Abstract
Human locomotion must be flexible in order to meet varied environmental demands. Alterations to the gait pattern occur on different time scales, ranging from fast, reactive adjustments to slower, more persistent adaptations. A recent study in humans showed the cerebellum to play a key role for slower walking adaptations in inter-limb coordination during split-belt treadmill walking, but not fast reactive changes. It is not known whether cerebral structures are also important in these processes, though some studies of cats have suggested that they are not. In this study, we used a split-belt treadmill walking task to test whether cerebral damage from stroke impairs either type of flexibility. Results showed that stroke involving cerebral structures did not impair either reactive or adaptive abilities and did not disrupt storage of new inter-limb relationships (i.e. after-effects). This suggests that cerebellar interactions with brainstem, rather than cerebral structures, comprise the critical circuit for this type of inter-limb control. Further, the after-effects from a 15-minute adaptation session could temporarily induce symmetry in subjects who demonstrated baseline asymmetry of spatio-temporal gait parameters. In order to re-establish symmetric walking, the choice of which leg is on the fast belt during split-belt walking must be based on the subject’s initial asymmetry. These findings demonstrate that cerebral stroke survivors are indeed able to adapt inter-limb coordination. This raises the possibility that asymmetric walking patterns post-stroke could be remediated utilizing the split-belt treadmill as a long-term rehabilitation strategy.
Keywords: stroke, motor, locomotion, movement
Introduction
Locomotion in humans must be flexible enough to accommodate changing environmental demands and task constraints. Achieving this is not trivial; it requires modification of intra- and inter-limb coordination without loss of stability. Modifications take place on different time scales: some are immediate reactions to a novel situation, and others are slower adaptive changes that last longer (Lam et al. 2006; Morton and Bastian 2006). Reactive changes rapidly occur using peripheral feedback (e.g.-increasing your step height to clear a curb after you catch your toe on it). Slower adaptive changes depend on practice and occur over minutes to hours. (e.g.- changing your walking pattern to adjust to new shoes). They result in new calibrations of feedforward motor commands, which cause after-effects that persist when the demands are removed.
Normally, both types of locomotor adjustments can be made with ease. For example, when people walk on a split-belt treadmill that moves each leg at a different speed, there is an immediate reaction such that the slower leg spends more time in stance and the faster leg spends less time (Reisman et al., 2005a). This reaction persists during split-belt walking, and then immediately reverses when the belts are returned to normal treadmill conditions (i.e. the belts tied at the same speed). In contrast, step lengths also are initially asymmetric, but an adaptive response occurs during split-belt walking that acts to re-establish symmetry via feedforward changes in phasing between legs. This adaptation induces an after-effect causing walking asymmetry when returned to normal treadmill conditions. A similar adaptive phenomenon occurs during circular treadmill locomotion, resulting in curved walking trajectories as after-effects (Gordon et al, 1995, Weber et al, 1998). When wearing a robotic gait orthosis that provides viscous resistance to the leg, subjects modulate the swing phase through feedback, but adapt feedforward control of the leg just prior to swing (Lam et al. 2006).
Which brain structures or pathways underlie these processes? Recent work in humans suggests that the cerebellum is required for slower adaptive changes in locomotion, but may not be as critical for reactive changes (Morton and Bastian 2006). In that study, subjects with cerebellar disorders were able to immediately adjust stance times to split-belt treadmill speeds, but could not adapt inter-limb phasing and showed no after-effects, suggesting that reactive changes rely on different descending commands or the interaction of spinal neural networks with the mechanical oscillation of the legs (Courtine and Schieppati 2004; Lacquaniti et al. 2002). Similar cerebellar deficits in adaptation have been shown in circular treadmill locomotion (Earhart et al. 2002). In cats, nitric oxide deprivation, which is thought to play a role in long-term depression in the cerebellum, abolishes locomotor adaptive capacity but not reactive ability (Yanagihara and Kondo 1996).
Studies of spinal and decerebrate cats suggest that both forms of flexibility occur in quadruped locomotion without cerebral inputs (Forssberg et al. 1980; Kulagin and Shik 1970; Yanagihara and Kondo 1996). Yet, the flexibility and stability requirements of human, bipedal gait are generally presumed to require some level of cerebral control (Armstrong 1986). Evidence from adult humans with stroke or spinal cord injury suggest that locomotor interlimb coordination is strongly influenced by cerebral inputs (Dietz et al. 2002; Kautz and Patten 2005; Kautz et al. 2006). Here we attempt to understand the role of cerebral structures in reactive modifications and/or adaptation of the locomotor pattern. Based on our previous results in subjects with cerebellar damage, we hypothesize that if cerebellar-cerebral motor connections play a predominant role in the adaptation then cerebral damage should impair adaptation. Alternatively, if plasticity at multiple neural levels or connections between the cerebellum and other motor areas are more important, then adaptation should be relatively normal.
