Abstract
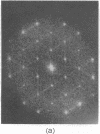
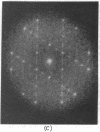
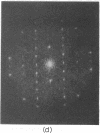
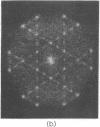
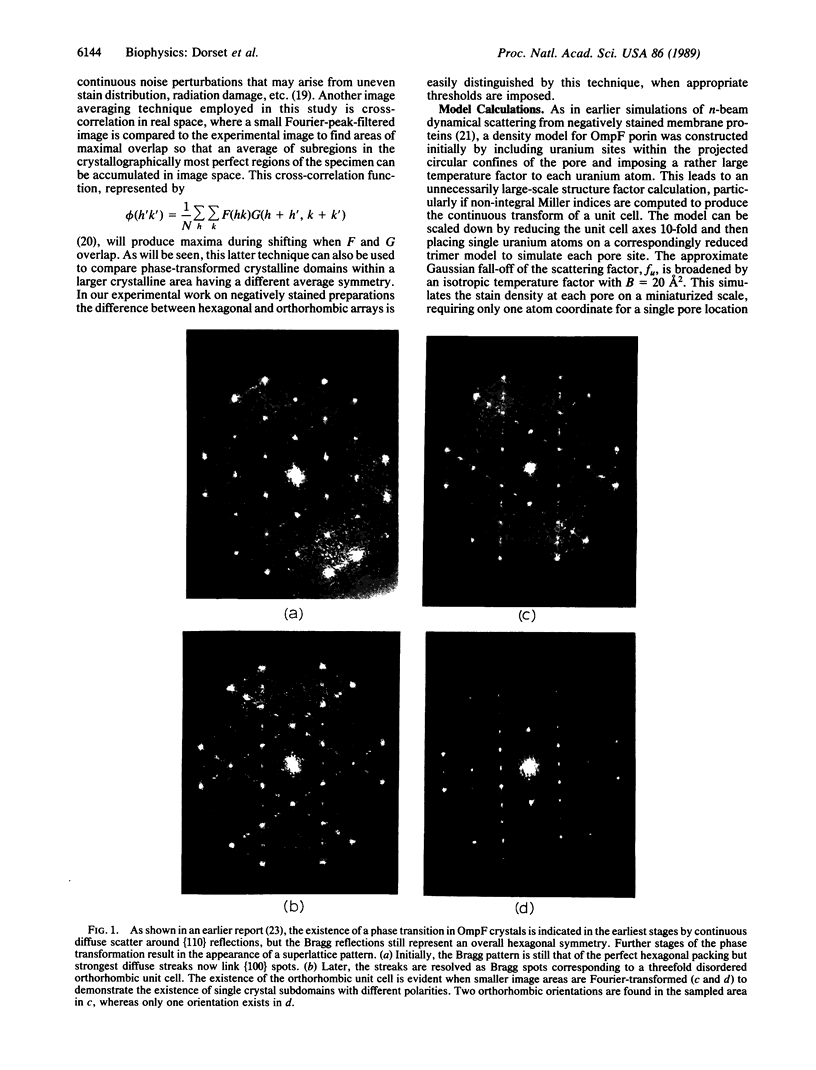
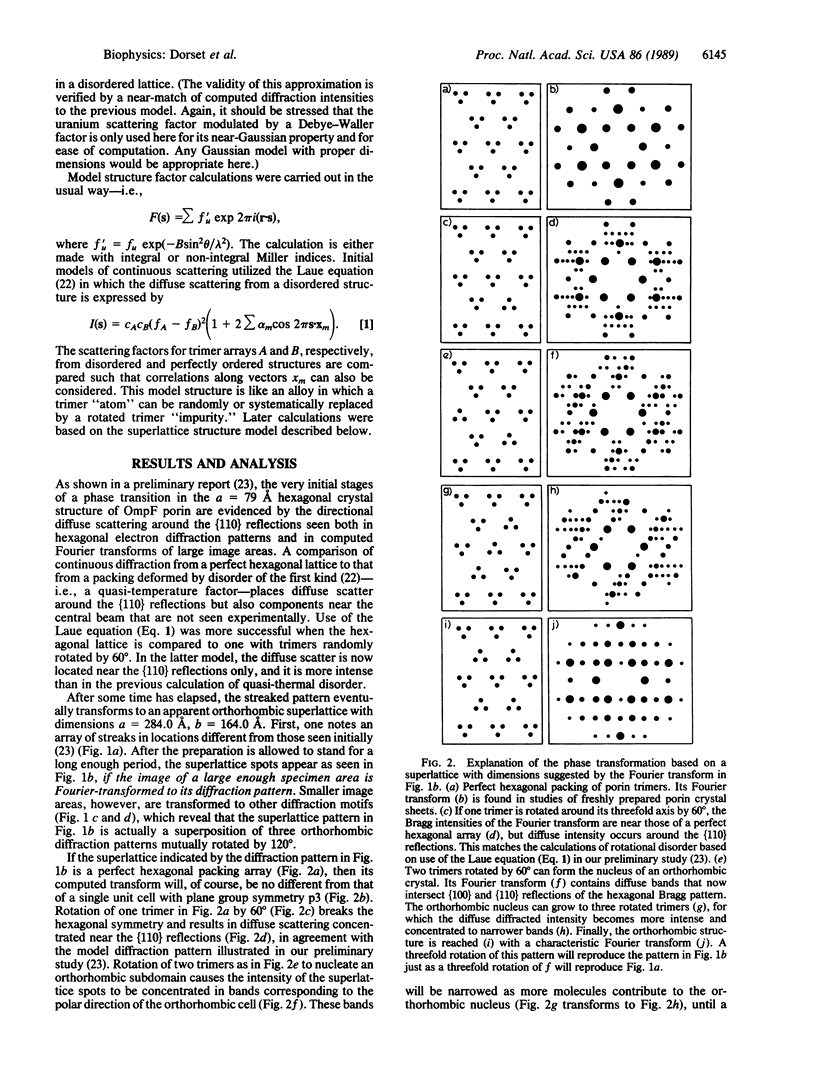
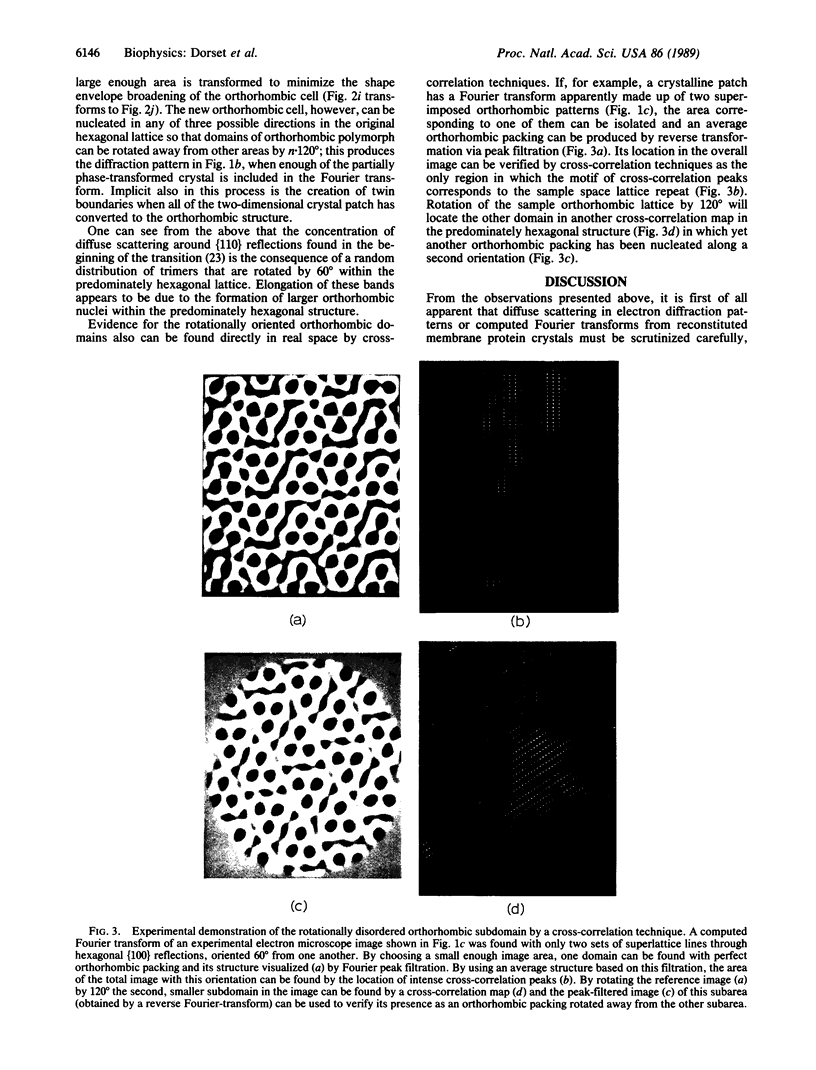
A hexagonal polymorph (a = 79 A) of OmpF matrix porin from Escherichia coli spontaneously transforms to a rectangular form (a = 79 A, b = 137 A) after several months' storage in the refrigerator. Nucleation of this second polymorph is first disclosed by diffuse streaks in electron diffraction patterns or in computer-generated Fourier transforms of electron microscope images. With time, this streaking is resolved as an apparent superlattice, and eventually domains of orthorhombic polymorph are detected in the parent hexagonal lattice that can be oriented in either of three directions, depending on the polarity of the orthorhombic crystal growth. Models for this phenomenon based on protein trimer rotation successfully explain the progress of the phase transition and, if protein-protein interactions are the most important interactions between adjacent trimers in the lipid matrix, the transition is quite similar to what occurs with molecular crystals.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney J. R., Braun J., Owicki J. C. Lateral interactions among membrane proteins. Implications for the organization of gap junctions. Biophys J. 1987 Sep;52(3):441–454. doi: 10.1016/S0006-3495(87)83233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Abney J. R., Owicki J. C. Lateral interactions among membrane proteins. Valid estimates based on freeze-fracture electron microscopy. Biophys J. 1987 Sep;52(3):427–439. doi: 10.1016/S0006-3495(87)83232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar D. L., Clarage J., Salunke D. M., Clarage M. Liquid-like movements in crystalline insulin. Nature. 1988 Apr 14;332(6165):659–662. doi: 10.1038/332659a0. [DOI] [PubMed] [Google Scholar]
