
| Intraoperative: | ||
| Blood loss (U) | 12.6±7.9 | 24.9±28.0 |
T. bil, total bilirubin; PT, prothrombin time; ICG (20′), indocyanine green dye retention test at 20 min (normal less than 5%); LCAT, lecithin:cholesterol acyltransferase.
Table 3.
Clinical data of patients with poor graft function after OLTx
| Case | Age (yr)/Sex | Indication for OLTx | Peak values within 5 days after OLTx |
Outcome | ||
|---|---|---|---|---|---|---|
| sGOT (IU/L) | sGPT (IU/L) | PT (sec) | ||||
| 1. | 70/F | PGNF, status after OLTx for PBC | 4010 | 4825 | 24.2 | Died of MOF(3)a |
| 2. | 23/F | PBC | 2677 | 2902 | 23.1 | ReTx for rejection (60), Doing well |
| 3. | 31/F | PNC (NANB) | 1818 | 1260 | 23.9 | Doing well |
| 4. | 61/M | Recurrent HB, status after OLTx | 6362 | 3603 | 31.6 | Died of sepsis (18) |
| 5. | 44/F | PNC (alcoholic) | 2840 | 1660 | 24.0 | Prolonged respiratory failure, CMV hepatitis, discharged (234), doing well |
| 6. | 55/F | PBC | 3035 | 2135 | 20.2 | ReTx for CMV hepatitis (31), died of sepsis (92) |
| 7. | 21/M | Cystic fibrosis | 400 | 413 | 22.7 | ReTx for HAT (1), died of pneumonia (15) |
| 8. | 29/F | PGNF, status after OLTx for BC | 2805 | 1820 | 34.2 | ReTx for HAT (3), died of MOF (11) |
| 9. | 54/F | PNC (NANB) | 13,874 | 5106 | 46.0 | ReTx for PGNF (5), doing well |
Number of postoperative days; OLTx, orthotopic liver transplantation; PT, prothrombin time; PGNF, primary, graft nonfunction; MOF, multiple organ failure; PBC, primary biliary cirrhosis; ReTx, retransplantation; PNC, postnecrotic cirrhosis; NANB: non-A-non-B hepatitis; HB, hepatitis B; CMV, cytomegalovirus; HAT, hepatic artery thrombosis; BC, Budd-Chiari syndrome.
Figure 1 and Table 4 depict the short-term changes of the LCAT activity after OLTx. Serial LCAT activity in the group with poor liver function was significantly lower than that of the group with adequate liver function at 6, 12, and 18 hr and at 3 days following OLTx (P< [at most] 0.05).
Figure 1.
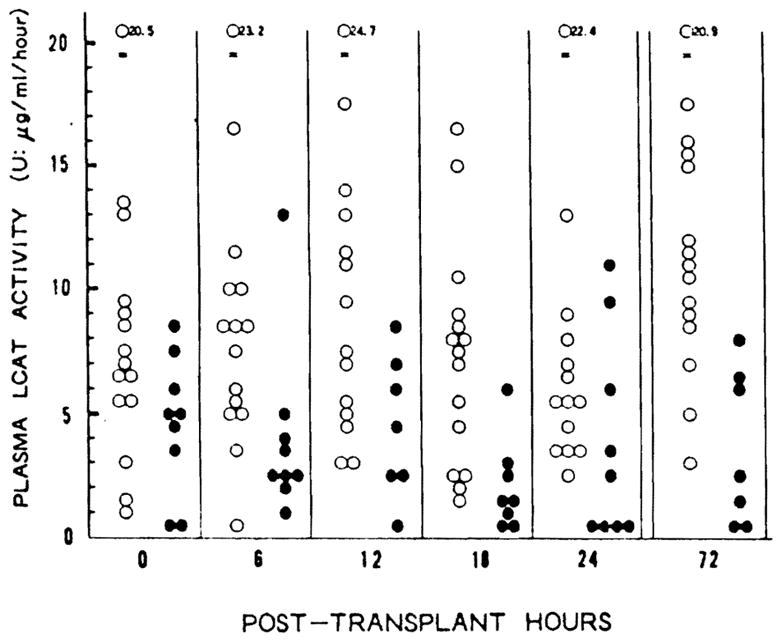
Plasma LCAT activity immediately following orthotopic liver transplantation. (○): adequate function group; (●): poor function group, (LCAT) lecithin:cholesterol acyltransferase. (U) μg/ml/hr at 37°C.
Table 4.
Plasma LCAT activity in adequate and poor function groups after OLTx (mean ± SD)
| Allograft function | Plasma LACT activity (U) in each posttransplant hour |
|||||
|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 18 | 24 | 72 | |
| Adequate (n=15) | 7.9±5.0 | 8.7±5.5b | 9.8±6.2a | 7.2±4.5b | 7.1±5.0 | 11.9±4.8b |
| Poor (<n=9) | 4.5±3.0 | 3.9±3.6b | 4.4±3.0a | 2.2±2.5b | 3.6±4.2 | 3.5±3.4b |
P<0.05.