Additionally, it is unknown if individuals with cerebral damage have the potential to alter their impaired walking pattern through short-term motor adaptations that result in after-effects. Damage to cerebral structures caused by stroke leaves many individuals with an asymmetric walking pattern (Hsu et al. 2003; Lamontagne and Fung 2004; Olney et al. 1994). If stroke survivors can show after-effects that reduce their walking asymmetry, even temporarily, following adaptive training on the split belt treadmill, it would demonstrate that their compromised nervous system is still capable of producing a more normalized (i.e. symmetric) pattern. This then raises the possibility that certain components of gait asymmetry might be remediated with specific rehabilitation strategies using the split-belt treadmill. Thus, a secondary purpose of this study is to investigate whether after-effects following split-belt treadmill walking lead to improvements in gait symmetry in subjects following stroke. Preliminary findings were published in abstract form (Reisman et al. 2005b).
Methods
Subjects
Thirteen individuals who had sustained a single stroke more than 6 months prior to the study (4 females and 9 males) and 13 age- and gender-matched healthy control subjects were recruited to participate in the study (Table 1). All subjects gave their informed consent prior to participating and a human studies committee approved the study. Subjects were excluded if they had other neurologic conditions, orthopedic conditions affecting the legs or back, uncontrolled hypertension, pacemaker or automatic defibrillator, active cancer, radiological and/or physical examination evidence of damage to the cerebellum or were unable to complete the task. Subjects who customarily wear an ankle-foot orthosis (AFO) were allowed to wear it during testing.
Table 1.
Stroke subject characteristics
| Subject | Age | Lesion location | LE Fugl- Meyer score |
Asymmetric Step length=S, Double support=DS |
Fast over ground walking speed (m/s) |
AFO* | Mono- filament threshold** |
|---|---|---|---|---|---|---|---|
| S1 | 48 | R hemisphere | 33/34 | S, DS | 1.1 | No | 4.31g |
| S2 | 63 | R frontal lobe | 21/34 | S | 1.3 | No | 4.56g |
| S3 | 47 | L hemisphere, hemorrhagic |
15/34 | S, DS | 0.82 | Yes | 4.56g |
| S4 | 49 | L basal ganglia, hemorrhagic |
21/34 | DS | 0.84 | No | >6.65g |
| S5 | 63 | L pons | 27/34 | 0.94 | No | 4.31g | |
| S6 | 53 | R basal ganglia | 28/34 | 1.1 | No | >6.65g | |
| S7 | 27 | L hemisphere | 21/34 | DS | 1.1 | Yes | 4.31g |
| S8 | 44 | R temporoparietal | 32/34 | 1.7 | No | 6.65g | |
| S9 | 70 | R parietal lobe | 32/34 | 1.5 | No | 4.31g | |
| S10 | 57 | L posterior temporoparietal |
22/34 | S, DS | 1.3 | No | 3.61g |
| S11 | 35 | R parietal lobe, hemorrhagic |
33/34 | 1.5 | No | >6.65g | |
| S12 | 62 | L caudate head, anterior limb internal capsule |
28/34 | S, DS | 0.66 | No | 3.61g |
| S13 | 55 | R putamen & cortical temporal lobe, hemorrhagic |
22/34 | S, DS | 0.85 | Yes | 4.56g |
Ankle-foot orthoses
Normal = 3.61 gm
Clinical examination
Subjects underwent a clinical examination including measurement of fast walking speed, the lower extremity portion of the FuglMeyer, a test of coordination, reflexes and the ability to move in and out of synergy (Fugl-Meyer et al. 1975), and tests of pressure sensitivity and proprioception. Fast walking speed was measured as the average of 3 trials along a 25-foot walkway. Sensation of the great toe was tested using graded monofilaments. The lowest gram filament that could correctly be detected on 4/5 trials was recorded. Proprioception was tested at the great toe, ankle, knee and hip by moving the joint approximately ten degrees and asking the subject to determine the direction of movement. The number of correct responses out of 5 trials was recorded and the percentage of trials correct at the great toe was used for subsequent analysis.
