Abstract
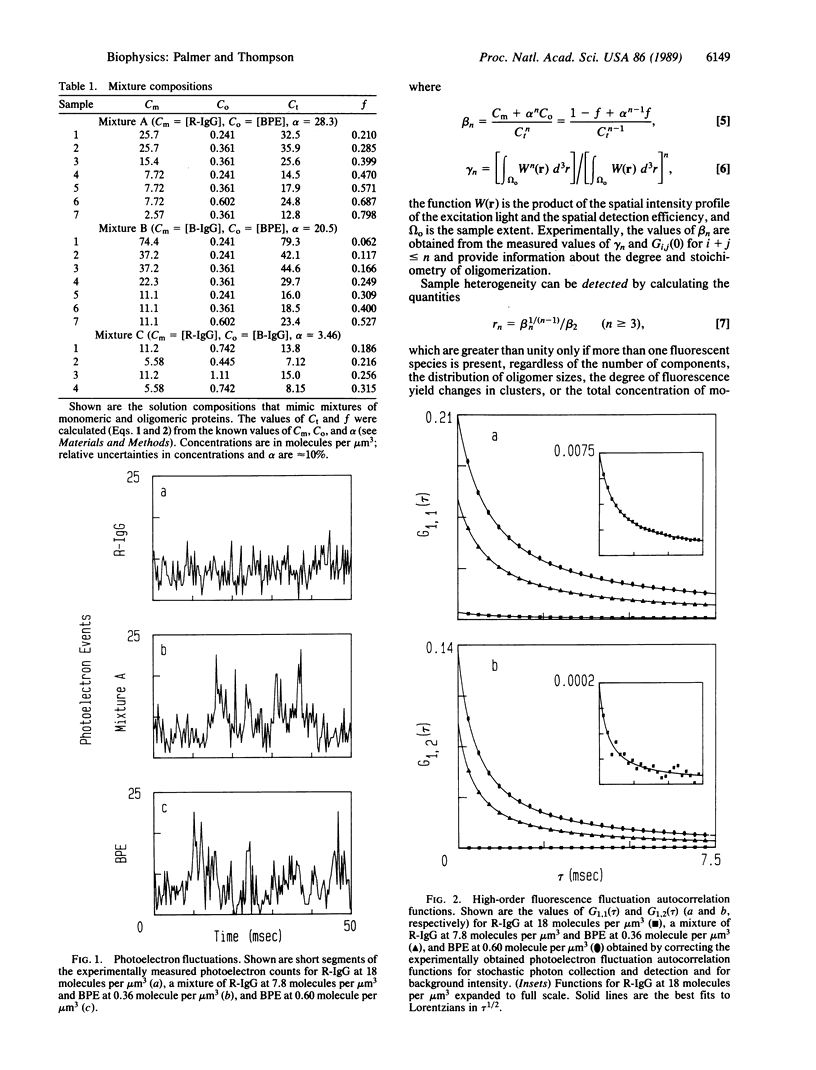
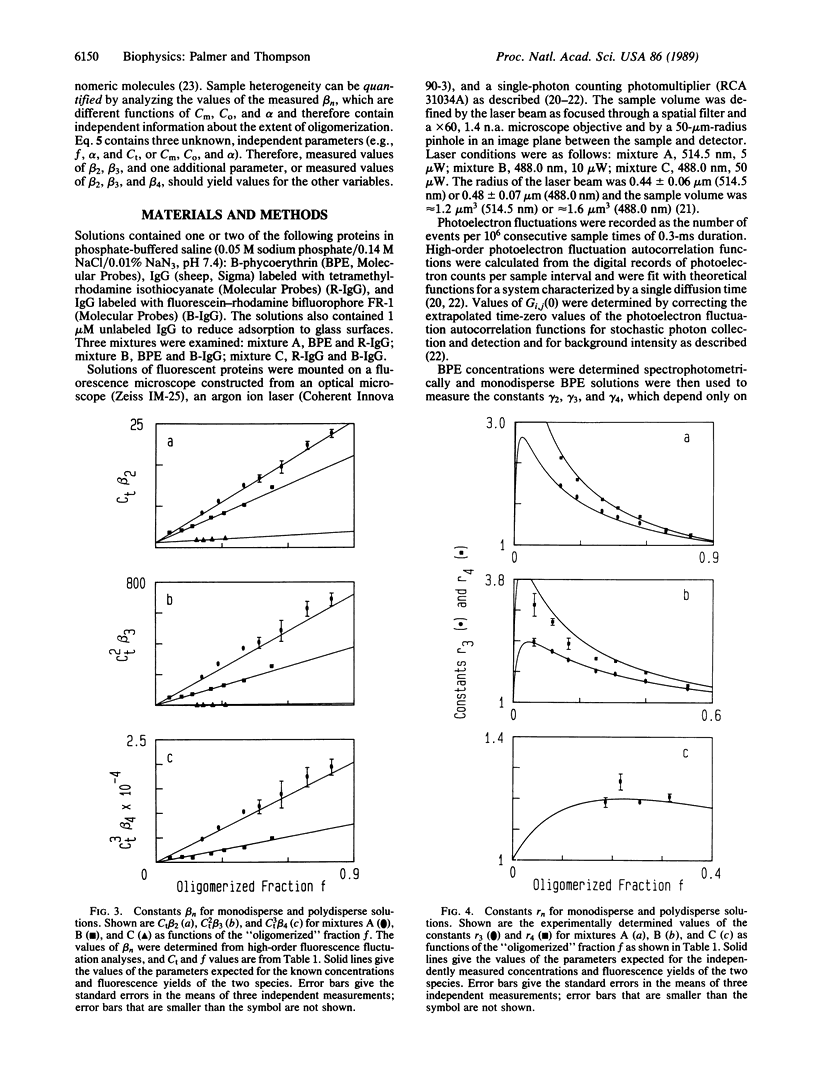
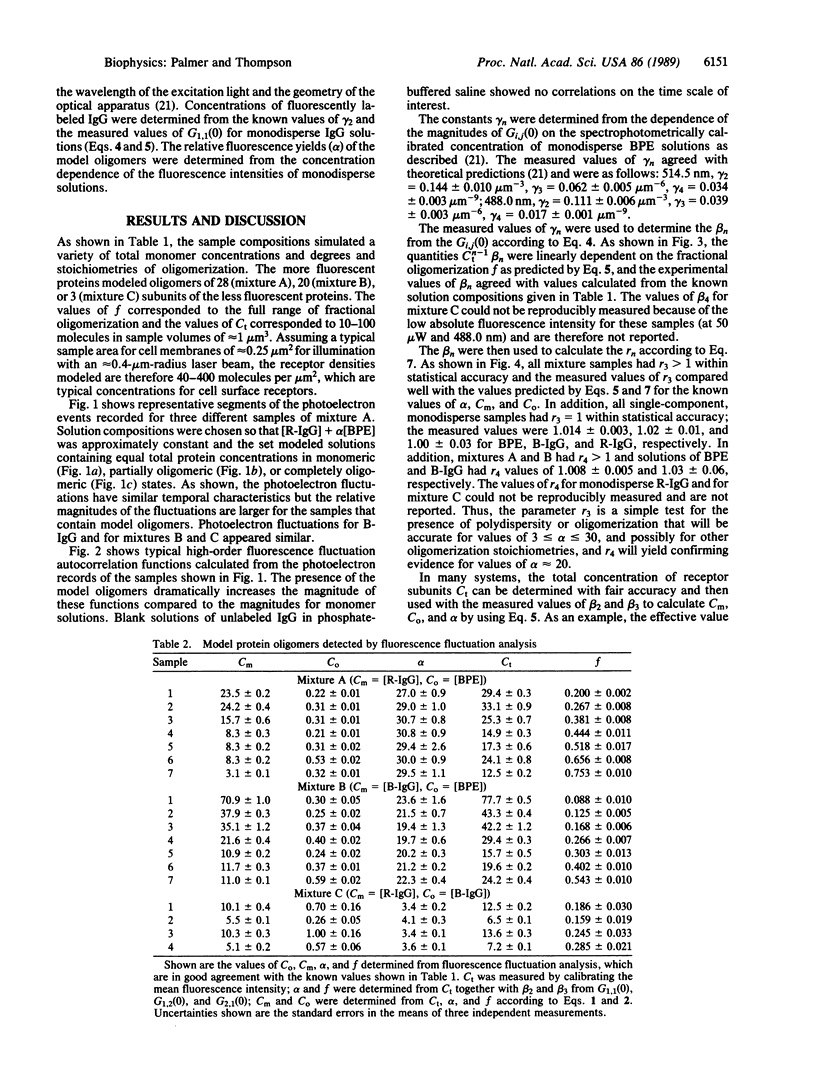
The technique of high-order fluorescence fluctuation autocorrelation for detecting and characterizing protein oligomers was applied to solutions containing two fluorescent proteins in which the more fluorescent proteins were analogues for clusters of the less fluorescent ones. The results show that the model protein clusters can be detected for average numbers of observed subunits (free monomers plus monomers in oligomers) equal to 10-100 and for relative fluorescent yields that correspond to oligomers as small as trimers. High-order fluorescent fluctuation analysis may therefore be applicable to cell surface receptor clusters in natural or model membranes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney J. R., Braun J., Owicki J. C. Lateral interactions among membrane proteins. Implications for the organization of gap junctions. Biophys J. 1987 Sep;52(3):441–454. doi: 10.1016/S0006-3495(87)83233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Ravdin P., Koppel D. E., Schlessinger J., Webb W. W., Elson E. L., Podleski T. R. Lateral motion of fluorescently labeled acetylcholine receptors in membranes of developing muscle fibers. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4594–4598. doi: 10.1073/pnas.73.12.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Developing concepts in receptor research. Drug Intell Clin Pharm. 