P<0.01.
Histologically, 12 grafts with pretransplant histology grade I had a mean LCAT activity within 24 hr after OLTx of 8.5±4.4 U, which was significantly higher than 4.6±1.9 U of 9 grafts with histology grade II (P<0.05).
DISCUSSION
This study demonstrated a statistically significant correlation between early allograft function and the plasma LCAT activity in OLTx recipients during the early postoperative period.
Numerous previous attempts have been made to assess the viability of the hepatic allograft prior to and immediately after transplantation. Fath et al. (11) reported a correlation between the ability of the hepatic allograft to reduce abnormal levels of total plasma amino acids immediately following revascularization and subsequent postoperative morbidity, defined as reoperation, death, or hepatic necrosis during the first 48 hr after OLTx. Jenkins et al. (12) demonstrated that a delayed recovery of the rate of central plasma clearance of amino acids following liver transplantation identifies recipients with an increased mortality. Taki et al. (13) reported in pigs that a failure to maintain a normal ratio of ketone bodies after OLTx correlated with hepatic energy charge and predicted a high mortality. Kamiike et al. (14) recently suggested that in humans the recovery of ATP levels in liver may be an indicator of early allograft function. Mora et al. (15) have demonstrated that the clearance of serum total bile acids can also serve as a putative marker of early allograft function (15). Persson et al. (16) have suggested that total-body oxygen consumption, which rapidly declines during the anhepatic phase of the transplant procedure and returns quickly to baseline levels immediately after revascularization of the graft, may also be a good indicator of early graft function and perfusion. All of these methods, however, are sophisticated, cumbersome, or invasive, or they require special equipment and expertise, and so cannot be used routinely and more widely.
Currently, measurement of the prothrombin time and determination of the levels of specific coagulation factors remain the most practical methods for the assessment of early allograft function after OLTx. Clinical features of malfunction of the liver allograft after OLTx include inability to awaken from anesthesia, low bile output or production of thin bile through a T tube, elevated transaminases and prothrombin time, and persistent or progressive hyperbilirubinemia, often followed by a rapid development of renal failure and other metabolic derangements. Nevertheless, as exhibited in Table 3, considerable variations exist in the clinical presentations of liver failure immediately after OLTx.
The prothrombin time is a practical and simple laboratory study that indirectly reflects the production of coagulation factors by the hepatic allograft. Transaminases, on the other hand, are indicators of hepatocellular breakdown rather than of allograft function that generally take several days to peak.
When the elevation of the prothrombin time (over 20 sec in adults and 30 sec in children) is identified immediately after OLTx, we administer fresh frozen plasma to avoid complications related to coagulopathy, such as intracranial or intraabdominal hemorrhage. Introduction of extrinsic coagulation factors, however, may complicate the interpretation of the prothrombin time as an indicator of allograft function.
Plasma LCAT activity, on the other hand, has been well accepted in Japan as an indicator of liver failure following hepatic resection or other surgical procedures for liver disease. LCAT is synthesized in the liver (22, 23), has a function half-life of 5–6 hr (24), and is not thought to be affected by the administration of fresh frozen plasma. Moreover, it can be measured within about 2 hr without any requirement for unique expertise or equipment. We therefore believe that it may be more useful than current methods—i.e., prothrombin time and coagulation factor levels—for evaluating early hepatic allograft function.
The relationship between the preimplantation histology of the liver allografts and the plasma LCAT activity in the recipient immediately following OLTx suggests that LCAT activity at this time may reflect the condition of the donor liver prior to organ procurement and the degree of preservation or reperfusion injury inflicted upon the allograft.
In summary, plasma LCAT activity assayed immediately following OLTx is a simple, reliable, and inexpensive parameter that may provide a rapid estimation of allograft function and prediction of outcome following OLTx.
Footnotes
Presented in part at the International Association for the Study of the Liver, November 1988, Toronto, Canada.
This work was supported by a Research Grant from the Veterans Administrations and by Project Grant A 29961 from the National Institutes of Health, Bethesda, MD.
Abbreviations: DAOS, sodium N-ethyl-N (2-hydroxy-3-sulfopropyl)-3,5-dimethoxyaniline; LCAT, lecithin:cholesterol acyltransferase; RPM, rounds per minute; UW, University of Wisconsin.