Testing Paradigm
Subjects were asked to walk on a custom-built treadmill (Woodway USA, Waukesha WI) comprised of two separate belts, each with its own motor, that permitted the speed of each belt (i.e., each leg) to be controlled independently. During different testing periods, subjects walked on the treadmill with the two belts either moving at the same speed (“tied” configuration) or different speeds (“split-belt” configuration). During the tied configuration, treadmill belt speeds were either “slow” (0.5 m/s) or “fast” (1.0 m/s). In the split-belt configuration, one treadmill belt was set at the slow speed while the other was set at the fast speed.
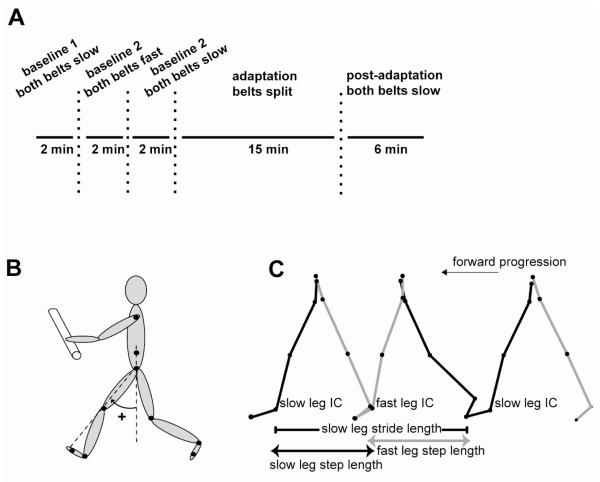
Stroke subjects participated in two testing sessions. In the first session, the leg assigned to the fast belt during the split-belt period was randomly determined as either the paretic or non-paretic leg. In the second session, the contralateral leg was tested on the fast belt during split-belt walking. Matched controls were randomly assigned to either their right or left leg on the fast belt and participated in only one testing session. Each session consisted of three testing periods. In the Baseline period, the belts were tied and moved first at the slow speed, then at the fast speed, and then again at the slow speed. In the Adaptation period, the treadmill belts were split (one belt fast, the other belt slow). In the Post-adaptation period, the belts were returned to the tied slow configuration. Figure 1a illustrates the experimental paradigm. The durations of each testing period were: Baseline 2 min (tied slow), 2 min (tied fast), 2 min (tied slow), Adaptation 15 min, and Post-adaptation 6 min (see Fig. 1a). Subjects were given standing or seated rest breaks every 5 minutes during Adaptation, or more frequently as requested. Three of the stroke survivors were only able to complete 6 minutes of Adaptation due to fatigue.
Figure 1.
A. Time course for the experimental paradigm showing Baseline, Adaptation, and Post-adaptation periods. B. Illustration of marker locations and the method used to calculate limb angle. C. Illustration of parameter calculations. Stride and step length depicted in over ground walking with forward progression. IC = initial contact.
Prior to data collection, subjects walked on the treadmill (in the tied condition) briefly at both the fast and slow speeds. They were not given any practice in the split-belt configuration, though they were told that the two belts would move at two different speeds at some point during the testing. For safety purposes while walking on the treadmill, all subjects held onto a front handrail and wore a ceiling mounted safety harness around the upper chest. The harness did not support body weight or interfere with subjects’ walking.
During testing, subjects were alerted when the treadmill was going to start, but were not instructed about belt speeds or coupling. Belt speeds were never changed while the belts were moving. Subjects were instructed to look straight ahead and refrain from looking down at the belts while walking so that they could not use visual information to determine belt speeds. An examiner stood by to monitor compliance with this instruction. At the beginning of each period, subjects were asked whether they felt the two belts were moving at the same speed or two different speeds. If they thought the belts were moving at different speeds, they were asked to indicate which belt (right or left) was moving faster.
Data Collection
Computerized gait analysis was performed using OPTOTRAK (Northern Digital, Waterloo ON) sensors that were used to record 3-dimensional position data from both sides of the body (Figure 1b). Infrared emitting diodes (IREDs) were placed bilaterally (Figure 1b) on the foot (5th metatarsal head), ankle (lateral malleolus), knee (lateral joint space), hip (greater trochanter), pelvis (iliac crest) and shoulder (acromion process). Foot contacts were determined using 4 contact switches per foot: 2 placed on the forefoot and 2 on the heel. Voltages reflecting treadmill belt speeds were recorded directly from treadmill motor output. Marker position and analog data (foot switches and treadmill speed) were synchronized and sampled simultaneously using OPTOTRAK software at 100 Hz and 1000 Hz, respectively.
