Abstract
Silyloxyallenes serve as highly useful α-acylvinyl anion equivalents. These latent allenolates undergo conjugate additions to alkylidene malonates in the presence of 10 mol% Sc(OTf)3. The reaction delivers intermolecular Rauhut–Currier products in excellent yields and regioselectivities for a wide scope of substrates. Notably, the formal cross-coupling of two different αβ-unsaturated carbonyl compounds (a cross Rauhut–Currier reaction) is achieved. Preliminary investigations have demonstrated good levels of enantioselectivity for the addition of a racemic silyloxyallene with a chiral Lewis acid.
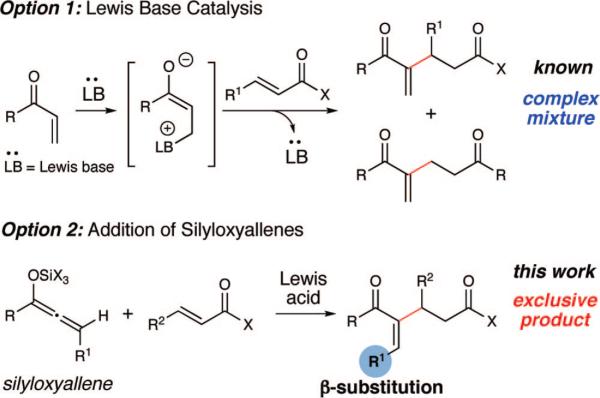
α-Acylvinyl anion equivalents are atypical nucleophiles that generate valuable α,β-unsaturated carbonyl compounds in a highly convergent manner.1 One method of interest that employs this type of unconventional reactivity is the Rauhut–Currier reaction in which the α-acylvinyl anion undergoes a Michael addition.2,3 Similar to the Morita–Baylis–Hillman reaction, the method involves the initial conjugate addition of a Lewis base catalyst to generate a zwitterionic enolate that then undergoes a conjugate addition onto a second equivalent of an electron-deficient olefin (Scheme 1, Option 1). While dimerizations of the activated alkenes (e.g., acrylates) are well-known, the reaction between two different α,β-unsaturated carbonyl compounds (a cross Rauhut–Currier) is much more challenging with few reported examples.4 The key problem is that self-condensations compete under the reaction conditions and the products, which are electron-deficient alkenes as well, are susceptible to polymerizations. In 2002, Krische and Roush independentlyreportedanintramolecularversionoftheRauhut–Currier employing bis-enone substrates.5 Miller has recently reported an elegant enantioselective intramolecular Rauhut–Currier variant with similar bis-enones using one equivalent of N-acetyl cysteine as the promoter.5k Despite the progress of the intramolecular manifold, a successful intermolecular Rauhut–Currier reaction has not emerged to date. A useful bimolecular process could indeed expand the utility of this bond-forming process. In this Communication, we disclose that the Lewis acid-catalyzed conjugate additions of silyloxyallenes afford a wide variety of 1,5-dicarbonyl compounds that map directly onto what would be products of an intermolecular Rauhut–Currier process (Scheme 1, Option 2).
Scheme 1.
Intermolecular Rauhut–Currier Strategies
Silyloxyallenes have emerged as versatile and useful R-acylvinyl anion equivalents.6,7 These latent enolates are prepared readily from the corresponding acylsilanes by way of the Kuwajima–Reich rearrangent of α-hydroxypropargylsilanes.8 Recently, we have demonstrated that silyloxyallenes undergo additions to aldehydes in the presence of Lewis acids and high yields are achieved for a wide scope of substrates with excellent control over the resulting double bond geometry. Encouaged by the full potential of these unusual nucleophiles, we have developed an enantioselective variant of this reaction using racemic silyloxyallenes and a chiral (salen)Cr(III) Lewis acid catalyst.9 In an effort to broaden the use of silyloxyallenes as nontraditional nucleophilic reagents, we have explored conjugate additions of these α-acylvinyl anion equivalents. This reaction, if successful, would generate products from the apparent coupling of two different α,β-unsaturated carbonyl compounds via an intermolecular Rauhut–Currier reaction.
