Abstract
Twenty-two consecutive liver allograft recipients, who tested positive for immunoglobulin G (IgG) lymphocytotoxicity were subjected to pretransplantion and posttransplantation immunologic monitoring of antidonor IgG lymphocytotoxic antibody titers, total hemolytic complement activity (CH100), circulating immune complexes (CIC), and platelet counts in an effort to improve our understanding of the preformed antibody state in clinical hepatic transplantation. Ten contemporaneous liver transplant recipients whose crossmatch results were negative and who experienced severe hepatocellular damage early after transplantation were included as controls. Crossmatch test results were negative 1 day after transplantation and during the 1 month follow-up remained negative in 14 of 22 (64%) sensitized recipients, most of whom had relatively low (≤ 1:16) antidonor IgG antibody titers before transplantation. After transplantation, this group and the control group experienced no thrombocytopenia, no increase of CIC, and a gradual increase in CH100 activity that reached normal levels within 1 week. A strong negative correlation between prothrombin time (PT) and CH100 activity in these groups of patients suggested that changes in CH100 activity (P < .0005) were tightly linked to liver synthetic function. In contrast, the crossmatch test results remained positive after transplantation in 8 of 22 (36%) sensitized recipients, all of whom had relatively high (>1:32 to 1024) pretransplantation titers of anti-donor IgG antibodies. After transplantation these patients developed a syndrome that was characterized by decreased CH100 activity and increased CIC compared with pretransplantation levels and refractory thrombocytopenia that was associated with a 50% allograft failure rate because of biopsy-proven humoral and acute (cellular) rejection. Moreover, the lack of a strong negative correlation between PT and CH100 activity (P = .1) in this group of patients suggested that the hypocomplementemia was not tightly linked to liver synthetic function. Before transplantation, determination of anti-donor antibody class (IgG) and titer alone showed a strong negative predictive value (100%) but less than optimal positive predictive value (67%) for identifying patients who experienced the posttransplantation syndrome described above. Therefore, evaluation of platelet counts, CH100 activity, CIC, persistence of anti-donor antibodies and results of a liver biopsy performed after transplantation assisted in identifying sensitized liver allograft recipients who suffered the adverse consequences of the preformed antibody state.
Although the resistance of liver allografts to humoral rejection is well known, we have recently reported a characteristic clinical 1,2 and pathological3 syndrome in sensitized primary liver allograft recipients. As a group, patients whose crossmatch results were testing positive for immunoglobulin G (IgG) lymphocytotoxicity are more likely to experience rejection and allograft failure. 1–3 yet it is difficult to predict these events before transplantation. Moreover, tangible evidence of type II/III hypersensitivity reactions have been difficult to obtain in either presensitized humans 1–7 or experimental animals.8–10
The mechanisms used to explain hepatic resistance to preformed antibody states have also been offered as reasoning for the difficulties in finding traces of humoral-related injury. Traditional explanations for this resistance are (1) release of soluble class I major histocompatibility complex antigens by the liver; (2) formation of immune complexes; (3) Kupffer cell phagocytosis of activated platelets and immune complexes; (4) the structurally and antigenically unique sinusoidal vasculature; and (5) the dual afferent hepatic blood supply. 11 More recently, the realization that complement-mediated lysis of a target cell is less efficient if the complement and the target cell have a common source is yet another possible explanation for the hepatic resistance.12 However, regardless of the defense mechanisms, experiments with animal 13,14 and clinical data 1–3, 15–18 now conclusively show that in some cases IgG lymphocytotoxic antibodies can override hepatic defenses and have a deleterious effect in liver transplantation, even if they do not precipitate “hyperacute” rejection.
The goal of this study was to determine if the functional consequences of presensitized states in clinical liver transplantation could be more precisely characterized by defining the level of sensitization before transplantation and looking for a syndrome marked by consumption of factors important in humoral rejection after transplantation. Therefore, we prospectively assayed donor-specific antibody subclass and titers, serum complement activity, platelet counts, and circulating immune complexes (CIC) in sensitized recipients before and after clinical liver transplantation. Because humoral rejection is dependent on complement activation, 19,20 and the liver is also the principal site of complement biosynthesis,21,22 the patients with positive crossmatch results were compared with a group of controls who had negative crossmatch results and experienced severe hepatocellular injury related to “preservation” injury.
PATIENTS AND METHODS
Patient Selection
Between March 1, 1991, and December 31, 1991, 22 of 277 (8%) adult patients (> 16 years of age) received a primary orthotopic liver allograft at the Pittsburgh Transplant Institute, University of Pittsburgh, from a donor whose crossmatch test results were positive. Selection of the contemporaneous control patients whose crossmatch test results were negative was based on the presence of severe “preservation” injury (aspartate transaminase [AST] > 2,500 U/mL on day 1 of posttransplantation.23 All donor livers were preserved with the University of Wisconsin solution.
Both groups were prospectively studied during the first month after transplantation for the presence of IgG antidonor lymphocytotoxic antibodies, the observation of total hemolytic complement activity (CH100), and the detection of circulating immune complexes. Blood samples for testing were drawn pretransplantation and 1-day posttransplantation on all patients. Thereafter, weekly samples (with a 2-day window) were obtained for 1 month, unless the patient died, experienced graft failure, or was discharged from the hospital. The results of the above tests were then correlated with patient and graft survival and the postoperative course.
Immunosuppression
The standard protocol consisted of FK506 (Fujisawa Pharmaceuticals, Japan) given via continuous intravenous infusion of 0.1 mg/kg/d and then converted to an oral dose of 0.15 mg/kg every 12 hours with the return of bowel function. Subsequent dosage adjustments were guided by the quality of graft function, rejection, toxicity, and FK506 plasma trough levels (usually < 2 ng/mL). All but three of the patients with positive crossmatch results received an intravenous operative dose of 1 g of methylprednisolone, followed by a 5-day taper from 200 mg to 20 mg (“recycling”). Humoral and acute rejection episodes were histologically confirmed3,11 and treated with either a 1-g bolus of methylprednisolone or a “recycling” of high-dose steroids. If rejection persisted, a 3- to 5-day course of 5 to 10 mg/d of OKT3 (Ortho Pharmaceuticals, Raritan, NJ) was administered.
