Abstract
Under FK506-based immunosuppression, 16 cadaveric small bowel transplantations were performed in 15 recipients with (n = 5) or without (n = 11) the large bowel. Twelve (80%) patients are alive after 1.5 to 19 months, 11 bearing their grafts, of which 4 include colon. The actuarial one-year patient and graft survivals are 87.5% and 65.9%, respectively. Five grafts were lost to acute (n = 4) or chronic (n = 1) rejection, and 3 of these patients subsequently died after 376, 440, and 776 days total survival. Six recipients developed severe CMV infection that was strongly associated with seronegative status preoperatively and receipt of grafts from CMV positive donors; 3 died, and the other 3 required prolonged hospitalization. Currently, 9 patients are free from TPN 1–18 months postoperatively, 2 require partial TPN, and one has returned to TPN after graft removal. The results show the feasibility of small bowel transplantation but emphasize the difficulty of managing these recipients not only early but long after their operation.
Until recently, patients with irreversible intestinal failure had only the socially restrictive option of parenteral nutrition, which is beset by annoying as well as life-threatening complications. Past experience with the potential alternative of isolated intestinal transplantation was not encouraging, because of the inability to control rejection, graft-versus-host disease or both (1).
Two years ago, we reported on the first five recipients of the small bowel treated with the new immunosuppressive agent, FK506; 4 of the intestines were in combination with the liver and one was alone (2). We describe here our experience with 16 isolated intestinal transplantations in 15 patients. In several cases, part of the colon also was included.
MATERIALS AND METHODS
The recipients
Five recipients were children and 10 were adults with a mean age of 5.1 ± 4.4 years and 36.5 ± 11.4 years, respectively. Indication for isolated intestinal transplantation was short-bowel syndrome in 13 patients and uncorrectable intestinal disease in 2. All of the recipients had been managed by total parenteral nutrition (TPN*) from 1 to 132 months preoperatively and had experienced more than one episode of TPN-related complications. Bacterial and/or fungal sepsis and multiple line replacements were seen in all but one patient (case 5). Eight had major vessel thrombosis that had already made venous access extremely difficult.
Abnormal histopathology of the liver was found in all 10 recipients from whom a biopsy was available, consisting of mild steatosis (n = 3) or mild portal fibrosis (n = 7). Five patients had been hospitalized and the remaining 10 were home-bound. These and other features of the recipients are summarized in Table 1.
TABLE 1.
Clinical summary of the recipients
| A. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pt. | Age (yr) |
Sex | Cause of intestinal failure |
Remaining intestine |
TPN | Transplantation | |||
| Duration (months) |
T.bil | Liver biopsy |
Date | Graft | |||||
| 1 | 31 | M | Gun-shot wound | Tca to rectum | 6 | 1.1 | Steatosis | 5/2/90 | Small bowel |
| 17 | — | 3/16/92 | Small bowel | ||||||
| 2 | 2.5 | F | Microvillus inclusion disease | Whole intestine | 29 | 0.4 | Fibrosis | 10/31/91 | Small bowel |
| 3 | 1.3 | M | Intestinal atresia | TC to rectum | 15 | 0.4 | — | 12/25/91 | Small bowel |
| 4 | 50 | F | Crohn’s disease | Jejunum (20 cm), rectum | 120 | 1 | — | 12/28/91 | Small bowel |
| 5 | 34 | F | Desmoid tumor | Whole intestine | 1 | 1.1 | — | 2/3/92 | Small bowel |
| 6 | 38 | M | Crohn’s disease | Jejunum (10 cm), TC to rectum | 120 | 1.8 | — | 3/4/92 | Small bowel |
| 7 | 10.2 | F | Pseudo-obstruction | Jejunum (20 cm), TC to rectum | 132 | 0.6 | Fibrosis | 3/6/92 | Small bowel |
| 8 | 22 | F | Crohn’s disease | Jejunum (48 cm), no colon or rectum | 36 | 0.9 | Steatosis | 3/12/92 | Small bowel |
| 9 | 20 | F | Traffic accident | Jejunum (20 cm), DC to rectum | 24 | 1.6 | — | 6/7/92 | Small bowel |
| 10 | 37 | F | Familial polyposis | Jejunum (60 cm), rectum | 32 | 2.8 | Fibrosis | 11/27/92 | Small bowel, colon |
| 11 | 39 | M | Crohn’s disease | Jejunum (30 cm), TC to rectum | 80 | 0.7 | Fibrosis | 2/15/93 | Small bowel |
| 12 | 35 | M | Crohn’s disease | Jejunum (15 cm), no colon or rectum | 17 | 0.8 | Steatosis | 2/21/93 | Small bowel, colon |
| 13 | 58 | F | Colon cancer and multiple intestinal resections | Jejunum (50 cm), no colon or rectum | 17 | 0.7 | Fibrosis | 3/3/93 | Small bowel, colon |
| 14 | 1.7 | M | Gastroschisis | Jejunum (15 cm), TC to rectum | 20 | 0.5 | Fibrosis | 3/18/93 | Small bowel, colon |
| 15 | 9.6 | M | Volvulus | Jejunum (15 cm), TC to rectum | 115 | 0.5 | Fibrosis | 3/28/93 | Small bowel, colon |
| B. | ||||||
|---|---|---|---|---|---|---|
| Hospital stay | Survival (days)b | Current status |
Comment |
|||
| ICU (days) |
Discharge (weeks) |
Patient | Graft | TPN | Location | |
| 14 | 28 | 776 | 668 | — | — | Retransplanted at 684 days, kidney transplanted at 626 days, died of sepsis |
| 71 | ||||||
| 7 | 11 | >567 | >567 | Free | Home | Severe cholestasis for 3 months |
| 5 | 7 | >512 | >512 | Free | Home | |
| 7 | 11 | >509 | >509 | Free | Hospital | CMV enteritis |
| 5 | 15 | 440 | 239 | — | — | Demyelination of the brain, graft removed at 239 days, died of pulmonary embolism |
| 6 | 15 | 376 | 366 | — | — | CMV hepatitis and enteritis, graft removed at 366 days, died of sepsis |
| 11 | 14 | >440 | >440 | Partial | Home | Gastrojejunostomy at 335 days |
| 4 | 8 | >434 | >434 | Free | Home | |
| 5 | 5 | >347 | >347 | Free | Hospital | CMV enteritis and retinitis |
| 7 | 7 | >174 | >174 | Free | Hospital | Fungal sinusitis, CMV enteritis |
| 9 | 9 | >94 | >94 | Free | Home | SMA reposition at 17 days |
| 72 | — | >88 | >88 | Partial | Hospital | CMV enteritis, pneumonitis |
| 6 | 6 | >78 | >78 | Free | Home | |
| 2 | 6 | >63 | 27 | — | Home | Respiratory syncitial virus, graft removed at 27 days |
| 6 | 4 | >53 | >53 | Free | Home | |
TC: transverse colon; DC: descending colon.
