Among the advantages of tacrolimus (Prograf FK506)-based immunosuppression in renal transplant patients has been the ability to withdraw steroids completely in the majority of successfully transplanted recipients. 1–4 The percentage of patients in whom steroids have been discontinued has varied with the length of follow-up, but has been as high as 70% or more.2,3 An important question, however, concerns the outcome in these patients, and to date, this issue has not been formally investigated. We looked at a large population of wellstudied patients entered into a prospective, randomized trial of two tacrolimus-based regimens, with and without azathioprine, to answer questions about the short- and medium-term safety of steroid withdrawal.
PATIENTS AND METHODS
Between August 1, 1991, and December 9, 1993, 395 adult patients undergoing 397 renal transplantations only were entered into a prospective, randomized trial of tacrolimus/prednisone versus tacrolimus/azathioprine/prednisone, without induction antilymphocyte therapy. The details and overall outcomes of this trial have been reported previously.1,3,5–7 In this analysis, we excluded the 18 cases who lost their allograft within 3 weeks after transplantation (and who were all still taking steroids at the time of graft loss), and studied the 379 remaining cases, focusing on steroid withdrawal. Patients were categorized into two groups, those in whom steroids were withdrawn and those in whom steroids were never withdrawn. A subgroup analysis was performed to examine differences in rates of steroid withdrawal. An additional analysis was performed to assess the outcomes in the steroid-withdrawn patients and in the patients still on steroids. A further analysis was performed to look at the timing of steroid withdrawal.
The idealized timetable for steroid withdrawal began with an intraoperative 1000 mg bolus of intravenous methylprednisolone in all patients, followed by a taper from 200 to 20 mg/d over 6 days. At 3 to 4 weeks, the prednisone dose was decreased to 15 mg/d, and by 2 to 3 months, the dose was tapered by 2.5 mg decrements to 10 mg/d. Thereafter, the prednisone dose was decreased by 2.5 mg/q4 to 6 weeks until complete discontinuation. There was considerable variability in these timetables; some patients never came off prednisone, some came off much later than 6 months after transplantation (in some cases as long as 3 years postoperatively), and a few patients came off steroids earlier.
This trial was performed before the US Food and Drug Administration approval of tacrolimus, and was approved, with yearly renewals, by the Biomedical Institutional Review Board of the University of Pittsburgh.
RESULTS
The mean follow-up was 33 ± 10 months.
Steroid Withdrawal
Two hundred eighty nine (76%) patients were taken off prednisone, and 90 (24%) never came off prednisone. The median time to steroid withdrawal was 10 months (Table 1).
Table 1.
Outcome After Steroid Withdrawal
| Withdrawn From Steroids |
Never Withdrawn |
|
|---|---|---|
| N | 289 (76%) | 90 (24%) |
| Median time to withdrawal (months) | 10 | – |
| Living donor | 92% | 8% |
| Cadaveric donor* | 75% | 25% |
| Donor age (y)* | 32.9 ± 19.8 | 38.8 ± 21.4 |
| Donors <60 y | 79% | 21% |
| Donors >60 y** | 53% | 47% |
| Tacrolimus/prednisone | 77% | 22% |
| Tacrolimus/prednisone/azathioprine | 76% | 23% |
| Delayed graft function | ||
| *** no | 81% | 19% |
| yes | 67% | 33% |
| Acute rejection | ||
| ** no | 87% | 13% |
| 65% | 35% | |
| Patient survival† | ||
| 1 y (actual) | 99% | 91% |
| 3 y (actuarial) | 98% | 80% |
| Graft survival† | ||
| 1 y (actual) | 98% | 77% |
| 3 y (actuarial) | 94% | 50% |
| Rejection† | 43% | 73% |
| Serum creatinine (mg/dL) | ||
| 1 y** | 1.6 ± 0.6 | 2.3 ± 1.5 |
| 2 y†† | 1.7 ± 0.7 | 2.3 ± 1.3 |
| Cytomegalovirus | 15% | 23% |
| Tacrolimus dose (mg/kg per d) | ||
| 1 y††† | 0.15 ± 0.11 | 0.19 ± 0.15 |
| 2 y†††† | 0.13 ± 0.08 | 0.18 ± 0.09 |
P < .02
P < .0001
P = .003
P < .0001
= .001
P < .01
P < .05
There were no differences in recipient sex, race, panel reactive antibodies, degree of HLA matching or mismatching, or immunosuppressive regimen between the two groups. There was also no difference in donor sex or race. A higher percentage of living donor cases (92%) than cadaver donor cases (75%) were taken off steroids (P < .03). Donor age was also an important factor. A much lower percentage of recipients of kidneys from donors over the age of 60 (53%) were taken off steroids, compared with recipients of kidneys from donors under the age of 60 (79%; P < .0004). As a corollary, the mean donor age was younger in the steroid-withdrawn group (32.9 ± 19.8 years) than in the never-withdrawn group (38.8 ± 21.4 years; P < .02).