Data Analysis
Three dimensional marker position data were low-pass filtered at 6 Hz. Custom software written in MATLAB (The MathWorks Inc., Natick MA) was used for all subsequent analyses. Based on our previous work, we measured spatial and temporal walking parameters that were expected to change rapidly using online feedback control and parameters that were expected to change more gradually using adaptive mechanisms (Reisman et al. 2005a). The rapidly-changing parameters were stride length and the percent time in stance phase; the adaptive parameters were step length and the percent time in double support (Fig. 1c). Stride and step lengths are spatial gait parameters while percent time in stance and double limb support are temporal measures. We calculated a third adaptive parameter, limb angle phasing, to quantify the phase relationship between the two limbs. All of these measures were calculated for both limbs. Stride length was the distance traveled by the ankle marker in the anterior-posterior direction from contact to lift-off of one limb (Figure 1c). This method of calculation could alter stride length if subject’s anterior-posterior position on the treadmill translated during walking. However, because subjects were required to hold on to the handrail, this was not a factor. The percent time in stance phase (the time from contact to lift-off) was expressed as a percentage of the stride time (the time from contact to next contact) for that limb. Step length was the anterior-posterior distance between the ankle markers at the time when each foot contacted the ground (Figure 1c). The percent time in double limb support was the time that both feet were in contact with the floor expressed as a percentage of the stride time for each leg. To calculate limb angle phasing, we first measured limb angle for each leg using the vector connecting the hip and metatarsal markers in the sagittal plane (see Fig. 1b). Phasing between the legs was calculated as the time difference (expressed as a percent of stride time) at the peak overlap of the cross-correlation between the two limb angles over three consecutive stride cycles.
Baseline asymmetry in the group of stroke survivors was determined individually for each subject. A stroke survivor was deemed asymmetric if the average of 5 strides in the second slow Baseline period exceeded two standard deviations of the mean asymmetry (0.02 m for step length and 0.01 percent double support) in the healthy control subjects during the same period. These calculations were completed separately for double support and step length.
Statistical analyses were completed using the averages of the first 5 strides (after the treadmill reaches steady state speed) in each of the Baseline periods, the first (after the treadmill reaches steady state speed) and last 5 strides of the Adaptation period (early and late Adaptation, respectively) and the first (after the treadmill reaches steady state speed) and last 5 strides of the Post-adaptation period (early and late Post- adaptation, respectively). Statistical comparisons were completed using MATLAB and Statistica (StatSoft, Tulsa OK) software. To compare results between groups we used a repeated measures Analysis of Variance (ANOVA) with a between-subjects factor of group (control and stroke) and a within-subjects (repeated measures) factor of testing period (late Baseline, early and late Adaptation, and early and late Post-adaptation) To compare differences between testing sessions in the paretic group we completed a repeated measures ANOVA with a within-subjects factor of leg (leg tested on the fast belt during split-belt walking). When the ANOVA yielded significant results, post hoc analyses were completed using Tukey’s Honest Significant Different test. Paired t-tests were used to determine changes in symmetry of double support and step length between Baseline and Post-adaptation for the sub-group of asymmetric stroke survivors. Pearson product-moment correlations were performed to test for relationships between impairment measures (LE Fugl-Meyer score, sensation and proprioception) and the magnitude of step length difference after-effect. For the limb angle phasing values, the Watson’s U2 statistic was used to test for differences across testing periods (Batschelet 1981). The level of statistical significance for all measures was set at p < 0.05.
Results
For all results, we refer to the legs on the slower and faster moving belts during the split-belt portion of the paradigm as the “slow” and “fast” legs, respectively. Overall, results of the control group were similar to that previously described (Reisman et al. 2005a). While there were individual differences in the responses of the stroke survivors, the group as a whole performed similarly to healthy controls.