As of 5/20/93.
Donor operation
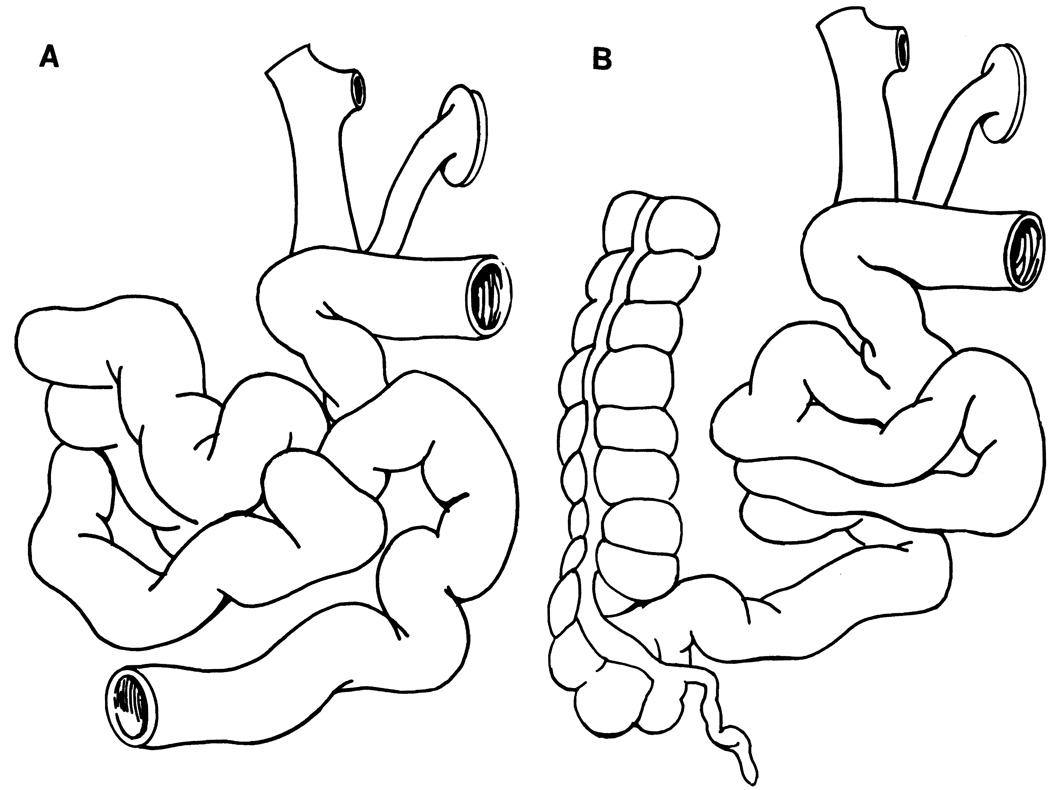
The 16 cadaveric donors had the same ABO blood type as the recipients and were slightly larger (n = 5), similar (n = 8), or smaller (n = 3) in size. All lymphocytotoxic crossmatches were negative, and HLA matching was universally poor. Selective bacterial decontamination of the donor was started immediately after the donor was accepted, as described before (3, 4). The grafts for patient 1 through patient 9 consisted of the entire small intestine except for short segments distal to the ligament of Treitz and proximal to the ileocecal valve (Fig. 1A). Because these patients tended to have high postoperative stomal output and diarrhea, the ascending colon, with or without the transverse colon, was included with the small bowel in patients 10, 12, 13, 14, and 15 to slow intestinal transit and facilitate water absorption (Fig. 1B). All grafts except one were flushed with 1 L University of Wisconsin solution via the abdominal aorta, and preserved for a mean duration of 6.5 ± 2.0 hr (ranging from 2.8 to 9.8 hr). The exception was the first graft for patient 1, which was excised and simply immersed in an ice bath for 10.5 hr until transplantation. Luminal flushing was performed only with grafts that consisted of both small bowel and colon. Depletion of immunocytes was not attempted in any of the grafts.
FIGURE 1.
Grafts for intestinal transplantation. (A) Entire small bowel except for short segments at each end on a vascular pedicle of the superior mesenteric artery and vein, (B) Small bowel plus ascending colon with or without the transverse colon.
Recipient operation
The technique for isolated intestinal transplantation was essentially the same as described before (5) but with modifications for venous reconstruction and restoration of gastrointestinal tract continuity. Arterial reconstruction was performed exclusively by end-to-side anastomosis of the graft superior mesenteric artery to the recipient infrarenal abdominal aorta. The portal vein at the hepatic hilum was chosen for venous reconstruction by mesenteric piggyback method in most of the cases (9/16) (6), but the distal end of the recipient superior mesenteric vein (4/16), or its confluence with the splenic vein (2/16) was also selected if feasible. Mesocaval anastomosis was needed on only one occasion at the time of retransplantation.