Patients with delayed graft function (67%) or who experienced acute rejection (65%) were less likely to he withdrawn from steroids than patients with immediate function (81%; P = .003) or who never had rejection (87%; P < .0001).
Patient Survival
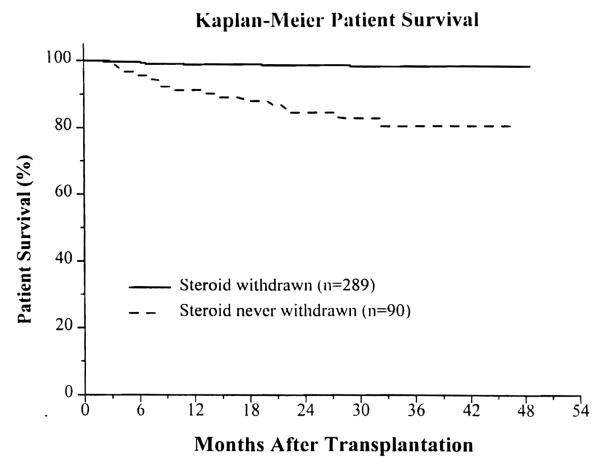
In the steroid-withdrawn patients, the 1-year actual and 3-year actuarial patient survival was 99% and 98%. In the group still on steroids, it was 91% and 80%, respectively (P < .00001) (Fig 1).
Fig 1.
Actuarial patient survival.
Graft Survival
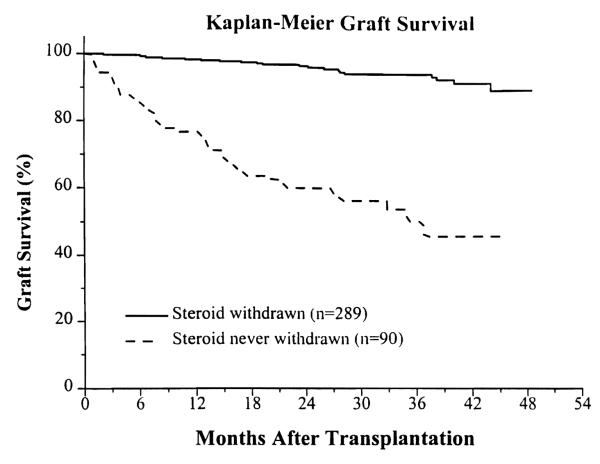
In the steroid-withdrawn group, the 1-year actual and 3-year actuarial graft survival was 98% and 94%; in the patients never withdrawn from steroids, it was 77% and 50%, respectively (P < .00001) (Fig. 2).
Fig. 2.
Actuarial graft survival.
Quality of Graft Function
In the steroid-withdrawn patients, the mean serum creatinine at 1 and 2 years was 1.6 ± 0.6 mgldL, and 1.7 ± 0.7 mgldL. In the patients still on steroids, the corresponding values were 2.3 ± 1.5 mgldL (P < .0001) and 2.3 ± 1.3 mgldL (P = .001).