- Chang C. F., Mizushima S., Glaeser R. M. Projected structure of the pore-forming OmpC protein from Escherichia coli outer membrane. Biophys J. 1985 May;47(5):629–639. doi: 10.1016/S0006-3495(85)83959-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorset D. L. Dynamical electron scattering from negatively stained protein microcrystals. Ultramicroscopy. 1984;13(3):311–324. doi: 10.1016/0304-3991(84)90209-2. [DOI] [PubMed] [Google Scholar]
- Dorset D. L., Engel A., Häner M., Massalski A., Rosenbusch J. P. Two-dimensional crystal packing of matrix porin. A channel forming protein in Escherichia coli outer membranes. J Mol Biol. 1983 Apr 25;165(4):701–710. doi: 10.1016/s0022-2836(83)80275-7. [DOI] [PubMed] [Google Scholar]
- Doucet J., Benoit J. P. Molecular dynamics studied by analysis of the X-ray diffuse scattering from lysozyme crystals. Nature. 1987 Feb 12;325(6105):643–646. doi: 10.1038/325643a0. [DOI] [PubMed] [Google Scholar]
- Engel A., Massalski A., Schindler H., Dorset D. L., Rosenbusch J. P. Porin channel triplets merge into single outlets in Escherichia coli outer membranes. Nature. 1985 Oct 17;317(6038):643–645. doi: 10.1038/317643a0. [DOI] [PubMed] [Google Scholar]
- Garavito R. M., Jenkins J., Jansonius J. N., Karlsson R., Rosenbusch J. P. X-ray diffraction analysis of matrix porin, an integral membrane protein from Escherichia coli outer membranes. J Mol Biol. 1983 Feb 25;164(2):313–327. doi: 10.1016/0022-2836(83)90079-7. [DOI] [PubMed] [Google Scholar]
- Jap B. K. High-resolution electron diffraction of reconstituted PhoE porin. J Mol Biol. 1988 Jan 5;199(1):229–231. doi: 10.1016/0022-2836(88)90393-2. [DOI] [PubMed] [Google Scholar]
- Jap B. K. Molecular design of PhoE porin and its functional consequences. J Mol Biol. 1989 Jan 20;205(2):407–419. doi: 10.1016/0022-2836(89)90351-3. [DOI] [PubMed] [Google Scholar]
- Kleffel B., Garavito R. M., Baumeister W., Rosenbusch J. P. Secondary structure of a channel-forming protein: porin from E. coli outer membranes. EMBO J. 1985 Jun;4(6):1589–1592. doi: 10.1002/j.1460-2075.1985.tb03821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella C. A. Phospholipase-induced crystallization of channels in mitochondrial outer membranes. Science. 1984 Apr 13;224(4645):165–166. doi: 10.1126/science.6322311. [DOI] [PubMed] [Google Scholar]