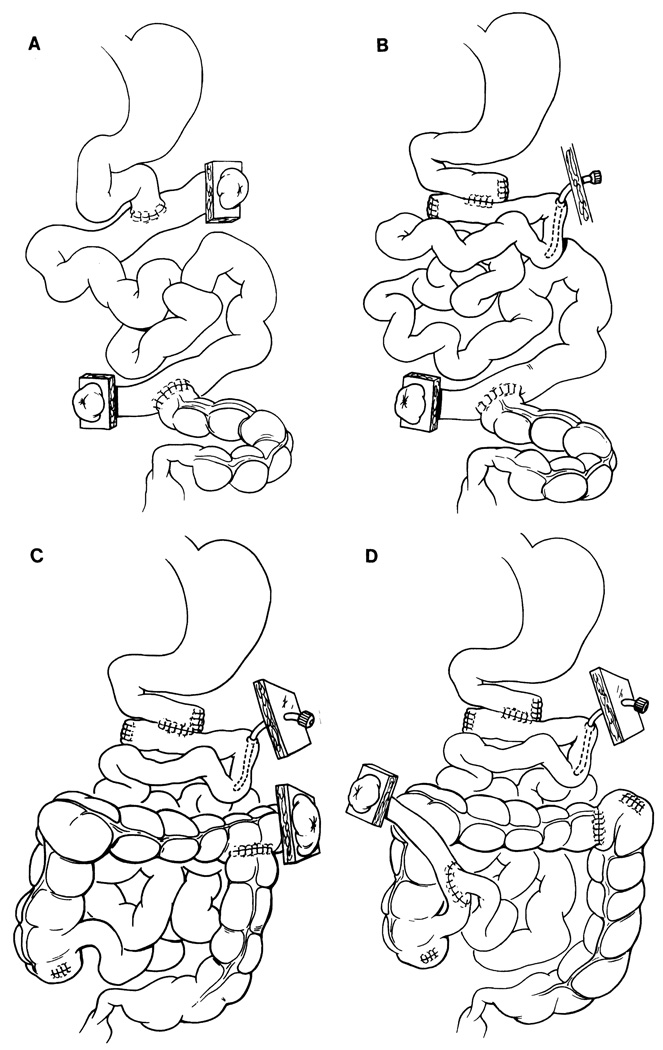
In reestablishing GI tract continuity, 4 different types of enterostomies were used for decompression and monitoring of the transplant. Initially, both ends of the graft were exteriorized by the chimney method (the first graft of patient 1, Fig. 2A). For the second graft of patient 1, patients 2–9, and patient 11, the proximal enterostomy was eliminated and replaced by a tube jejunostomy (Fig. 2B). In patients 10 and 12–14, a distal enterostomy was made with the transverse colon (Fig. 2C). Patient 15 had a distal ileal loop exteriorized by the Bishop-Koop method (Fig. 2D). A tube jejunostomy was added for intestinal decompression and enteral feeding in all recipients except after the first transplantation in patient 1. Cholecystectomy always was performed.
FIGURE 2.
Reconstruction of gastrointestinal continuity and position of enterostomies. (A) Each end of the graft is exteriorized by the chimney method. (B) The proximal enterostomy is eliminated and replaced by a tube-jejunostomy. (C) Distal transverse colostomy. (D) Bishop-Koop method to exteriorize distal ileum for biopsy monitoring.
Immunosuppression
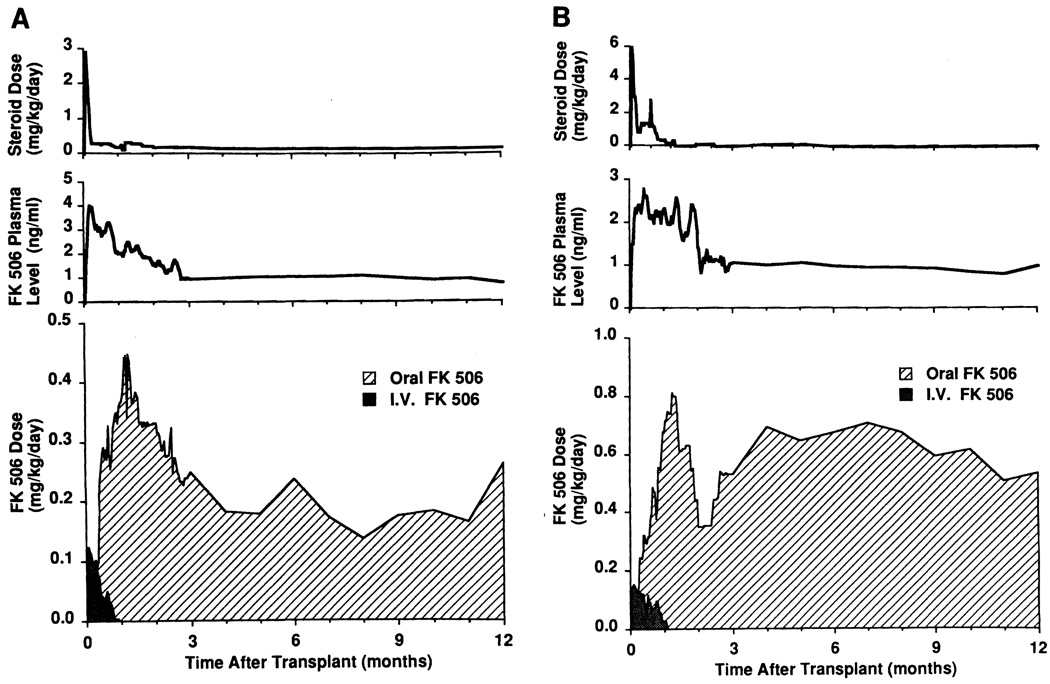
FK506, steroids, and prostaglandin E1 (Prostin) (Fig. 3) were used for postoperative immunosuppression, as is routine at our center for liver recipients (7). Immediately after graft reperfusion, FK506 at 0.1 to 0.15 mg/kg/day was given intravenously, and then switched to oral FK506, 0.3 mg/kg/day, or usually less, when the recipients became tolerant to enteral feedings. To ensure enteric absorption, oral and i.v. doses of FK506 were allowed to overlap for several days with gradual weaning of the i.v. route. FK506 dose was adjusted to maintain trough plasma levels of 2–3 ng/ml for the first month, 1–2 ng/ml until the third month, and 1 ng/ml thereafter. These target levels are higher than for liver transplantation. The FK506 dose was lowered if toxic side effects appeared. Methylprednisolone 1 g in adults or hydrocortisone in children was given intravenously in the operating room, and followed by rapid tapering of prednisone over 5 days after transplantation. Maintenance steroids were given thereafter or stopped when possible. Prostaglandin E1 was started at 0.2 µg/kg/hr soon after the graft was revascularized, gradually increased to 0.6 to 0.8 µg/kg/hr if the recipient was stable, and continued until intravenous FK506 was stopped.