Treatment With Prostaglandin E
Fourteen patients with positive crossmatch results and all the control patients with negative crossmatch results and hepatocellular damage received treatment with prostaglandin E1 (PGE1) (Prostin VR, UpJohn, Kalamazoo, MI) 0.2 to 0.6 µg/kg/h intravenously for 5 to 7 days after transplantation.
Crossmatch Test
Pretransplantation sera was drawn immediately before transplantation and tested for cytotoxic activity before and after treatment with dithiothreitol, which inactivates IgM antibodies.24 Donor T lymphocytes isolated from spleen or lymph nodes using CD3-conjugated dynabeads (Dynal, Inc., Great Neck, NY) were used as targets.
The cytotoxicity test was done according to National Institutes of Health standards with one wash: 1 µL of 2 × 106/mL T lymphocytes was placed into 1 µL of serum, followed by a 1-hour incubation at room temperature. The titer of antibodies present was determined by a 1:2 serial dilution of the sera with RPMI 1640. After one wash, addition of 5 µL of rabbit complement for 1 hour at room temperature produced lysis that was evaluated using trypan blue exclusion. Crossmatch test results were considered positive if more than 50% donor lymphocytes were killed after treatment of the serum with dithiothreitol.
Total Complement Activity Test
Measurement of total complement activity was based on the ability of complement to lyse sensitized red blood cells (Kallestad, Inc., Austin, TX). The test serum radially diffused from wells in an agarose gel that contained standardized sheep erythrocytes that were sensitized with hemolysin. The extent of lysis caused by the test serum sample compared with that caused by reference sera run simultaneously provided an estimate of total complement activity (CH100). The results were reported in units/mL (normal value > 60 µ/mL).
Detection of Circulating Immune Complexes
CIC were qualitatively detected using zone electrophoresis on agarose gels as reported by Kelly et al.25 In principle, an antibody-antigen immune complex has a net surface charge different from the isolated constituents. This property, together with the clonal restriction of the antibody response, causes distinctive patterns that are apparent in stained agarose gels after routine zone electrophoresis.
Statistical Analysis
The Wilcoxon test for two independent samples was used to compare the characteristics of both crossmatch groups before transplantation and the CH100 activity before and after transplantation. The Fisher's exact test was used to compare the incidence of CIC before and after transplantation. One-way ANOVA was used to compare AST, total bilirubin, and prothrombin time (PT) during the first 4 weeks after transplantation. The possibility of a relationship, if any, between CH100 and PT was determined by linear regression analysis.
RESULTS
Patient Immunologic Profiles Before Transplantation
Table 1 shows the pretransplantation profile of the 22 patients with positive crossmatch results and the 10 controls with negative crossmatch results. As expected, panel-reactive antibodies in sera pretreated with dithiothreitol were higher in the patients with positive crossmatch results: 80.0 ± 29.3% versus 3.5 ± 4.7% (P < .001). Consistent with the method of patient selection, mean serum AST 1 day after transplantation was 1,434 ± 1,040 U/mL in the group with positive crossmatch results and 6,094 ± 3,700 U/mL in the controls (P < .001). Pretransplantation anti-donor IgG lymphocytotoxic Ab titers available in 20 of 22 patients whose crossmatch results were positive showed levels greater than 1:32 in 12 patients (60%), 1:16 in 1 patient (5%), greater than 1:8 in 2 patients (10%), 1:2 in 3 patients (15%), and 1: 1 in 2 patients (10%). There were no statistically significant differences between the two groups in total CH100 activity, circulating immune complexes, age, sex, cold ischemic time, or nature of the original disease. More females had positive crossmatch results. as expected.
TABLE 1.
IgG Lymphocytotoxic Crossmatch Positive Cases and Crossmatch Negative Controls With Hepatocellular Damage
| Positive Crossmatch Results |
Negative Crossmatch Results |
P | |
|---|---|---|---|
| No. of patients | 22 | 10 | NS |
| Age* | 47 ± 13 | 54 ± 14 | NS |
| Male/female | 7/15 | 6/4 | NS |
| Cold ischemic time (hr) | 12.0 ± 4.6 | 13.3 ± 3.9 | NS |
| PRA %* | 80 ± 29 | 4 ± 2 | <.001 |
| AST on day 1* | 1.434 ± 1.040 | 6.094 ± 3.700 | <.001 |
| Original disease | |||
| Hepatocellular | 15 | 7 | NS |
| Cholestatic | 7 | 3 | NS |
Abbreviations: NS, not significant; PRA, Panel reactive antibodies.
Mean ± SD.
Posttransplantation Crossmatch Testing and Correlation With Pretransplantation Antibody Titers
Analysis of the posttransplantation crossmatch test results separated the sensitized recipients into two groups. Repeat crossmatch testing results were negative 1 day after transplantation and remained negative in 14 of 22 (64%) patients whose crossmatch result was positive before engraftment. In the remaining 8 (36%) patients with positive crossmatch results, donor-specific IgG lymphocytotoxic antibodies persisted for 4 weeks in 5 patients and for 3 weeks in 2 patients after transplantation. One patient required retransplantation on day 2.
The pretransplantation antibody titer was greater than 1:32 in all 8 patients with persistently positive crossmatch results after transplantation. However, the crossmatch test results were negative 1 day after transplantation and remained negative in 4 other patients with pretransplantation titers greater than 1:32 and in all of the patients with pretransplantation titers of less than 1:16. A pretransplantation titer less than or equal to 1:16 had a 100% negative-predictive value and 67% positive-predictive value for persistently positive cross match results after transplantation. The pretransplantation PRA did not show a statistically significant difference between patients with persistently positive crossmatch results and patients whose crossmatch results became negative: 75.1% ± 34.1% (range 10% to 100%) versus 77.3% ± 23.0% (range 40% to 100%), respectively. Five of 8 patients (63%) with persistent positive crossmatch results and 9 of 14 patients (64%) whose crossmatch results became negative after transplantation underwent treatment with PGE1.