Reactive changes in walking
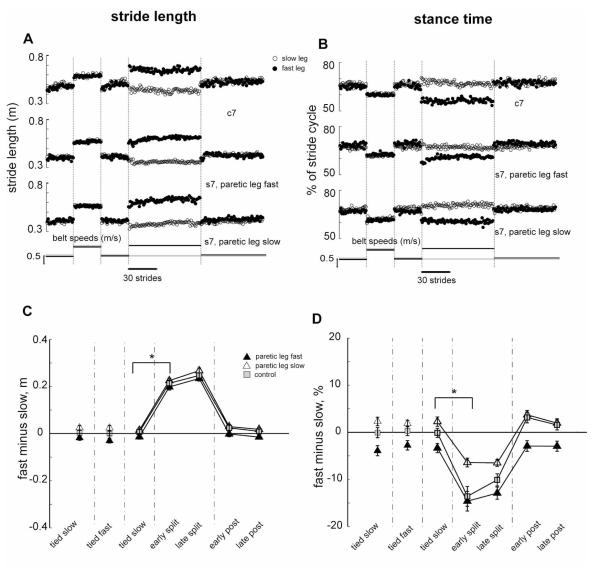
Figure 2 illustrates the walking parameters that normally change rapidly during the split belt period, and show no after-effects. Stride lengths are plotted stride-by-stride for a typical control subject and for a stroke subject in both testing conditions (Fig. 2a). All are symmetric during the Baseline period, and rapidly change during early Adaptation so the fast stride is longer and the slow stride is shorter. These alterations were maintained throughout Adaptation, and switched back to baseline levels immediately during the Post-adaptation period. Group data for stride length symmetry showed a main effect of testing period (p<0.0001), but no group differences or group by testing period interactions (Fig. 2c). All groups made comparable, rapid changes in stride symmetry when going from Baseline to Adaptation (i.e. split belt) conditions (p<0.001), but then immediately returned to Baseline levels of symmetry during Post-adaptation, with no difference between those two periods (p=0.87, stroke; p=0.78, control),
Figure 2.
Rapidly changing parameters. A, B. Stride length (A) and stance time (B) values for sequential strides on the treadmill from a typical control (top row) and matched stroke (bottom 2 rows) subject across all testing periods. For the stroke subject the middle row is from the session with the paretic leg on the fast belt and vice versa for the bottom row. Open and filled circles indicate values for the slow and fast legs, respectively. C, D. Average stride length (C) and stance time (D) differences for the stroke subjects in the paretic leg slow session (open triangles), the paretic leg fast session (filled triangles) and for the control (squares) group. Each data point represents values averaged over the first five strides from the early or late portions of each testing period for each control and stroke subject individually and then averaged across all subjects in a group. Error bars indicate ± 1 SE.
Figure 2b shows percent stance time for a control and for both sessions from a stroke subject. Some stroke subjects showed a stance time asymmetry during the Baseline period with a shorter stance time on the paretic leg. Just as was observed for stride length, subjects rapidly changed the time spent in the stance phase in response to changes in the treadmill speeds. During Adaptation, stance time on the fast leg quickly decreased and stance time on the slow leg quickly increased. In Post-adaptation, both legs’ stance time rapidly returned to Baseline levels. Group data also show the stance time asymmetry of stroke survivors (Fig. 2d); they spent less time on the paretic leg. As such, there was a main effect of group (p<0.01) in addition to the main effect of testing period (p<0.0001). There was also a group × testing period interaction (p<0.01), due to a reduced ability to increase the paretic stance time when it was the slow leg during Adaptation (Fig. 2d, open triangles). However, the basic reactive pattern still held in the Adaptation and Post-adaptation periods for all groups. There was a significant difference between Baseline and early Adaptation periods (p<0.001), but only slight, non-significant differences between the Baseline and early Post-adaptation periods (p=0.98, stroke; 0.30, control). Furthermore, there were no differences between early and late Adaptation periods (p=1.0, stroke; 0.21, control). Thus, both groups rapidly changed percent stance time on each leg in response to split belts and then almost immediately returned to baseline levels when the belts were returned to the same speeds (Figure 2d).
When comparing results from the two sessions in stroke subjects (for both stride length and stance time), the statistical analyses revealed a main effect for testing period (p<0.0001 for both stride length and stance time), and for leg tested (p<0.01, stance time only) but no interaction between testing period and leg tested, indicating that the stroke survivors showed similar patterns of performance for the rapidly changing parameters, regardless of which leg walked on the fast belt during the Adaptation period (p>0.1 for both).
In summary, during split-belt treadmill locomotion, subjects with cerebral damage were able to rapidly change their walking pattern to accommodate different belt speed relationships. This occurred regardless of whether the paretic or non-paretic leg walked on the fast belt during the Adaptation period.