FIGURE 3.
Postoperative immunosuppression for intestinal transplant recipients. Values are expressed as median. (A) Adult patients. (B) Pediatric patients. Note striking differences in FK506 doses (greater in children) and prednisone (less in children).
Graft rejection was monitored by a combination of clinical findings, endoscopic observation, and histopathologic examination of endoscope-guided mucosal biopsies. The treatment of graft rejection, based upon clinical, endoscopic, and mucosal biopsy findings, has been described elsewhere (5, 8).
Nutritional management
Methods for nutritional management after intestinal transplantation have been described elsewhere (9). In brief, total parenteral nutrition was continued postoperatively. After confirming the integrity of gastrointestinal reconstruction by an upper GI series (usually at 7–10 postoperative days), enteral feeding with an isoosmolar elemental diet (Peptamen) containing peptides and a small amount of long-chain triglycerides was begun via a jejunostomy tube. Enteral feedings and oral intake were gradually increased with a reciprocal decrease in parenteral nutrition.
Prophylaxis of infection
Selective decontamination of the GI tract was continued for 4–6 weeks postoperatively and supplemented with i.v. ampicillin and cefotaxin for the first 5 days. Frequent cultures of the blood, stool, wound, urine, sputum, and ostomy discharge were obtained to monitor changes in flora and to detect evidence suggesting translocation. Ganciclovir was continued for 3 to 6 months for prophylaxis of cytomegalovirus infection. If severe CMV infection occurred, foscarnet or CMV immunoglobulin was added to the ganciclovir treatment.
Assessment of graft function
Body weight, volume of stomal output, and frequency and nature of the stool were common indices used to evaluate intestinal function. D-xylose absorption test and 72-hr fecal fat secretion were also studied periodically.
RESULTS
Postoperative course
Postoperative recovery was uneventful for most of the recipients, with a median ICU stay of 6 days (4 to 72 days, Table 1). The postoperative course of two adult recipients was complicated by bouts of rejection, sepsis, and renal failure. Patient 1 had repeated episodes of rejection following drug noncompliance. Patient 12 suffered from a poor pretransplant general condition due to his 25-year history of Crohn’s disease. Enteral feeding was started a median of 9 days postoperatively (range 3 to 17), and TPN was discontinued at a median of 30.5 days (range 18 to 300) with one exception. Patients were discharged after 4 to 28 weeks, as shown in Table 1 for each case. After discharge, the patients with a >1-month follow-up were readmitted a median of 3 times for a median stay of 15 days. High stomal output and diarrhea resulting in dehydration were the leading indications for readmission, but CMV infection and rejection were also prevalent indications. Patients were in the hospital a median of 32% of the time after their transplant admission.
Survival
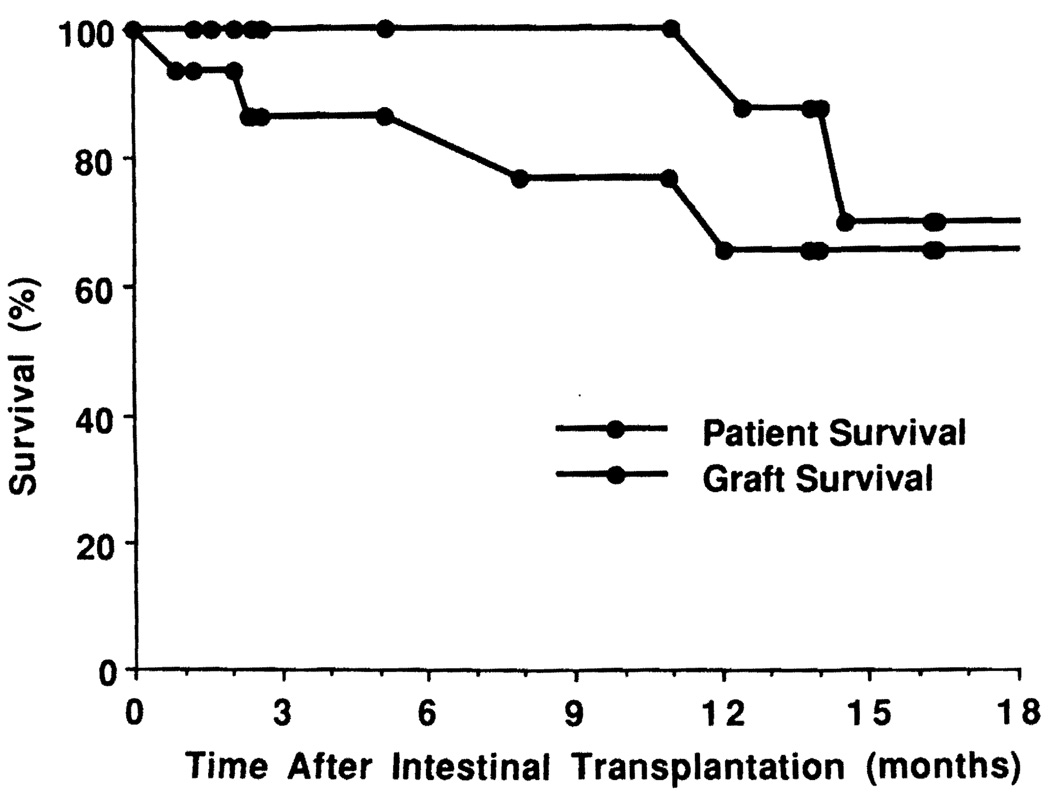
The 5 pediatric recipients are alive at home after 53 to 567 days, and 7 of the 10 adult patients are alive after 78 to 499 days. Three of the surviving 7 adult patients are at home while 4 are currently hospitalized for the treatment of CMV infection (n = 3) or fungal sinusitis (n = 1). Thus patient survival is 12/15 (80%). By a life-table analysis using the Kaplan-Mayer method, actuarial patient survival rates at 6, 12, and 18 months are 100%, 87.5%, and 70%, respectively (Fig. 4).