Total Complement Activity and Relationship to Liver Synthetic Function and Liver Injury Tests After Transplantation
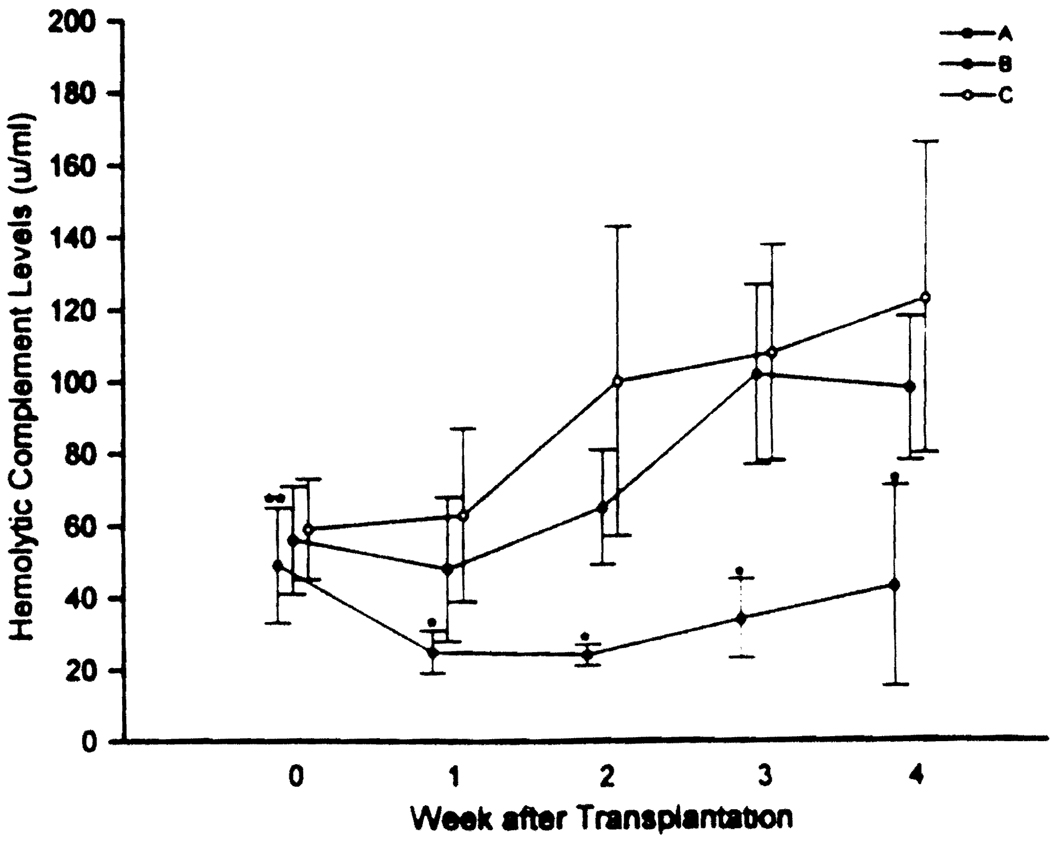
After transplantation, patients with persistently positive crossmatch results showed a significant decrease in CH100 activity during the first 2 weeks after transplantation in comparison with pretransplantation levels (Fig. 1). These patients also showed significantly less CH100 activity during the first 4 weeks in comparison to patients whose crossmatch results were negative before transplantation and patients whose crossmatch results became negative after transplantation (Fig. 1). No differences were found at any time between the latter two groups, although there was a trend toward less complement activity in patients whose crossmatch results changed from positive to negative.
FIG. 1.
Relationship between total CH100 and time after transplantation in patients whose crossmatch results remained positive after transplantation (group A; dotted circle), patients whose crossmatch results became negative after transplantation (group B; closed circles), and patients with negative crossmatch results (group C; open circles).
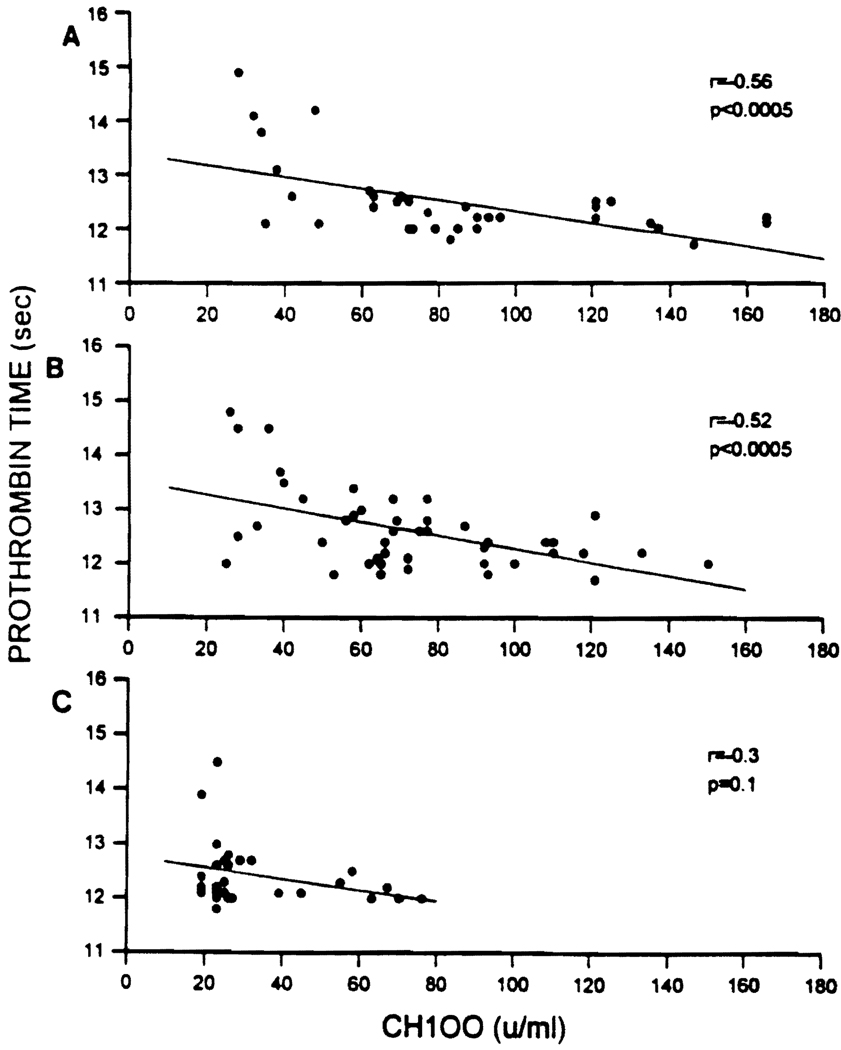
The relationship of CH100 activity to liver synthetic function was quantitatively assessed by a linear regression analysis between CH100 levels and PT. CH100 showed a significant negative correlation with the PT in patients with negative crossmatch results (r − .56; P < .0005) (Fig. 2A) and in patients whose crossmatch results became negative after transplantation (r − .52; P < .0005) (Fig. 2B). In contrast, a definite negative correlation was not found in patients with persistently positive crossmatch results (r − .3; P = .1) (Fig. 2C).