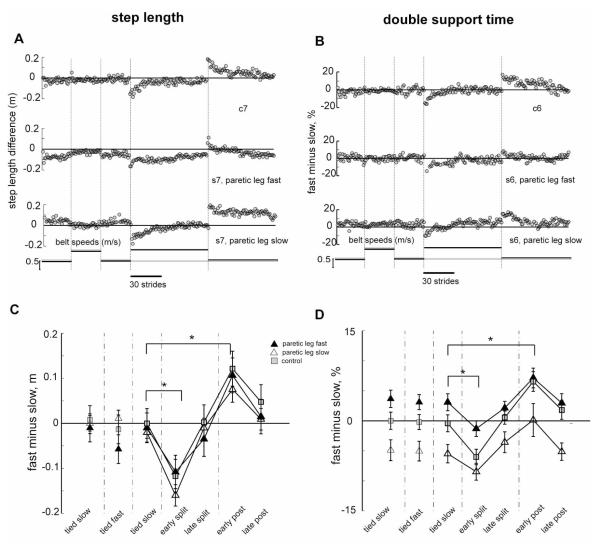
Slowly adapting changes in walking
Figure 3 shows the walking parameters that we expected to change more slowly over the course of Adaptation: step length and double support time. Step length changes show the same general pattern in controls and people with hemiparesis, regardless of which leg is made to go faster during adaptation (Fig. 3a). Here we report the difference in step length (fast minus slow leg). In the Baseline period, the control subject showed step length differences at or near zero indicating symmetry. The stroke subject shown in this example (Figure 3a) tended to take shorter steps with the paretic leg, which is why Baseline values are not zero. However, some stroke subjects show the opposite step length asymmetry (see Changes in asymmetry section below). During split-belt Adaptation, the example subjects initially had asymmetric step lengths that slowly adapted towards symmetry (i.e. zero). With the return to tied belts in Post-adaptation, both subjects initially showed the reverse asymmetry (negative after-effect) which slowly returned to baseline levels. Figure 3c shows group data indicating only a significant effect of testing period (p< 0.0001). Thus, control and stroke groups performed similarly over the different testing periods. Post-hoc tests showed significant changes from baseline to early Adaptation, improved symmetry over the course of adaptation, and significant after-effects (Baseline vs. early Post-adaptation, p < 0.001).
Figure 3.
Slowly adapting parameters. A, B. Step length (A) and double support time (B) values for sequential strides on the treadmill from a typical control (top row) and matched stroke (bottom 2 rows) subject across all testing periods. For the stroke subject the middle row is from the session with the paretic leg on the fast belt and vice versa for the bottom row. Filled grey circles indicate the difference between the legs (fast leg minus slow leg) in step length and double support time values. C, D. Average step length (C) and double support time (D) differences for the stroke subjects in the paretic leg slow session (open triangles), the paretic leg fast session (filled triangles) and for the control (squares) group. Each data point represents values averaged over the first five strides from the early or late portions of each testing period for each control and stroke subject individually and then averaged across all subjects in a group. Error bars indicate ± 1 SE.
Figure 3b shows double support times, also expressed as differences (fast minus slow). Both subjects had double support time differences near zero during the Baseline period, indicating symmetry. Some stroke subjects showed double support differences that were not zero, indicating an asymmetry of the two periods of double support. When this occurred, the double support period at the end of paretic leg stance was always longer than double support at the end of non-paretic stance (see Changes in asymmetry section below). In Adaptation, the subjects initially showed substantial asymmetry that gradually was adjusted towards symmetry. In Post-adaptation, the subjects demonstrated the expected reverse asymmetry (negative after-effect), which was also gradually corrected to baseline levels. Group data showed main effects for group (p<0.01) and testing period (p<0.0001), but no interaction. The group effect was due to the fact that stroke survivors had asymmetries in double support time as described above. However, this bias did not affect the general pattern of adaptation and after-effects. Post-hoc analyses showed that both groups changed double support difference from Baseline to early Adaptation (p<0.05), modified it over the course of Adaptation, and showed significant after-effects (Baseline vs. early Post-adaptation, p < 0.05 stroke, p<0.001, control).