FIGURE 4.
Actuarial patient and graft survival rates after intestinal transplantation.
Causes of graft loss and mortality
The grafts were removed before the deaths of the 3 adult recipients (patients 1, 5, and 6), and in patient 1 the mortality followed subsequent retransplantation. A pediatric patient (patient 14) who underwent graft removal at 27 days is alive after returning to TPN. Thus, graft survival is 11/16 (68.8%). Actuarial graft survivals at 6, 12, and 18 months are 86.5%, 65.9%, and 65.9% respectively (Fig. 4).
The 5 graft losses were from refractory rejection. Patient 1, who had a stormy course during the immediate postoperative period, lost his graft at 668 days from chronic rejection after several episodes of drug noncompliance. A second graft was lost to acute rejection 71 days later and he died of sepsis 22 days after its removal, for a total survival of 776 days. The graft of patient 5 was removed 239 days after transplantation because of acute rejection that followed withdrawal of immunosuppression because of a neurologic syndrome caused by demyelination of the white matter of the brain. She was discharged from the hospital on TPN but died 201 days later from a pulmonary embolism that occurred during an operation to replace the TPN line.
The postoperative course of patient 6 was uneventful until he developed recurrent Crohn’s disease in his native colon 300 days after transplantation, and then acute rejection of the intestinal graft. This patient died of sepsis at 376 days, 10 days after the graft was removed. Patient 14, who received an intestine and colon transplant with a colostomy (Fig. 2C), developed clinical signs of graft rejection on postoperative day 12, but histopathologic confirmation of the diagnosis and initiation of treatment were delayed because an ileal biopsy could not be obtained. Biopsies of the transplanted colon remained normal until rejection became advanced. By this time, respiratory syncitial virus infection precluded augmentation of immunosuppression. The graft was removed at 27 days, and this child is alive on TPN.
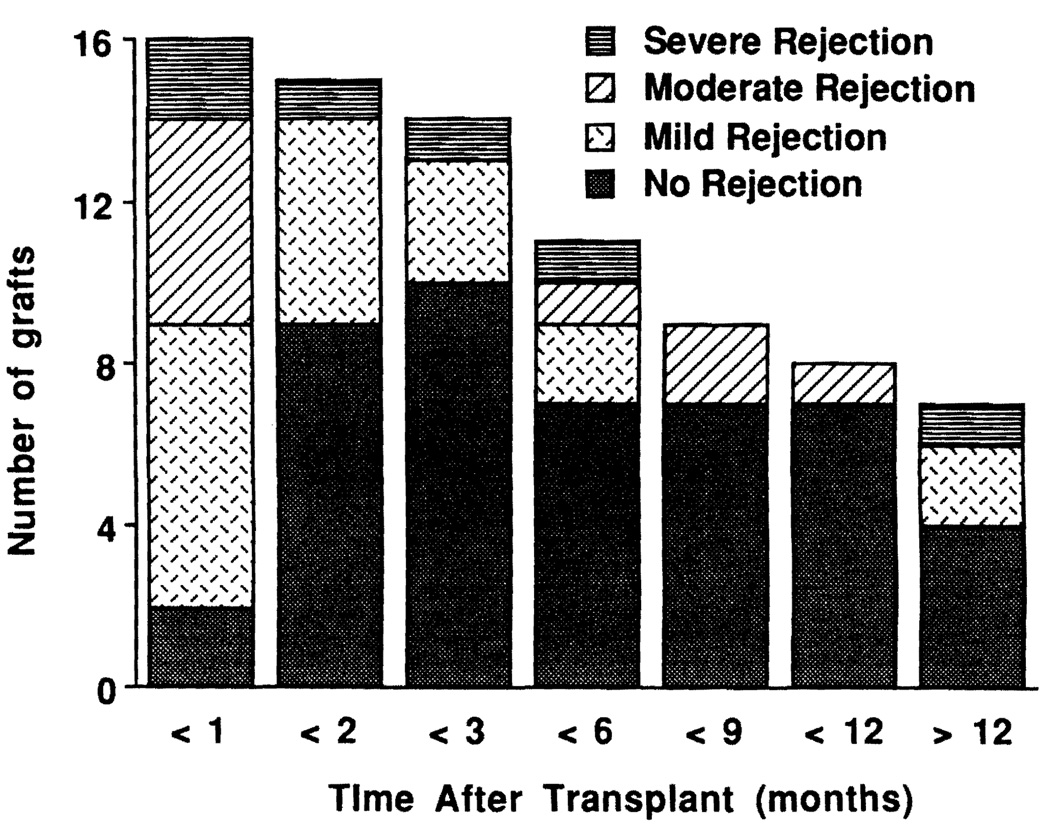
Rejection
Of the 16 grafts, only one (patient 3) developed no evidence of rejection. Thus, the overall incidence of graft rejection was 93.8% (15/16). The risk of acute rejection was highest at 87.5% (14/16) during the first month and decreased to 28.6% (4/14) at 3 months and 36.4% (4/11) at 6 months, but was still high at 42.9% (3/7) after 12 months (Fig. 5). Moderate-to-severe acute graft rejection was treated by OKT3. Patient 1 received two courses of OKT3 for his first graft and one for the second. Patients 10, 14, and 15 also received a course of OKT3 treatment. Chronic rejection was seen in two removed grafts (the first graft of patient 1 and patient 5). Characteristics of clinical course and angiographic and pathological changes have been described elsewhere (5). Graft-versus-host disease was not seen in any of these patients.