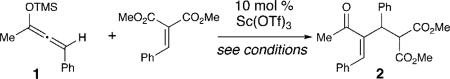
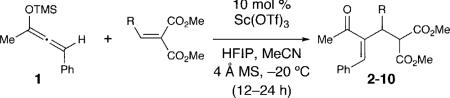
Given the mild nucleophilicity of silyloxyallenes, alkylidene malonates were examined as conjugate acceptors with racemic 1.10 After surveying potential Lewis acids at 10 mol%, we discovered that scandium triflate promotes the addition of 1 in only 9% yield (Table 1, entry 1). Although only weakly encouraging, we postulated that catalyst turnover was rate limiting with these conditions. After surveying an assortment of additives, the addition of hexafluoroisopropanol (HFIP) improves the yield to 54% (entry 2). Changing the solvent to acetonitrile provides the optimal balance of rate and yield (entry 6).11 Finally, lowering the temperature to –20 °C in acetonitrile with 10 mol% Sc(OTf)3 and HFIP (1 equiv) delivers the Rauhut–Currier product in 95% yield and >20:1 regioselectivity favoring the Z-isomer (entry 8).
Table 1.
Optimization of Michael Additiona
 | |||||
|---|---|---|---|---|---|
| entry | additive | solvent | temp (°C) | time (h) | yield (%)b |
| 1 | none | CH2Cl2 | 23 | 18 | 9 |
| 2 | (CF3)2CHOH | CH2Cl2 | 23 | 18 | 54 |
| 3 | (CF3)2CHOH | THF | 23 | 24 | 0 |
| 4 | (CF3)2CHOH | Et2O | 23 | 48 | 71 |
| 5 | (CF3)2CHOH | PhCH3 | 23 | 24 | 55 |
| 6 | (CF3)2CHOH | MeCN | 23 | 18 | 61 |
| 7 | (CF3)2CHOH | MeCN | 0 | 18 | 77 |
| 8 | (CF3)2CHOH | MeCN | –20 | 18 | 95 |
| 9 | (CF3)2CHOH | MeCN | –40 | 18 | 71 |
1 (1.5 equiv), 1 equiv of alkylidene malonate.
Isolated yield.
With these optimized bond-forming conditions in hand, the electrophilic scope of the transformation was explored (Table 2). Various alkylidene malonates were found to be reactive partners. A wide range of aromatic β-substituents is tolerated in good to excellent yields (entries 1-4). Aliphatic substituents are also accommodated with excellent selectivities and yields including two examples of R-branched substituents (entries 7 and 8). However, the more hindered tert-butyl substituted alkylidene malonate is unreactive under the reaction conditions (entry 9).
Table 2.
Addition of Silyloxyallene 1 to Alkylidene Malonates
 | ||||
|---|---|---|---|---|
| entry | alkylidene malonate | product | Z:Ea | yield (%)b |
| 1 |
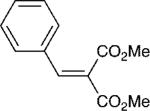
|
2 | 20:1 | 95 |
| 2 |
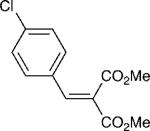
|
3 | 20:1 | 80 |
| 3 |
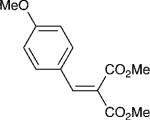
|
4 | 20:1 | 74 |
| 4 |
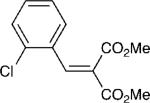
|
5 | 20:1 | 90 |
| 5 |
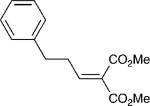
|
6 | 20:1 | 81 |
| 6 |
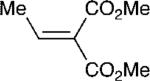
|
7 | 20:1 | 92 |
| 7 |
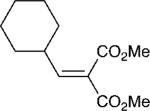
|
8 | 20:1 | 92 |
| 8 |
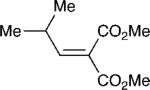
|
9 | 20:1 | 85 |
| 9 |

|
10 | NAc | 0 |
Determined by 500 MHz 1H NMR spectroscopy.