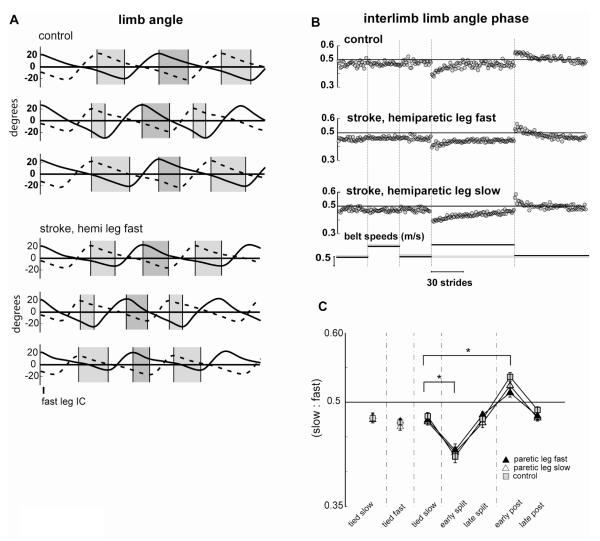
Interlimb phasing
Our previous work in healthy subjects showed that a small phase shift in limb motions can abolish asymmetries of step length and double support observed in early Adaptation (Reisman et al. 2005a). Figure 4a shows the limb angles of the fast and slow legs during slow Baseline, early Adaptation, and early Post-adaptation periods for a control and a stroke subject. When the interlimb phase is 0.5, the legs are moving reciprocally and the widths of the light and dark grey shaded areas are equal (top trace for both subjects). In early Adaptation, both subjects showed a phase shift whereby the fast leg was phase advanced relative to the slow leg (light < dark grey region). This phase advance is slowly corrected stride by stride (Fig. 4b). In early Post-adaptation there is a negative after-effect with the opposite phase shift (dark<light grey bars).
Figure 4.
Limb angle interlimb phase. A. Limb angles on the slow (dashed line) and fast (solid line) legs plotted over two successive strides from a typical control (top 3 pairs of traces) and stroke (bottom 3 pairs of traces) subject. Pairs of strides are from the Baseline (top), early Adaptation period (middle) and the early Post-adaptation period (bottom). All strides are aligned on the first initial contact (IC) on the fast leg. Light grey bars show the duration from peak limb flexion on the slow leg to peak limb extension on the fast leg; dark grey bars show the duration from peak limb flexion on the fast leg to peak limb extension on the slow leg. During symmetric walking, these two durations are equal (top trace); note the clear temporal shift in limb angles that occurs during the early Adaptation (middle trace) and early Post-adaptation (bottom trace) periods. These phase shifts are quantified over the duration of the limb angle cycle in the cross-correlation measures. B. Limb angle interlimb phasing values for sequential strides on the treadmill from the same control and stroke subject shown in A above. C. Average limb angle interlimb phasing values for the stroke subjects in the paretic leg slow session (open triangles), the paretic leg fast session (filled triangles) and for the control (squares) group. Each data point represents values averaged over the first five strides from the early or late portions of each testing period for each control and stroke subject individually and then averaged across all subjects in a group. Error bas indicate ± 1 SE.
Interlimb phase relationship was determined using a cross-correlation function. Figure 4b depicts the lag time at the peak in the cross-correlation function calculated between the two limb angles for the same control and stroke subjects shown in Figure 5a. Throughout the Baseline period, the limb angle interlimb phasing values for both subjects were near 0.5. In early Adaptation, both subjects initially showed a phase shift reflecting the phase advancement of the fast leg, which was gradually corrected over the course of Adaptation and showed a negative after-effect in Post-adaptation. Group results depicted in Figure 4c show that limb phasing changed from Baseline to early Adaptation (p<0.05) and the new phase relationship was stored, resulting in an after-effect in the Post-adaptation period (compare Baseline to early Post-adaptation Figure 5c, p<0.01). Thus, individuals with stroke appear to use the same means for adapting inter-limb coordination as control subjects.
Figure 5.
Changes in asymmetry. A. Step length of a stroke subject. Step length on the paretic (solid circles) and non-paretic (open circles) legs shown for consecutive strides in all periods. Note the marked baseline asymmetry when the belts are tied, the increase in asymmetry when the belts are split (because the paretic leg is on the fast belt, thus exaggerating the baseline asymmetry) and the symmetry when the belts are tied in Post-adaptation. B. Double support times for a stroke subject. Double support at the end of paretic stance is indicated with open circles and double support at the end of non-paretic leg stance indicated with solid circles. Note the marked baseline asymmetry when the belts are tied, the increase in asymmetry when the belts are split and the symmetry when the belts are tied in Post-adaptation. C. Step length difference for individual subjects in the Baseline and Post-adaptation periods when the paretic leg is on the slow (left figure) or fast (right figure) belt during the split-belt period. Grey lines represent subjects who at baseline take a longer step on the paretic leg and black lines represent subjects who take a shorter step on the paretic leg at baseline. Note that the after-effects in Post-adaptation serve to either increase or decrease step length asymmetry, depending on the direction of the asymmetry at baseline. D. Changes in step length and double support from Baseline to Post-adaptation periods for subjects who demonstrated significant (see Methods) baseline asymmetry. Slow leg indicated by open circles and fast leg indicated by closed circles. Note the improvement in asymmetry from the Baseline to Post-adaptation period. Each data point represents values averaged over the first five strides from the Baseline or Post-adaptation period. Error bas indicate ± 1 SE.