- Markovics J., Glass L., Maul G. G. Pore patterns on nuclear membranes. Exp Cell Res. 1974 Apr;85(2):443–451. doi: 10.1016/0014-4827(74)90148-7. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Effect on solute size on diffusion rates through the transmembrane pores of the outer membrane of Escherichia coli. J Gen Physiol. 1981 Feb;77(2):121–135. doi: 10.1085/jgp.77.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson L. T., Chan S. I., Lewis B. A., Engelman D. M. Pair distribution functions of bacteriorhodopsin and rhodopsin in model bilayers. Biophys J. 1983 Aug;43(2):167–174. doi: 10.1016/S0006-3495(83)84337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson L. T., Edelman J., Chan S. I. Statistical mechanics of lipid membranes. Protein correlation functions and lipid ordering. Biophys J. 1984 May;45(5):863–871. doi: 10.1016/S0006-3495(84)84232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. P., Hui S. W., Stewart T. P. Correlative statistical analysis and computer modelling of intramembraneous particle distributions in human erythrocyte membranes. Biochim Biophys Acta. 1979 Nov 2;557(2):265–282. doi: 10.1016/0005-2736(79)90326-2. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Fillers J. P., Cohen C. Motions of tropomyosin. Crystal as metaphor. Biophys J. 1980 Oct;32(1):485–502. doi: 10.1016/S0006-3495(80)84985-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein from Escherichia coli outer membranes forms voltage-controlled channels in lipid bilayers. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3751–3755. doi: 10.1073/pnas.75.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein in planar membranes: clusters of channels in a native environment and their functional reassembly. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2302–2306. doi: 10.1073/pnas.78.4.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven A. C., Heggeler B., Müller R., Kistler J., Rosenbusch J. P. Ultrastructure of a periodic protein layer in the outer membrane of Escherichia coli. J Cell Biol. 1977 Feb;72(2):292–301. doi: 10.1083/jcb.72.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]