1983 May;17(5):357–366. doi: 10.1177/106002808301700507. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Metzger H. Structural basis of antibody function. Annu Rev Immunol. 1983;1:87–117. doi: 10.1146/annurev.iy.01.040183.000511. [DOI] [PubMed] [Google Scholar]
- Gross D., Webb W. W. Molecular counting of low-density lipoprotein particles as individuals and small clusters on cell surfaces. Biophys J. 1986 Apr;49(4):901–911. doi: 10.1016/S0006-3495(86)83718-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magde D., Elson E. L., Webb W. W. Fluorescence correlation spectroscopy. II. An experimental realization. Biopolymers. 1974 Jan;13(1):29–61. doi: 10.1002/bip.1974.360130103. [DOI] [PubMed] [Google Scholar]
- Meyer T., Schindler H. Particle counting by fluorescence correlation spectroscopy. Simultaneous measurement of aggregation and diffusion of molecules in solutions and in membranes. Biophys J. 1988 Dec;54(6):983–993. doi: 10.1016/S0006-3495(88)83036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A. G., 3rd, Thompson N. L. Molecular aggregation characterized by high order autocorrelation in fluorescence correlation spectroscopy. Biophys J. 1987 Aug;52(2):257–270. doi: 10.1016/S0006-3495(87)83213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N. O., Johnson D. C., Schlesinger M. J. Scanning fluorescence correlation spectroscopy. II. Application