References
- 1.Starzl TE, Marchioro TL, Von Kaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659. [PMC free article] [PubMed] [Google Scholar]
- 2.Iwatsuki S, Starzl TE, Todo S, et al. Experience in 1,000 liver transplants under cyclosporine-steroid therapy; a survival report. Transplant Proc. 1988;20:498. [PMC free article] [PubMed] [Google Scholar]
- 3.Busuttil RW, Colonna JO, II, Hiatt JR, et al. The first 100 liver transplants at UCLA. Ann Surg. 1987;206:387. doi: 10.1097/00000658-198710000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Klintmalm GBG, Porter KA, Iwatsuki S, Schroter GP. Liver transplantation with use of cyclosporine-A and prednisone. N Engl J Med. 1981;305:266. doi: 10.1056/NEJM198107303050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw BW, Jr, Martin DJ, Marquez JM, et al. Venous bypass in clinical liver transplantation. Ann Surg. 1984;200:524. doi: 10.1097/00000658-198410000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepalology. 1982;2:614. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Kalayoglu M, Sollinger HW, Stratta RJ, et al. Extended preservation of the liver for clinical transplantation. Lancet. 1988;1:617. doi: 10.1016/s0140-6736(88)91416-x. [DOI] [PubMed] [Google Scholar]
- 9.Shaw BW, Gordon RD, Iwatsuki S, Starzl TE. Hepatic retransplantation. Transplant Proc. 1985;17:264. [PMC free article] [PubMed] [Google Scholar]
- 10.Makowka L, Gordon RD, Todo S, et al. Analysis of donor criteria for the prediction of outcome in clinical liver transplantation. Transplant Proc. 1987;19:2378. [PMC free article] [PubMed] [Google Scholar]
- 11.Fath J, Ascher NL, Konstantinideds FN, et al. Metabolism during hepatic transplantation: indicators of allograft function. Surgery. 1984;96:664. [PubMed] [Google Scholar]
- 12.Jenkins R, Bosari S, Khettry U, Clowes GHA, Jr, Pearl RH, Trey C. Survival from hepatic transplantation: relationship of protein synthesis to histological abnormalities in patient selection and postoperative management. Ann Surg. 1986;204:364. doi: 10.1097/00000658-198610000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taki Y, Ukikusa M, Morimoto T, et al. Short-term changes in blood ketone body ratio in the phase immediately after liver transplantation. Transplantation. 1987;43:350. doi: 10.1097/00007890-198703000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Kamiike W, Burdelski M, Steinhoff G, Ringe B, Lauehart W, Pichlmayr R. Adenine nucleotide metabolism and its relation to organ viability in human liver transplantation. Transplantation. 1988;45:138. doi: 10.1097/00007890-198801000-00030. [DOI] [PubMed] [Google Scholar]
- 15.Mora NP, Cienfuegos JA, Codoceo R, et al. Monitoring of serum total bile acids as an early indicator of graft function in clinical and experimental liver transplantation. Transplant Proc. 1987;19:3840. [PubMed] [Google Scholar]
- 16.Persson H, Karlberg I, Svensson K, et al. Rapid indication of allograft function in liver transplantation. Transplant Proc. 1987;19:3545. [PubMed] [Google Scholar]
- 17.Glomset JA. The mechanism of the plasma cholesterol esterification reaction: plasma fatty acid transferase. Biochim Biophys Acta. 1962;65:128. doi: 10.1016/0006-3002(62)90156-7. [DOI] [PubMed] [Google Scholar]
- 18.Glomset JA. Physiological role of lecithinxholesterol acyltransferase. Am J Clin Nutr. 1970;23:1129. doi: 10.1093/ajcn/23.8.1129. [DOI] [PubMed] [Google Scholar]
- 19.Takenaka K, Kanematsu T, Sugimachi K, Inokuchi K. Serum lecithinxholesterol acyltransferase (LCAT) activity is an accurate predictor of post-operative hepatic failure. Disease Marker. 1984;2:501. [Google Scholar]
- 20.Gjone E, Blomhoff JP, Wiencke I. Plasma lecithinxholesterol acyltransferase activity in acute hepatitis. Scand J Gastroenterol. 1971;6:161. doi: 10.3109/00365527109180686. [DOI] [PubMed] [Google Scholar]
- 21.Nagasaki T, Akanuma Y. A new colorimetric method for the determination of plasma lecithinxholesterol acyltransferase activity. Clinica Chimica Acta. 1977;75:371. doi: 10.1016/0009-8981(77)90355-2. [DOI] [PubMed] [Google Scholar]
- 22.Osuga T, Port man OW. Origin and disappearance of plasma lecithinxholesterol acyltransferase. Am J Physiol. 1971;220:735. doi: 10.1152/ajplegacy.1971.220.3.735. [DOI] [PubMed] [Google Scholar]
- 23.Simon JB, Boyer JL. Production of lecithin:cholesterol acyltransferase by the isolated perfused rat liver. Biochim Biophys Acta. 1971;218:549. doi: 10.1016/0005-2760(70)90020-2. [DOI] [PubMed] [Google Scholar]
- 24.Sabesin SM, Kuiken LB, Ragland JB. Lipoprotein abnormalities in galactosamine hepatitis: A model of experimental lecithin:cholesterol acyltransferase deficiency. Scand J Clin Lab Invest. 1978;38(suppl 150):187. [PubMed] [Google Scholar]