FIG. 2.
Relationship between CH100 and PT in liver transplant recipients with negative crossmatch results and severe hepatocellular damage (A), liver transplant recipients with positive IgG lymphocytotoxic crossmatch results pretransplantation that became negative posttransplantation (B), and liver transplant recipients with positive IgG lymphocytotoxic crossmatch results pretransplantation and posttransplantation (C).
Table 2 shows the mean AST, total bilirubin, and PT during the first 4 weeks after transplantation. Controls whose crossmatch results were negative and with preservation injury and negative crossmatch results experienced higher AST and total bilirubin values as well as lower PT compared with both groups of patients with positive crossmatch results.
TABLE 2.
AST, Total Bilirubin, and PT During the First Month After Transplantation
| Week 1 | Week 2 | Week 3 | Week 4 | |
|---|---|---|---|---|
| AST (U/L) | ||||
| A | 1.059 ± 956* | 73 ± 61 | 49 ± 27 | 56 ± 41* |
| B | 373 ± 298 | 48 ± 40 | 35 ± 17 | 38 ± 21 |
| C | 2.134 ± 1.825† | 114 ± 103† | 70 ± 69† | 58 ± 42* |
| Total bilirubin (mg/dL) | ||||
| A | 7.7 ± 6.9 | 9.3 ± 8.8* | 6.7 ± 5.5* | 4.4 ± 3.5 |
| B | 6.2 ± 3.7 | 4.4 ± 3.8 | 3.6 ± 3.3 | 3.3 ± 2.5 |
| C | 12.2 ± 8.1† | 10.5 ± 8.5* | 7.6 ± 6.7* | 6.3 ± 5.8* |
| PT(s) | ||||
| A | 14.6 ± 2.5 | 12.9 ± 0.6* | 12.7 ± 0.8 | 12.6 ± 0.6 |
| B | 13.8 ± 1.6 | 12.5 ± 0.7 | 12.7 ± 0.6 | 12.7 ± 0.7 |
| C | 16.6 ± 3.7† | 13.1 ± 1.1* | 12.8 ± 0.8 | 12.9 ± 0.9 |
NOTE. Data represent Mean ± SD. Patients are grouped as follows: A, patients with positive IgG lymphocytotoxic crossmatch results pretransplantation and posttransplantion; B, patients with pretransplantation positive crossmatch results that became negative posttransplantation; C, controls with negative crossmatch results.
P < .05 with group B.
P < .05 with group A and B.
Immune Complex Detection
Although no difference between the three groups of patients in the incidence of CICs was detected before transplantation (22% to 33%), after transplantation all (100%) of the patients with persistently positive crossmatch results developed CIC (P < .02 compared with pretransplant) that persisted for 3 weeks. In contrast, CICs were detected on weeks 1, 2, and 3 in only 50%. 33%, and 30% of the patients whose crossmatch results became negative and in 20%, 20%, and 33% of the controls with negative crossmatch results (P < .05 compared with patients with persistently positive crossmatch results).
Blood Product Usage and Platelet Counts
There were no statistically significant differences among the three groups in the intraoperative or postoperative blood product requirements, except that the patients with positive crossmatch results needed more platelets (data not shown) during transplantation and for the first 4 weeks (data not shown) after transplantation (P < .001). Despite increased platelet transfusions, the mean platelet counts in patients with persistently positive crossmatch results were still significantly less (data not shown) during the first 4 weeks after transplantation than patients with negative crossmatch results and those whose crossmatch results became negative after transplantation (P < .001).
Clinicopathological Course After Transplantation
As shown in Table 3, at least seven of the eight (88%) patients with persistently positive crossmatch results had biopsy results that confirmed rejection, which resulted in allograft failure requiring retransplantation in four patients (50%). Six of these eight patients had biopsy changes that resembled preservation injury and/or large duct stricturing that previously had been attributed to humoral rejection.3 No biopsy specimens from the early posttransplantation course were available in the remaining patient, although the failed allograft removed on day 31 showed evidence of both humoral and acute rejection. There was one graft failure from pure humoral rejection on day 3, and three others failed from severe acute and humoral rejection on days 10, 35, and 39. The patient whose allograft failed because of pure humoral rejection had a pretransplantation crossmatch titer 1:1,024. The other three patients who experienced allograft failure did not receive posttransplantation steroid “recycling” or treatment with PGE1 because of early infectious complications.
TABLE 3.