to virus glycoprotein aggregation. Biophys J. 1986 Apr;49(4):817–820. doi: 10.1016/S0006-3495(86)83710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez M., Axelrod D. Polarized fluorescence photobleaching recovery for measuring rotational diffusion in solutions and membranes. Biophys J. 1988 Apr;53(4):575–591. doi: 10.1016/S0006-3495(88)83137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., Gaub H. E., McConnell H. M. T-cell-mediated association of peptide antigen and major histocompatibility complex protein detected by energy transfer in an evanescent wave-field. Nature. 1986 Mar 13;320(6058):179–181. doi: 10.1038/320179a0. [DOI] [PubMed] [Google Scholar]
- Williams L. T. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]
- Wright L. L., Palmer A. G., 3rd, Thompson N. L. Inhomogeneous translational diffusion of monoclonal antibodies on phospholipid Langmuir-Blodgett films. Biophys J. 1988 Sep;54(3):463–470. doi: 10.1016/S0006-3495(88)82979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Schlessinger J. Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor. Biochemistry. 1987 Mar 10;26(5):1443–1451. doi: 10.1021/bi00379a035. [DOI] [PubMed] [Google Scholar]
- Young J. D., Unkeless J. C., Young T. M., Mauro A., Cohn Z. A. Role for mouse macrophage IgG Fc receptor as ligand-dependent ion channel. Nature. 1983 Nov 10;306(5939):186–189. doi: 10.1038/306186a0. [DOI] [PubMed] [Google Scholar]