FIGURE 5.
Incidence and severity of acute rejection after intestinal transplantation and at progressively later times postoperatively.
Infection
All except patient 8 experienced more than one episode of postoperative bacterial or fungal infection: line infection (n = 7), abdominal wound (n = 5), peritonitis (n = 2), evidence of translocation (n = 2), and others (n = 6).
Cytomegalovirus infection after transplantation was strongly influenced by the preoperative serological status (Table 2). For example, 7 of the 9 adult patients, who were negative for CMV preoperatively and received grafts from CMV-positive donors developed severe clinical disease at 1.5 to 4 months after transplantation. Three patients required frequent hospital readmission for CMV infection treatment. Five patients with CMV infection developed graft rejection due to reduction of immunosuppression, and 3 of the 5 went on to graft removal. One pediatric recipient who was seropositive before transplant and received the graft from a seronegative donor (patient 2) had positive culture of CMV in urine and sputum, but developed no clinical symptoms. In contrast, there were no episodes of CMV infection in the 6 CMV-seronegative patients who received CMV-seronegative intestinal grafts.
TABLE 2.
CMV Infection after isolated intestinal transplantation
| Donor | Recipient | (n) | Pediatric/ adult |
CMV Infection | ||
|---|---|---|---|---|---|---|
| Positive culture | Severe infection | |||||
| − | − | 6 | 4/2 | 0 | 0 | |
| − | + | 1 | 1/0 | 1 | 0 | |
| + | − | 9 | 0/9 | 7 | 6a | |
| + | + | 0 | 0/0 | 0 | 0 | |
Three patients required prolonged hospitalization, and three grafts were lost to rejection.
Graft function
Nine of the 11 surviving patients with functioning grafts are free of TPN and on an unrestricted oral diet. One patient (patient 7), who was transplanted for pseudoobstruction, had a gastrojejunostomy placed surgically 335 days after transplantation and requires partial nutritional support by TPN because of dysmotility of the stomach. Patient 12 is currently undergoing weaning from parenteral to enteral feeding.
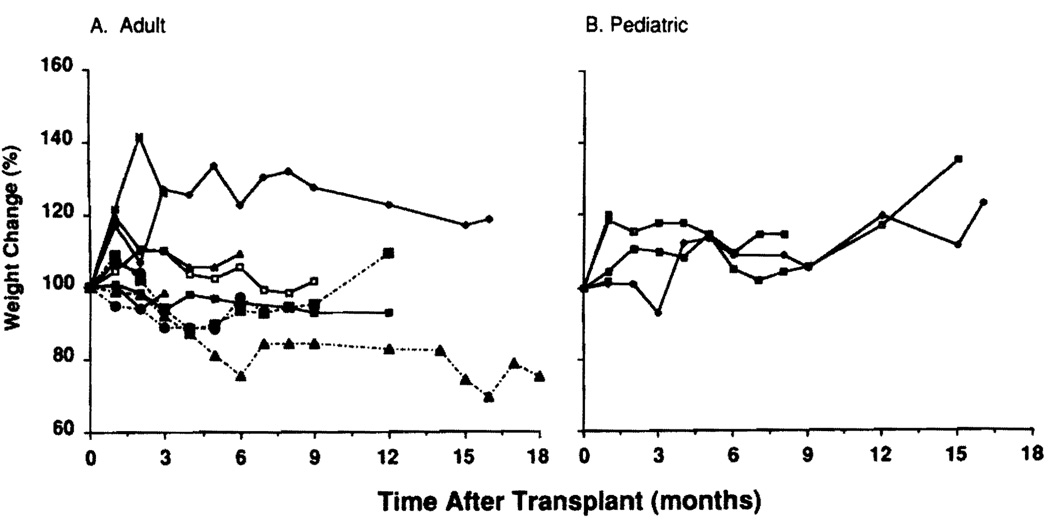
All recipients except for patient 1 gained or maintained body weight after 3 months (Fig. 6) and had improved d-xylose absorption.
FIGURE 6.
Body weight changes after intestinal transplantation. (A) Adults. (B) Infants and children.
None of the four Crohn’s disease patients has developed disease recurrence in the transplanted intestine to date. One patient (case 4) had recurrence of Crohn’s disease to the native rectum 12 months after transplantation and was medically treated. One patient with desmoid tumor (case 5) also has had no recurrence for 14 months.
DISCUSSION
Before the introduction of cyclosporine, the longest survival of an intestinal transplant was 76 days (10) using immunosuppressive regimens that included the combination of azathioprine and prednisone, to which antilymphocyte globulin or thoracic duct drainage were added in some cases (1, 11, 12). After the advent of cyclosporine, isolated intestinal grafts were lost to rejection in Toronto (n = 1) (13), Chicago (n = 1) (14), Paris (n = 7) (15), Kiel (n = 2) (16, 17), London, Ontario (n = 1) (18), and Uppsala (n = 1) (19). However, the other 2 recipients, one of an intestinal segment from a living-related donor (17) and the other of a full small bowel from a cadaveric donor (20), are currently alive and maintained totally by an unrestricted oral diet. In our series with FK506 immunosuppression, patient and graft survival rates improved to 80% and 68.8%, respectively. Although the follow-up period is still limited, this experience indicates that FK506 has moved isolated intestinal transplantation toward clinical practicality.
Because of tolerogenicity induced by the liver, Grant et al. (21) suggested that the intestinal graft be transplanted together with the liver even in patients who have normal liver function. We confine the indication for isolated intestinal transplantation only to patients who have irreversible intestinal failure without hepatic abnormalities. However, since it is still in the developmental stage, we perform this procedure in highly selected patients, such as those whose venous accesses are running out from major vessel thrombosis or those who have a long history of active Crohn’s disease that is refractory to any conventional treatments. In spite of intensive management by TPN, no microvillus inclusion disease patients survived for more than a few years (22), compared with our patient (case 2) who is well with an unrestricted oral diet for 19 months after transplantation.