Pretransplantation and Posttransplantation Crossmatch Results and Clinicopathological Follow-up of Patients With Positive Crossmatch Results
| Patient | Pretransplant Crossmatch (titer) |
Posttransplant Crossmatch (titer) |
PGE1 | Histological Evidence of Rejection (d) |
Graft Survival (d) | Clinicopathological Follow-up (d) |
|---|---|---|---|---|---|---|
| 1 | Pos (> 1:32) | Pos (> 1:32) | Yes | Yes (16) | Died (350) | Duct strictures (116), metastatic hepatoma (350) |
| 2 | Pos (> 1:32) | Pos (1:32) | Yes | Yes (10) | Functioning (621) | Segmental duct necrosis and biliary sludge (8) |
| 3 | Pos (1:128) | Pos (> 1:32) | Yes | Yes (4) | Functioning (595) | Stricturelike changes (portal edema with cholangiolar proliferation and acute pericholangitis) and relapsing acute and chronic rejection (355) |
| 4 | Pos (> 1:32) | Pos (> 1:32) | No | Yes (7) | Failed (35) | Humoral and acute rejection (35) |
| Died (52) | Fungal sepsis (52) | |||||
| 5 | Pos (> 1:32) | Pos (1:32) | Yes | Yes (12) | Functioning (566) | Relapsing acute rejection with stricturelike changes (41) and central vein sclerosis (56) |
| 6 | Pos (1:64) | Pos (1:1) | No | NA | Failed (31) | Humoral and acute rejection with duct strictures (31) |
| Died (400) | Hepatitis B virus infection with liver failure | |||||
| 7 | Pos (1:1024) | Pos (> 1:32) | Yes* | Yes | Failed (3) | Humoral rejection (3) |
| Functioning (452) | ||||||
| 8 | Pos (> 1:32) | Pos (> 1:32) | No | Yes (7) | Failed (10) | Humoral and acute rejection (10) |
| Died (36) | Fungal sepsis | |||||
| 9 | Pos (1:2) | Neg | No | Yes (12) | Functioning (659) | Nonspecific changes (174) |
| 10 | Pos (> 1:8) | Neg | Yes | Yes (26) | Functioning (637) | Viral hepatitis (57) |
| 11 | Pos (> 1:8) | Neg | No | None | Functioning (609) | Nonspecific changes on biopsy (54) |
| 12 | Pos (1:64) | Neg | Yes | Yes (97) | Died (113) | Cytomegalovirus hepatitis (46); respiratory arrest (113) |
| 13 | Pos (1:1) | Neg | No | None | Functioning (559) | Nonspecific changes on biopsy (153) |
| 14 | Pos (NA) | Neg | Yes | Yes (11) | Functioning (546) | NA |
| 15 | Pos (1:1) | Neg | No | Yes (6) | Functioning (533) | Stricturelike changes on biopsy (50) |
| 16 | Pos (1:16) | Neg | Yes | Yes (5) | Died (44) | Bacterial sepsis |
| 17 | Pos (1:512) | Neg | Yes | Yes (11) | Functioning (504) | NA |
| 18 | Pos (1:2) | Neg | No | Yes (9) | Died (45) | Stricturelike changes (26); biliary leak and reconstruction (39); bacterial sepsis (45) |
| 19 | Pos (NA) | Neg | Yes | Yes (13) | Functioning (498) | Chronic rejection (440) after withdrawal of immunosuppression (Epstein-Barr virus infection) |
| 20 | Pos (1:256) | Neg | Yes | Yes (14) | Functioning (458) | Persistent acute rejection (4–22) with infarcts and inflammatory arteritis |
| 21 | Pos (1:1024) | Neg | Yes | Yes (13) | Functioning (422) | Developed septal duct necrosis (2); early chronic rejection (99) |
| 22 | Pos (1:2) | Neg | Yes | None | Functioning (420) | NA |
Abbreviations: NA, not available; Pos, positive; Neg, negative.
Patient received PGE1 with allograft no. 1, but not until day 2 when the liver was already severely damaged and failing.
Ten of the thirteen (77%) patients whose crossmatch results became negative after transplantation and who were subjected to biopsy in less than 30 days had acute rejection. Although there were no graft failures in this group, 5 of 13 (38%) patients developed a particularly severe form of rejection, including 2 patients who had relatively high pretransplantation antibody titers (Table 3), In the crossmatch negative controls, histologically documented severe “preservation” injury developed in 7 of 10 (70%) patients, whereas 2 patients showed mild preservation injury and no biopsy specimens were available in the remaining patient. One of the grafts failed from primary dysfunction on day 17. Histologically proven rejection was seen in 5 of 8 (63%) patients.
DISCUSSION
Characterization of the immunoglobulin class (IgG) and titer of lymphocytotoxic anti-donor antibodies before transplantation, and monitoring of platelet counts, CH100 activity, CICs and evaluation of a liver biopsy specimen after transplantation provided a more accurate assessment of the significance of allosensitization in clinical liver allografting. Using these monitoring tests, the liver allograft recipients whose crossmatch results were positive were roughly separated into two subpopulations. Preformed IgG lymphocytotoxic antibodies disappeared within 1 day after liver transplantation in the first group that consisted of a majority (14 of 22 [64%]) of the sensitized patients. They did not develop thrombocytopenia after transplantation and showed no significant increase of CIC or decrease in CH100 activity compared with pretransplantation levels. These results confirm that liver allografting can reverse alloimmunity as was previously shown in experimental animals 8–10,26 and in combined liver-kidney transplantation in humans.27, 28
In contrast, donor-reactive IgG antibodies persisted for several weeks after transplantation in 8 of 22 (36%) sensitized recipients. After transplantation these patients also developed refractory thrombocytopenia and showed a decrease of CH100 activity compared with pretransplantation levels and a significant increase of CIC. The concomitantly high incidence of allograft failure in this subgroup because of biopsy results that showed humoral and acute (cellular) rejection strongly suggests that the alloantibodies contributed to the injury via type II hypersensitivity reactions, which also consumed platelets and complement.