Isolated yield.
Not applicable.
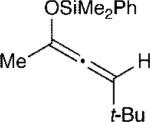
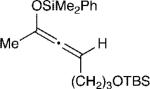
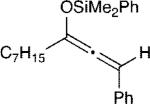
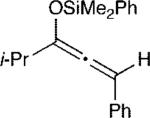
The effect of the silyloxyallene on the reaction was also investigated (Table 3). A broad range of substituents was incorporated at the β-position of the allene (R2) providing products in excellent yields and regioselectivities. A long chain alkyl group is tolerated as well as sterically encumbered trimethylsilyl and tert-butyl groups (entries 1-3). A silyloxyallene with a protected alcohol at the β-position was also demonstrated to be reactive (entry 4). In addition, various R1 substituents were explored with a heptyl and iso-propyl silyloxyallene undergoing addition in excellent yield and E/Z selectivity (entries 5 and 6).
Table 3.
Silyloxyallene Scope
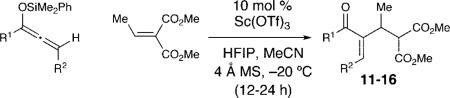 | ||||
|---|---|---|---|---|
| entry | silyloxyallene | product | Z:Ea | yield (%)b |
| 1 |
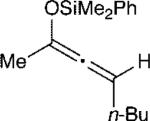
|
11 | 20:1 | 92 |
| 2 |
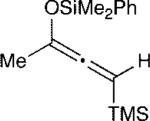
|
12 | 20:1 | 96 |
| 3 |
|
13 | 20:1 | 77 |
| 4 |
|
14 | 20:1 | 83 |
| 5 |
|
15 | 20:1 | 92 |
| 6 |
|
16 | 20:1 | 99c |
Determined by 500 MHz 1H NMR spectroscopy.
Isolated yield.
Reaction performed at 23 °C.
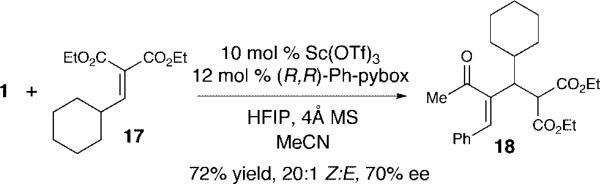
To render this highly selective reaction with alkylidene malonates enantioselective, our preliminary investigations have focused on the additions of racemic silyloxyallenes with chiral Lewis acids. After a survey of chiral ligands and reaction conditions,12 silyloxyallene 1 adds to alkylidene malonate 17 with 10 mol% Sc(OTf)3•(R,R)-Ph-Pybox in 72% yield and 70% ee (Scheme 2).13
Scheme 2.
Enantioselective Addition of Silyloxyallene 1
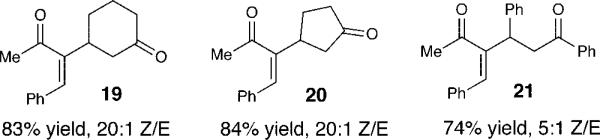
To widen the scope of this new process, we examined additional conjugate acceptors. Less reactive α,β-unsaturated ketones do not afford desired products with the scandium(III) conditions, but the combination of a stronger Lewis acid (1 equiv of TiCl4) and 1 in the presence of cyclohexenone, cyclopentenone or chalcone affords good yields of the desired Rauhut–Currier products (19, 20, 21, respectively) with excellent to good Z-alkene selectivity (Figure 1).
Figure 1.
Additions to α,β-Unsaturated Ketones.