Although isolated intestinal transplantation has thus become feasible under improved immunosuppression, this achievement has been far from easy. The occurrence of severe diarrhea and high stomal output have been particularly troublesome during the first 3 to 6 months after transplantation, necessitating frequent readmissions for dehydration. The cause of diarrhea after intestinal transplantation is multifactorial, and is enhanced by decreased intestinal transit time, increased osmolarity of luminal contents, increased water and electrolyte secretion, bacterial overgrowth, and steatorrhea (23). Intestinal denervation, ischemic damage, interruption of lymphatics, malabsorption, and rejection also could be factors of cumulative impact.
Antidiarrheal medications, such as opiates, loperamide, and kaolin-pectin mixture, were not always effective, and in 5 recent cases an attempt was made to reduce the problem by including the ileocecal valve and at least the ascending colon in the graft. This approach was mentioned by Lillehei 25 years ago (24), and has been used in our multivisceral recipients (4, 25). Although still inconclusive because of the small number of patients, small bowel and colon transplant recipients have tended to have less stomal output and more semiformed stool. However, the use of a colostomy instead of a distal ileostomy has made passage of the endoscope difficult for monitoring by ileal biopsies and was responsible for one graft loss in a child whose colon biopsies failed to reflect the more proximal acute rejection. In the last patient to receive a small and large bowel graft, the distal colostomy was replaced with a Bishop-Koop ileostomy (at the suggestion of Dr. Adrian Bianchi, Manchester, England). This permitted easy access to the ileum and did not increase the amount of stomal discharge.
Several (26–28) but not all (29) experimental studies have suggested the metabolic and/or immunologic advantages of draining the venous effluent of the intestinal graft through the liver (mesoportal reconstruction) rather than resorting to a mesocaval shunt, which may be easier. In the 11 historical cases in which information on the operative procedures was available (1, 11), portal drainage was used in only two instances, all others having a mesosystemic shunt. We have been able to routinely accomplish the more physiologic mesoportal reconstruction, reserving the mesosystemic shunt anastomosis for the eventuality of retransplantation. The significance of the method of venous drainage remains unresolved. No serious metabolic complications have developed from mesocaval shunt in one of our liver-intestine recipients at almost 3 years postoperatively (5) or in the long-surviving isolated intestinal recipients in studies by Kiel (17) and Paris (20).
CMV has been the most frequent cause of serious infectious complications in our patients, with a specific risk for those who converted to CMV-positive serology after transplantation. Six of the 8 developed severe CMV disease, 5 had episodes of rejection, 3 required prolonged hospitalization, and 3 underwent graft removal and eventually died. Prophylaxis and active therapy were ineffective. In contrast, the patients who did not develop CMV infection had a smooth recovery and stable postoperative course. Posttransplant disease can occur by reactivation of preexisting CMV or by infection from blood products—and perhaps most important, by transmission from infected organs.
This experience recapitulates that with other kinds of organ transplants. The incidence of CMV pneumonia in seronegative recipients after transplantation of the lung from seropositive donors was 80%, with mortality exceeding 50% (30). When seropositive grafts were given to seropositive recipients, the incidence of CMV pneumonitis decreased to 20%. After kidney transplantation with prophylaxis with acyclovir and hyperimmunoglobulin, CMV disease occurred in 10% of the seronegative patients receiving CMV-infected organs, while it decreased to 0.8% when CMV-negative grafts were given to seronegative patients (31). These same trends, but in an exaggerated form, were seen in our transplant recipients. These findings suggest the advisability of avoiding transplantation of isolated intestinal grafts from seropositive donors to seronegative recipients. However, the shortage of otherwise suitable donors and the urgent need of some of the recipients requires case-by-case decision making.
Our experience has addressed the question of whether the liver should be transplanted simultaneously with the intestine if recipient liver function is normal in order to exploit the so called hepatic tolerogenicity that extends to concomitantly transplanted organ(s) from the same donor (32). Although this has been a matter for discussion since the first successful combined intestine and liver transplantation by Grant et al. (21), our earlier report comparing the evolution of isolated intestinal versus composite grafts (containing both liver and intestine) showed better results with the intestine alone (4). This trend has continued with our subsequent experience. Therefore, we perform combined liver and intestinal transplantation only for patients who have failure of both organs. Further observations will be required to establish the validity of this policy, particularly because its application selects the sickest patients for the composite procedure and therefore biases the results for comparison.
Footnotes
Presented at the 19th Annual Meeting of the American Society of Transplant Surgeons, May 19–21, 1993, Houston, TX.
This work was supported by Research Grants from the Veterans Administration and by Project Grant DK-29961 from the National Institute of Health, Bethesda, MD.
Abbreviation: TPN, total parenteral nutrition.