Alternate explanations for the low posttransplantation complement levels in patients with persistently positive crossmatch results are poor liver synthetic function or sepsis. We think these explanations are unlikely for the following reasons. First, there was a strong negative correlation between CH100 and PT in patients whose crossmatch results were negative before transplantation and in those whose crossmatch results became negative after transplantation. In contrast, no strong correlation between these two different measures of liver synthetic function was seen in the patients with persistently positive crossmatch results. This suggests that CH100 activity was tightly linked to liver synthetic function in the former two groups but not in the latter one. Because the fresh frozen plasma and packed red blood cell requirements were the same in all three groups, blood component replacement therapy cannot explain the differences in complement levels. Secondly, the CH100 levels decreased after transplantation only in the patients with persistent antibodies, despite comparable pretransplantation levels and no differences in cold ischemic times. The validity of the controls with negative crossmatch results as damaged organs was confirmed functionally (e.g., higher bilirubin values and an increase in PT and in AST; Table 2) and histologically and supported by one graft failure from primary dysfunction and the development of the biliary sludge syndrome in two patients included in this group.29 Lastly, the incidence of sepsis was the same in all three groups.
A more likely explanation for the low CH100 activity is immune consumption caused by activation by antidonor antibodies bound in the graft and by CIC.8–11,30–32 Complement fixation to antibody directly bound to the allograft is a well-recognized mechanism of allograft damage and complement consumption. However, immune complexes also bind complement that target the immune complexes for clearance. In sensitized liver allograft recipients, it is likely that increased CIC formed by anti-donor antibodies and soluble HLA antigens 8–11,30–32 shed by the allograft leads to complement binding and clearance. If decreased clearance were responsible for elevated CIC, one would expect that the same increase of CIC would occur in the controls with negative crossmatch results.
Consumption within the allograft and increased destruction and clearance because of antibody binding are also the most likely explanations for the refractory thrombocytopenia.3,33 It is well known that platelets express HLA class (I) antigens, and specific alloimmunization with these antigens is a major cause of thrombocytopenia refractoriness.34,35 Recently, crossmatching with platelet targets has been used to avoid primary nonfunction in renal transplantation and to minimize false-positive reactions of the lymphocytotoxic crossmatch.36 In addition, several previous publications have shown increased platelet sequestration in liver allografts of sensitized recipients.3,33
In our study, the pretransplantation antibody titer analysis alone had a good negative-predictive value (100%) but a less than optimal positive-predictive value (67%) for determining the persistence of anti-donor antibodies and humoral rejection after transplantation. This suggests that the anti-donor antibody level is certainly important, but factors other than the titer can influence the subsequent functional consequences and determine whether the antibodies persist or disappear after transplantation. Similar observations were made in experimental animal models.13,33,37 where liver allograft failure in presensitized rodent recipients was dependent not only on antibody titer37 but also on antibody class (IgG) and specificity.37 We have previously shown the importance of antibody subclass (IgG) in clinical liver transplantation.1–3 Moreover, combined humoral and acute (cellular) rejection mediated liver allograft failure in the sensitized animal model,33,37 and this form of rejection is more responsive to increased immunosuppression. In other clinical studies of sensitized liver allograft recipients, Karuppan et al 16 and Ogura et al.38 have reported a higher incidence of early posttransplantation allograft dysfunction and failure in patients with positive crossmatch results. The Mayo Clinic group reports no early complications,39 but they do note a higher incidence of “chronic” rejection in sensitized recipients.40 Lobo et al,41 on the other hand, found no adverse consequences of sensitization in liver allograft recipients, even those with high titer (> 1:64) IgG lymphocytotoxic anti-HLA antibodies. It is likely that a combination of factors account for the discrepancies. These factors include a more precise characterization of the posttransplantation crossmatch state described herein, recognition of an antibody mediated insult, and local differences in immunosuppressive therapy, such as the use of “induction” therapy with antileukocyte antibodies and/or high-dose steroids. All of the treatments mentioned can lessen the injury associated with humoral rejection. 1–3,42
In our study, only one patient whose crossmatch results were persistently positive after liver transplantation and whose graft failed because of humoral rejection received high-dose steroids and PGE1 therapy, but she was not treated until day 2 (Table 3), when the liver was already severely damaged. Since recognizing the increased immunologic risk,1–3 we routinely include these agents in all patients with positive crossmatch results.42 In addition to inherent immunosuppressive43 and cytoprotective44 qualities. PGE1 treatment could ameliorate the intense vasoconstriction that occurs during antibody-mediated rejection.3,45, including xenograft models like hamster-to-rat46 and pig-to-dog.47
In summary, patients with anti-donor IgG lymphocytotoxic antibodies in titers greater than 1:32 are more likely to show persistently positive crossmatch results after liver transplantation and develop a syndrome manifest by low-CH100 activity, increased CIC, and refractory thrombocytopenia. Evaluation of a liver biopsy specimen in this group of patients will help identify humoral and acute (cellular) rejection as a cause of the higher incidence of allograft failure. Identification of “at risk” sensitized patients can be used to guide immunosuppressive therapy. However, one must remember that these patients are a relatively small percentage of the total recipient population (3% in this series). In contrast, low-level anti-donor IgG antibodies usually do not significantly influence the posttransplantation course, even though they may cause a positive result in lymphocytotoxic crossmatch tests.
Acknowledgments
Supported by Research Grant DK 29961 from the National Institutes of Health, Bethesda, MD.