Rejection
The incidence of rejection in the steroid-withdrawn group was 43%. In the never-withdrawn group, it was 73% (P < .00001). The majority of these rejections occurred in the early posttransplant period, long before steroids were withdrawn.
Cytomegalovirus
The incidence of cytomegaloviral infection in the steroid-withdrawn group was 15%; in the never-withdrawn group, it was 23% (P = ns).
Tacrolimus Dosage
After 1 and 2 years, the mean tacrolimus dose in the steroid-withdrawn patients was 0.15 ± 0.11 mg/kg/d and 0.13 ± 0.08 mg/kgld. In the never-withdrawn group, the corresponding dosages were 0.19 ± 0.15 mg/kg/d (P < .01) and 0.18 ± 0.09 mg/kg per d (P < .05).
DISCUSSION
The primary intent of this analysis was to assess the safety of steroid withdrawal in renal transplant recipients receiving tacrolimus-based immunosuppression. Viewed strictly from that perspective, steroid withdrawal was associated with excellent patient and graft survival and stable renal function. The incidence of rejection, while high, was not inconsistent with our previous reports of this study1,3 and has parenthetically stimulated other subsequent studies to attempt to reduce the incidence of acute rejection in our transplant population.8 The poor outcomes in the patients never taken off steroids were somewhat unexpected. An argument can be made that these differences in outcomes between steroid-withdrawn and never-withdrawn patients is merely a reflection of differences between a group of patients who did well and a group of patients with ongoing problems after transplantation. An alternative, but not necessarily conflicting, argument is that steroid withdrawal facilitated the mutual acceptance between donor and recipient, consistent with the role of chimerism in long-term graft survival.9–12 Unfortunately, this cohort of patients was not routinely analyzed for chimerism.
Ultimately, of course, a rigorous analysis of the effect of steroid withdrawal will have to be made with a prospective, randomized trial. While the patients entered into this trial were randomly assigned to double or triple therapy, steroid withdrawal was not randomized, and, indeed, was the theoretic goal in all patients.
The main lesson from this analysis is that steroid withdrawal in renal transplant patients receiving tacrolimus-based immunosuppression is possible most of the time and seems to be reasonably safe, in terms of short- and medium-term patient and graft survival and stability of renal function. More follow-up will be required to assess the longer-term outcome.
REFERENCES
- 1.Shapiro R, Jordan ML, Scantlebury VP, et al. Transplant Proc. 1995;27:814. [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro R, Jordan ML, Scantlebury VP, et al. Transplant Proc. 1996;28:2117. [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro R, Scantlebury VP, Jordan ML, et al. Transplantation. 1996;62:1752. doi: 10.1097/00007890-199612270-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro R, Scantlebury VP, Jordan ML, et al. Pediatr Nephr. 1995;9:S43. doi: 10.1007/BF00867683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro R, Jordan M, Scantlebury V, et al. Transplant Proc. 1993;25:669. [PMC free article] [PubMed] [Google Scholar]
- 6.Shapiro R, Jordan M, Scantlebury V, et al. Clin Trans. 1994;8:508. [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro R, Jordan ML, Scantlebury VP, et al. Transplantation. 1995;59:485. doi: 10.1097/00007890-199559040-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro R, Jordan ML, Scantlebury VP, et al. Presented at the 15th Annual American Society of Transplant Physicians Scientific Meeting; Chicago. May, 1997; Abst #293. [Google Scholar]
- 9.Starzl TE, Demetris AJ, Murase N, et al. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Demetris AJ, Trucco M, et al. Hepatology. 1993;17:1127. [PMC free article] [PubMed] [Google Scholar]
- 11.Starzl TE, Demetris AJ, Trucco M, et al. Transplantation. 1993;55:1272. doi: 10.1097/00007890-199306000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE, Demetris AJ, Murase N, et al. Immunology Today. 1993;14:326. doi: 10.1016/0167-5699(93)90054-o. [DOI] [PMC free article] [PubMed] [Google Scholar]