In conclusion, silyloxyallenes undergo conjugate additions to alkylidene malonates in excellent yields and Z-alkene selectivity. The process accommodates wide substitution patterns for both silyloxyallenes and conjugate acceptors. Importantly, this new Lewis acid-catalyzed bond-forming reaction is the first general and selective solution to access compounds arriving from an intermolecular Rauhut–Currier reaction. These unique products cannot be generated cleanly or efficiently using traditional Rauhut–Currier conditions nor by using a more stepwise approach such as a Michael addition followed by aldol condensation. Employing a chiral scandium complex as the Lewis acid affords good levels of enantioselectivity and using titanium(IV) promotes the reaction with α,β-unsaturated ketones. Further studies of silyloxyallenes as R-acylvinyl anion equivalents and applications of the 1,5-dicarbonyl products are currently underway.
Supplementary Material
Acknowledgment
Financial support for this work has been provided by the NIGMS (GM73072) and the NSF (CAREER). K.A.S. thanks the Sloan Foundation, Abbott, Amgen, AstraZeneca, GlaxoSmithKline, and Boehringer-Ingelheim for unrestricted support and Wacker Chemical and FMCLithium for generous reagent support. T.E.R. is a recipient of a 2007-2008 ACS Division of Organic Chemistry fellowship sponsored by Bristol-Myers Squibb. M.S.B. thanks Northwestern for a Summer Research Fellowship. Funding for the NU Integrated Molecular Structure Education and Research Center (IMSERC) has been furnished in part by the NSF (CHE-9871268).
Footnotes
Supporting Information Available: Experimental procedures and spectral data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
OL800745Q
References
- 1.Chinchilla R, Najera C. Chem. Rev. 2000;100:1891–1928. doi: 10.1021/cr9900174. [DOI] [PubMed] [Google Scholar]
- 2.Rauhut MM, Currier H. U.S. Patent 307499919630122. American Cyanamid Co.; 1963. [Google Scholar]
- 3.a McClure JD. J. Org. Chem. 1970;35:3045–3048. [Google Scholar]; b Basavaiah D, Gowriswari VVL, Bharathi TK. Tetrahedron Lett. 1987;28:4591–4592. [Google Scholar]; c Amri H, Rambaud M, Villieras J. Tetrahedron Lett. 1989;30:7381–7382. [Google Scholar]; d Jenner G. Tetrahedron Lett. 2000;41:3091–3094. [Google Scholar]
- 4.a Hwu JR, Hakimelahi GH, Chou CT. Tetrahedron Lett. 1992;33:6469–6472. [Google Scholar]; b Evans CA, Miller SJ. J. Am. Chem. Soc. 2003;125:12394–12395. doi: 10.1021/ja0377798. [DOI] [PubMed] [Google Scholar]; c Evans CA, Cowen BJ, Miller SJ. Tetrahedron. 2005;61:6309–6314. [Google Scholar]; d Dadwal M, Mohan R, Panda D, Mobin SM, Namboothiri INN. Chem. Commun. 2006:338–340. doi: 10.1039/b512267h. [DOI] [PubMed] [Google Scholar]; e Yin YB, Zhang Q, Li J, Sun SG, Liu Q. Tetrahedron Lett. 2006;47:6071–6074. [Google Scholar]