REFERENCES
- 1.Kirkman RT. Small bowel transplantation. Transplantation. 1984;37:429. doi: 10.1097/00007890-198405000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Todo S, Tzakis AG, Abu-Elmagd K, et al. Cadaveric small bowel and small bowel-liver transplantation in humans. Transplantation. 1992;53:369. doi: 10.1097/00007890-199202010-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casavilla A, Selby R, Abu-Elmagd K, et al. Logistics and technique for combined hepatic-intestinal retrieval. Ann Surg. 1992;216:85. doi: 10.1097/00000658-199211000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Todo S, Tzakis A, et al. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172:335. [PMC free article] [PubMed] [Google Scholar]
- 5.Todo S, Tzakis AG, Abu-Elmagd K, et al. Intestinal transplantation in composite visceral grafts or alone. Ann Surg. 1992;216:223. doi: 10.1097/00000658-199209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tzakis AG, Todo S, Reyes J, Nour B, Fung JJ, Starzl TE. Piggyback orthotopic intestinal transplantation. Surg Gynecol Obstet. 1993;176:297. [PMC free article] [PubMed] [Google Scholar]
- 7.Takaya S, Iwaki Y, Starzl TE. Liver transplantation in positive cytotoxic crossmatch cases using FK506, high-dose steroids, and prostaglandin E1. Transplantation. 1992;54:927. doi: 10.1097/00007890-199211000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu-Elmagd K, Tzakis A, Todo S, et al. Monitoring and treatment of intestinal allograft rejection in humans. Transplant Proc. 1993;25:1202. [PMC free article] [PubMed] [Google Scholar]
- 9.Reyes J, Tzakis AG, Todo S, et al. Nutritional management of intestinal transplant recipients. Transplant Proc. 1993;25:1200. [PMC free article] [PubMed] [Google Scholar]
- 10.Fortner JG, Sichuk G, Litwin SD, Beattie EJ., Jr Immunological responses to an intestinal allograft with HL-A-identical donor-recipient. Transplantation. 1972;14:531. doi: 10.1097/00007890-197211000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Okumura M, Mester M. The coming age of small bowel transplantation: a historical perspective. Transplant Proc. 1992;24:1241. [PubMed] [Google Scholar]
- 12.Grant D. Intestinal transplantation: current status. Transplant Proc. 1989;21:2869. [PubMed] [Google Scholar]
- 13.Cohen Z, Silverman RE, Wassaf R, et al. Small intestinal transplantation using cyclosporine: report of a case. Transplantation. 1986;42:613. doi: 10.1097/00007890-198612000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Tattersall C, Gebel H, Haklin M, Hartsell W, Williams J. Lymphocyte responsiveness after irradiation in canine and human intestinal allografts. Curr Surg. 1989;46:16. [PubMed] [Google Scholar]
- 15.Revillon Y, Jan D, Goulet O, Ricour C. Small bowel transplantation in seven children: preservation technique. Transplant Proc. 1991;23:2350. [PubMed] [Google Scholar]
- 16.Hansmann ML, Deltz E, Gundlach M, Schroeder P, Radzun HJ. Small bowel transplantation in a child. Am J Clin Pathol. 1989;920:686. doi: 10.1093/ajcp/92.5.686. [DOI] [PubMed] [Google Scholar]
- 17.Deltz E, Schroeder P, Gebhardt H, et al. Successful clinical small bowel transplantation: report of a case. Clin Transplant. 1989;3:89. [Google Scholar]
- 18.Grant D, Sommerauer J, Mimeault R, et al. Treatment with continuous high-dose intravenous cyclosporine following intestinal transplantation: a case report. Transplantation. 1989;48:151. doi: 10.1097/00007890-198907000-00036. [DOI] [PubMed] [Google Scholar]
- 19.Wallander J, Ewald U, Lackgren G, Tufveson G, Wahlberg J, Meurling S. Extreme short bowel syndrome in neonates: an indication for small bowel transplantation. Transplant Proc. 1992;24:1230. [PubMed] [Google Scholar]
- 20.Goulet O, Revillon Y, Brousse N, et al. Successful small bowel transplantation in an infant. Transplantation. 1992;53:940. doi: 10.1097/00007890-199204000-00046. [DOI] [PubMed] [Google Scholar]
- 21.Grant D, Wall W, Mimeault R, et al. Successful small-bowel/liver transplantation. Lancet. 1990;335:181. doi: 10.1016/0140-6736(90)90275-a. [DOI] [PubMed] [Google Scholar]
- 22.Cutz E, Rhoads JM, Drumm B, Sherman PM, Durie PR, Forstner GG. Microvillus inclusion disease: an inherited defect of brush-border assembly and differentiation. New Engl J Med. 1989;320:646. doi: 10.1056/NEJM198903093201006. [DOI] [PubMed] [Google Scholar]
- 23.Quigley EMM, Thompson JS, Rose SG. The long-term function of canine jejunoileal autotransplants—insights into allograft physiology. Transplant Proc. 1992;24:1105. [PubMed] [Google Scholar]
- 24.Lillehei RC, Idezuki Y, Feemster JA, et al. Transplantation of stomach, intestine and pancreas: experimental and clinical observations. Surgery. 1967;62:721. [PubMed] [Google Scholar]
- 25.Starzl TE, Rowe M, Todo S, et al. Transplantation of multiple abdominal viscera. JAMA. 1989;26:1449. [PMC free article] [PubMed] [Google Scholar]
- 26.Schraut WH, Abraham VS, Loe KKW. Portal versus caval venous drainage of small bowel allografts: technical and metabolic consequences. Surgery. 1986;99:193. [PubMed] [Google Scholar]
- 27.Koltun WA, Madara JL, Smith RJ, Kirkman RL. Metabolic aspects of small bowel transplantation in inbred rats. J Surg Res. 1987;42:341. doi: 10.1016/0022-4804(87)90167-3. [DOI] [PubMed] [Google Scholar]
- 28.Murase N, Demetris AJ, Furuya T, et al. Comparison of the small intestine after multivisceral transplantation and the small intestine transplanted with portal versus caval drainage. Transplant Proc. 1992;24:1143. [PMC free article] [PubMed] [Google Scholar]
- 29.Shaffer D, Diflo T, Love W, Clowes GHA, Maki T, Monaco AP. Immunologic and metabolic effects of caval versus portal venous drainage in small-bowel transplantation. Surgery. 1988;104:518. [PubMed] [Google Scholar]
- 30.Hutter JA, Scott JA, Wreghitt T, et al. The importance of cytomegalovirus in heart-lung transplant recipients. Chest. 1989;95:627. doi: 10.1378/chest.95.3.627. [DOI] [PubMed] [Google Scholar]
- 31.Nicol D, McDonald AS, Belitsky P, et al. Reduction by combination prophylactic therapy with CMV hyperimmune globulin and acyclovir of the risk of primary CMV disease in renal transplant recipients. Transplantation. 1993;55:841. doi: 10.1097/00007890-199304000-00030. [DOI] [PubMed] [Google Scholar]
- 32.Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]