Abbreviations
- IgG
immunoglobulin G
- CIC
circulating immune complexes
- AST
aspartate transaminase
- CH100
hemolytic complement activity
- PGE1
prostaglandin E1
- PT
prothrombin time
REFERENCES
- 1.Takaya S, Duquesnoy R, Iwaki Y, Demetris AJ, Yagihashi A, Bronsther O, Iwatsuki Y, et al. Positive crossmatch in primary human liver allografts under cyclosporine or FK506 therapy. Transplant Proc. 1991;23:396–399. [PMC free article] [PubMed] [Google Scholar]
- 2.Takaya S, Bronsther O, Iwaki Y, Nakamura K, Abu-Elmagd K, Yagihashi A, Demetris AJ, et al. The adverse impact on liver transplantation of using positive cytotoxic crossmatch donors. Transplantation. 1992;53:400–406. doi: 10.1097/00007890-199202010-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demetris AJ, Nakamura K, Yagihashi A, Iwaki Y, Takaya S, Hartman G, Murase N, et al. A clinicopathologic study of human liver allograft recipients harboring preformed IgG lymphocytotoxic antibodies. Hepatology. 1992;16:671–681. doi: 10.1002/hep.1840160310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Ishikawa M, Putnam CW, Porter KA, Picache R, Husberg BS, Halgrimson CG, et al. Progress in and deterrents to orthotopic liver transplantation, With special reference to survival, resistance to hyperacute rejection and biliary duct reconstruction. Transplant Proc. 1974;6 suppl:129–139. [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Koep LT, Halgrimson CG, Hooj J, Schroter GPJ, Porter KA, Weil R. Fifteen years of clinical liver transplantation. Gastroenterology. 1979;77:375–388. [PMC free article] [PubMed] [Google Scholar]
- 6.Iwatsuki S, Rabin BS, Shaw BW, Jr, Starzl TE. Liver transplantation against T cell-positive warm crossmatches. Transplant Proc. 1984;16:1427–1429. [PMC free article] [PubMed] [Google Scholar]
- 7.Andres GA, Anzell ID, Halgrimson CG, Hsu KC, Porter KA, Starzl TE, Accini L, et al. Immunopathological studies of orthotopic liver allografts. Lancet. 1972;1:275–281. doi: 10.1016/s0140-6736(72)90288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houssin D, Blanche B, Brunaud MD, Gugenheim J, Settaf A, Meriggi F, Emond J. Interactions between liver allografts and lymphocytotoxic alloantibodies in inbred rats. Hepatology. 1986;6:994–998. doi: 10.1002/hep.1840060531. [DOI] [PubMed] [Google Scholar]
- 9.Gugenheim J, Le Thai B, Rouger P, Gigou M, Gane P, Vial MC, Charpentier B, et al. Relationship between the liver and lymphocytotoxic alloantibodies inbred rats. Transplantation. 1988;45:474–478. doi: 10.1097/00007890-198802000-00046. [DOI] [PubMed] [Google Scholar]
- 10.Orosz CG, Zinn NE, Sirinek LP, Ferguson RM. Delayed rejection of heart allografts in hypersensitized rats by extracorporeal donor specific liver hemoperfusion. Transplantation. 1986;41:398–404. doi: 10.1097/00007890-198603000-00025. [DOI] [PubMed] [Google Scholar]
- 11.Demetris AJ, Murase N, Nakamura K, Iwaki Y, Yagihashi A, Valdivia L, Todo S, et al. Immunopathology of antibodies as effectors of orthotopic liver allograft rejection. Semin Liver Dis. 1992;12:51–59. doi: 10.1055/s-2007-1007376. [DOI] [PubMed] [Google Scholar]
- 12.Starzl TE, Valdivia LA, Murase N, Demetris AJ, Fontes P, Rao AS, Manez R, et al. The biologic basis of and strategies for clinical xenotransplantation. Immunol Rev. 1994;141:213–244. doi: 10.1111/j.1600-065x.1994.tb00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knechtle S, Kolbeck PC, Tsuchimoto S, Coundouriotis A, Sanfilippo F, Bollinger RR. Hepatic transplantation into sensitized recipients. Transplantation. 1992;43:8–12. doi: 10.1097/00007890-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Gubernatis G, Lauchart W, Jonker M, Steinhoff G, Bornscheuer A, Nehaus P, van Es AA, et al. Signs of hyperacute rejection in liver grafts in rhesus monkeys after donor-specific presensitization. Transplant Proc. 1987;19:1082–1093. [PubMed] [Google Scholar]
- 15.Starzl TE, Demetris AJ, Todo S, Kang Y, Tzakis A, Duquesnoy R, Makowka L, et al. Evidence of hyperacute rejection of human liver grafts. The case of the canary kidneys. Clin Transplant. 1989;3:37–45. [PMC free article] [PubMed] [Google Scholar]
- 16.Karuppan S, Ericzon BG, Moller E. Relevance of a positive crossmatch in liver transplantation. Transpl Int. 1991;4:18–25. doi: 10.1007/BF00335511. [DOI] [PubMed] [Google Scholar]
- 17.Hanto DW, Snover DC, Sibley RK, Noreen HJ, Gajl-Pezalska KF, Najarian JS, Ascher NL. Hyperacute rejection of a human orthotopic liver allograft in a presensitized recipient. Clin Transplant. 1987;1:304–310. [Google Scholar]
- 18.Bird G, Friend P, Donaldson P, O'Grady J, Portmann B, Calne R, Williams R. Hyperacute rejection in liver transplantation: a case report. Transplant Proc. 1989;21:3742–3744. [PubMed] [Google Scholar]
- 19.Pruitt SK, Bollinger RR. The effect of soluble complement receptor type 1 on hyperacute allograft rejection. J Surg Res. 1991;50:350–355. doi: 10.1016/0022-4804(91)90202-w. [DOI] [PubMed] [Google Scholar]
- 20.Knetchtle SJ, Halperin EC, Murphy CE, Saad T, Abernethy K, Miller D, Bollinger RR. The effect of cyclosporine, total lymphoid irradiation, and cobra venom factor on hyperacute rejection. J Heart Transplant. 1985;4:541–545. [PubMed] [Google Scholar]
- 21.Alper CA, Raum D, Awdeh Z, Petersen BH, Taylor PD, Starzl TE. Studies of hepatic synthesis in vivo of plasma proteins including oromucosoid, transferrin, al-antitrypsin, C8 and factor B. Clin Immunol Immunopathol. 1980;16:84–89. doi: 10.1016/0090-1229(80)90169-5. [DOI] [PubMed] [Google Scholar]
- 22.Morris KM, Aden DP, Knowles BB, Colten HR. Complement biosynthesis by the human hepatoma-derived cell line, Hep-G2. J clin Invest. 1982;70:906–913. doi: 10.1172/JCI110687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mor E, Klintmalm GB, Gonwa TA, Solomon H, Holman MJ, Gibbs JF, Watemberg I, et al. The use of marginal donors for liver transplantation. A retrospective study of 365 liver donors. Transplantation. 1992;53:383–386. doi: 10.1097/00007890-199202010-00022. [DOI] [PubMed] [Google Scholar]