- 5.a Wang LC, Luis AL, Agapiou K, Jang HY, Krische MJ. J. Am. Chem. Soc. 2002;124:2402–2403. doi: 10.1021/ja0121686. [DOI] [PubMed] [Google Scholar]; b Frank SA, Mergott DJ, Roush WR. J. Am. Chem. Soc. 2002;124:2404–2405. doi: 10.1021/ja017123j. [DOI] [PubMed] [Google Scholar]; c Brown PM, Kappel N, Murphy PJ. Tetrahedron Lett. 2002;43:8707–8710. [Google Scholar]; d Mergott DJ, Frank SA, Roush WR. Org. Lett. 2002;4:3157–3160. doi: 10.1021/ol026540d. [DOI] [PubMed] [Google Scholar]; e Agapiou K, Krische MJ. Org. Lett. 2003;5:1737–1740. doi: 10.1021/ol030035e. [DOI] [PubMed] [Google Scholar]; f Methot JL, Roush WR. Org. Lett. 2003;5:4223–4226. doi: 10.1021/ol0357550. [DOI] [PubMed] [Google Scholar]; g Couturier M, Menard F, Ragan JA, Riou M, Dauphin E, Andresen BM, Ghosh A, Dupont-Gaudet K, Girardin M. Org. Lett. 2004;6:1857–1860. doi: 10.1021/ol049392v. [DOI] [PubMed] [Google Scholar]; h Luis AL, Krische MJ. Synthesis. 2004:2579–2585. [Google Scholar]; i Mergott DJ, Frank SA, Roush WR. Proc. Nat. Acad. Sci. 2004;101:11955–11959. doi: 10.1073/pnas.0401247101. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Brown PM, Kappel N, Murphy PJ, Coles SJ, Hursthouse MB. Tetrahedron. 2007;63:1100–1106. [Google Scholar]; k Aroyan CE, Miller SJ. J. Am. Chem. Soc. 2007;129:256–257. doi: 10.1021/ja067139f. [DOI] [PubMed] [Google Scholar]
- 6.a Reynolds TE, Bharadwaj AR, Scheidt KA. J. Am. Chem. Soc. 2006;128:15382–15383. doi: 10.1021/ja0653674. [DOI] [PubMed] [Google Scholar]; b Reynolds TE, Stern CA, Scheidt KA. Org. Lett. 2007;9:2581–2584. doi: 10.1021/ol0710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For previous examples of silyloxyallenes as nucleophiles, see: Merault G, Bourgeoi P, Dunogues J, Duffaut N. J. Organomet. Chem. 1974;76:17–27. Fleming I, Perry DA. Tetrahedron. 1981;37:4027–4034. Kato M, Kuwajima I. Bull. Chem. Soc. Jpn. 1984;57:827–830. Reich HJ, Eisenhart EK, Olson RE, Kelly MJ. J. Am. Chem. Soc. 1986;108:7791–7800. doi: 10.1021/ja00284a051. Stergiades IA, Tius MA. J. Org. Chem. 1999;64:7547–7551. Li GG, Wei HX, Phelps BS, Purkiss DW, Kim SH. Org. Lett. 2001;3:823–826. doi: 10.1021/ol000377+. Yoshizawa K, Shioiri T. Tetrahedron. 2007;63:6259. Mueller AJ, Jennings MP. Org. Lett. 2007;9:5327–5329. doi: 10.1021/ol702546w.
- 8.a Kuwajima I, Kato M. Tetrahedron Lett. 1980;21:623–626. [Google Scholar]; b Reich HJ, Olson RE, Clark MC. J. Am. Chem. Soc. 1980;102:1423–1424. [Google Scholar]
- 9.Reynolds TE, Scheidt KA. Angew. Chem., Int. Ed. 2007;46:7806–7809. doi: 10.1002/anie.200702818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For selected additions to alkylidene malonates, see: Evans DA, Rovis T, Kozlowski MC, Downey CW, Tedrow JS. J. Am. Chem. Soc. 2000;122:9134–9142. Lassaletta JM, Vazquez J, Prieto A, Fernandez R, Raabe G, Enders D. J. Org. Chem. 2003;68:2698–2703. doi: 10.1021/jo026557+. Betancort JM, Sakthivel K, Thayumanavan R, Tanaka F, Barbas CF. Synthesis. 2004:1509–1521. Prieto A, Fernandez R, Lassaletta JM, Vazquez J, Alvarez E. Tetrahedron. 2005;61:4609–4613. Cao CL, Sun XL, Zhou JL, Tang Y. J. Org. Chem. 2007;72:4073–4076. doi: 10.1021/jo070070p.
- 11.Although diethyl ether gave the highest yield, the reaction was slower than with acetonitrile.
- 12.See Supporting Information.
- 13.Ph-pybox =(+)-2,6-bis[(4R)-4-phenyl-2-oxazolin-2-yl]pyridine. Cu(OTf)2 or Cu(SbF6)2 bis-oxazoline complexes as the Lewis acid afforded no desired products.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.