- 24.Iwaki Y, Lau M, Terasaki PI. Successful transplants across T warm positive crossmatches due to IgM antibodies. Clin Transplant. 1988;2:81–94. [Google Scholar]
- 25.Kelly RH, Scholl MA, Harvey S, Devenyi AG. Qualitative testing for circulating Immune complexes by use of zone electrophoresis on agarose. Clin Chem. 1980;26:396–402. [PubMed] [Google Scholar]
- 26.Kamada N, Davies HS, Roser BJ. Reversal of transplantation immunity by liver grafting. Nature. 1981;292:840–842. doi: 10.1038/292840a0. [DOI] [PubMed] [Google Scholar]
- 27.Fung JJ, Griffin M, Duquesnoy R, Shaw BW, Starzl TE. Successful sequential liver kidney transplantation in patient with preformed lymphocytotoxic antibodies. Transplant Proc. 1987;19:767–768. [PMC free article] [PubMed] [Google Scholar]
- 28.Flye MN, Duffy BF, Phelan DL, Ratner LE, Mohanakumar T. Protective effects of liver transplantation on a simultaneously transplanted kidney in a highly sensitized patient. Transplantation. 1990;50:1051–1054. [PubMed] [Google Scholar]
- 29.Sanchez-Urdazpal L, Gores GJ, Ward EM, Maus TP, Wahlstrom HE, Moore SB, Wiesner RH, et al. Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology. 1992;16:49–53. doi: 10.1002/hep.1840160110. [DOI] [PubMed] [Google Scholar]
- 30.Calne R, Sells R, Perna J. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;233:472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 31.Kamada N, Brons G, Davies HS. Fully allogeneic liver grafting in rats induces a state of systemic non reactivity to donor transplantation antigens. Transplantation. 1980;29:429–435. doi: 10.1097/00007890-198005000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Ruddy S. In: Manual of Clinical Laboratory Immunology. Rose NR, Demacario EC, Fahey JL, Friedman H, Penn GM, editors. Washington. DC: American Society of Microbiology; 1992. pp. 114–123. [Google Scholar]
- 33.Nakamura K, Murase N, Becich MJ, Furuya T, Todo S, Fung JJ, Starzl TE, et al. Liver allograft rejection in sensitized recipients: observations in a clinically relevant small animal model. Am J Pathol. 142(5):1383–1391. [PMC free article] [PubMed] [Google Scholar]
- 34.Manno IR, Weber T, Kang YG, Esquivel C, Starzl TE, Duquesnoy R. HLA allioimmunization and blood requirements in orthotopic liver transplantation. Transplant Proc. 1989;21:789–79l. [PMC free article] [PubMed] [Google Scholar]
- 35.Dutcher JP, Schiffer CA, Aisner J, Wiernik PH. Alloimmunization following platelet transfusion: the absence of a dose-response relationship. Blood. 1981;57:395–398. [PubMed] [Google Scholar]
- 36.Wang GX, Terashita GY, Terasaki PI. Platelet crossmatching for kidney transplantation by flow cytometry. Transplantation. 1989:959–961. doi: 10.1097/00007890-198912000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Furuya T, Murase N, Nakamura K, Woo J, Todo S, Demetris AJ, Starzl TE. Preformed lymphocytotoxic antibodies: the effect of class, titer and specificity on liver Vs, heart allografts. Hepatology. 1992;16:1415–1422. doi: 10.1002/hep.1840160618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogura K, Koyama H, Takemoto S, Terasaki PI, Busuttil RW. Significance of a positive crossmatch on outcome in human liver transplantation. Transplant Proc. 1992;24:1465. [PubMed] [Google Scholar]
- 39.Moore SB, Wiesner RH, Perkins JD, Nagorney OM, Sterioff S, Krom RAF. A positive lymphocyte cross-match and major histo-compatibility complex mismatching do not predict early rejection of liver transplants in patients treated with cyclosporine. Trans plant Proc. 1987;19:2390–2391. [PubMed] [Google Scholar]
- 40.Batts KP, Moore SB, Perkins JD, Wiesner RH, Grambsch PM, Krom RA. Influence of positive lymphocyte crossmatch and HLA mismatching on vanishing bile duct syndrome in human liver allografts. Transplantation. 1988;45:376–379. doi: 10.1097/00007890-198802000-00026. [DOI] [PubMed] [Google Scholar]
- 41.Lobo I, Spencer C, Douglas MT, Stevenson WC, Pruett TL. The lack of long-term detrimental effects on liver allografts caused by donor-specific anti-HLA antibodies. Transplantation. 1993;55:1063–1066. doi: 10.1097/00007890-199305000-00023. [DOI] [PubMed] [Google Scholar]
- 42.Takaya S, Iwaki Y, Starzl TE. Liver transplantation in positive cytotoxic crossmatch cases using FK506, high-dose steroids, and prostaglandin E1. Transplantation. 1992;54:927–929. doi: 10.1097/00007890-199211000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strom TB, Carpenter CB. Prostaglandin as an effective antirejection therapy in rat renal allograft recipients. Transplantation. 1983;35:279–281. doi: 10.1097/00007890-198304000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Greig PD, Woolf GM, Sinclair SB, Abecassis M, Strasberg SM, Taylor BR, Blendis LM, et al. Treatment of primary liver graft nonfunction with prostaglandin E1. Transplantation. 1989;48:447–453. doi: 10.1097/00007890-198909000-00020. [DOI] [PubMed] [Google Scholar]
- 45.Terada Y, Ueno A. Hyperacute renal allograft rejection in the rabbit: vasoconstriction demonstrated by microangiography. Transplantation. 1983;35:205–208. doi: 10.1097/00007890-198303000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Shaw JRL. Role of prostaglandins in transplantation. In: Cohen MM, editor. Biological protection with prostaglandins. Vol 1. Boca Raton, FL: CRC; 1985. pp. 111–128. [Google Scholar]
- 47.Makowka L, Miller C, Chapchap P, Podesta L, Pan C, Pressley D, Mazzaferro V, et al. Prolongation of pig-to-dog renal xenograft survival by modification of the inflammatory mediator response. Ann Surg. 1987;206:482–495. doi: 10.1097/00000658-198710000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]