Changes in asymmetry
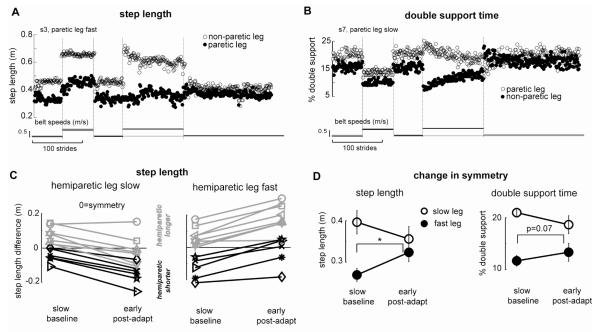
Some of our stroke subjects showed asymmetries in step length and/or double support times during baseline walking. Here we tested whether we could induce after-effects that altered those asymmetries. Since after-effects are assessed Post-adaptation (i.e. belts tied at the same speed), alterations in the walking pattern would be due to changes in motor commands; they would not simply be a mechanical phenomenon, as might be seen during split-belt portions of the paradigm. Figure 5a illustrates an improvement in step length asymmetry for a stroke subject who, at baseline, takes a shorter step with his paretic leg (compare open and closed circles in the three Baseline periods). By effectively increasing this asymmetry during early Adaptation period, we present a situation that drives adaptation of motor commands to ultimately reduce the asymmetry. Thus, in the Post-adaptation period we see after-effects that result in symmetrical step lengths on the two legs (compare open and closed circles in the Post-adaptation period).
Figure 5b illustrates a similar case for double support time. In the Baseline periods, this stroke subject spends longer in the double support period at the end of paretic leg stance (compare open and closed circles in the three Baseline periods). Again, this asymmetry is increased during the early Adaptation period and the subject adapts his motor commands. In the Post-adaptation period we again see that the after-effect has improved symmetry (compare open and closed circles in the Post-adaptation period). Similar effects were found in general for the subjects with hemiparesis that demonstrated a baseline asymmetry (see Figure 5d).
People with hemiparesis can show step length asymmetries in either direction (Chen et al. 2005a; Olney et al. 1994; Roth et al. 1997). In order to re-establish symmetric walking, the training pattern (i.e. which leg is on the fast belt during Adaptation) must therefore be based on the subject’s initial asymmetry. This is illustrated for all subjects in Figure 5c. Subjects who take a longer paretic step during Baseline are trained with the paretic leg on the slow belt to induce an after-effect that leads to greater symmetry (grey lines, left figure). Subjects who take a shorter paretic step in Baseline, are trained with the paretic leg on the fast belt to induce an after-effect that leads to greater symmetry (black lines, left figure). If we train in the wrong direction, we get after-effects that worsen their step length asymmetry. And, if there was no asymmetry to start with, we induce after-effects that cause asymmetry.
We tested whether the after-effects following split-belt treadmill walking could lead to improved symmetry of step length and double support for the group of subjects who demonstrated a baseline asymmetry. Table 1 shows that 6 subjects met our criteria for asymmetry of step length at baseline, 3 with a longer paretic step and 3 with a longer non-paretic step. Seven subjects were found to have baseline double support asymmetries; all subjects spent longer in the double support period at the end of paretic leg stance. We analyzed the conditions that would theoretically induce symmetric after-effects. Results of the paired t-test reveal that there was a significant difference between the step length asymmetry in the slow Baseline period compared to the early Post-adaptation period (p=0.01), with subjects becoming more symmetric in Post-adaptation (left side of Figure 5d). A similar trend was found for double support, with subjects becoming more symmetric in Post-adaptation (p=0.07, right side of Figure 5d).
Impairment level and after-effect magnitude
An important question is whether any of the stroke survivors’ impairments relate to their ability to adapt inter-limb coordination. Our subjects had widely varying sensory and motor impairments (Table 1). We were surprised that we found no significant correlations between lower extremity impairment scores (i.e. Fugl-Meyer score, sensation, proprioception) and the magnitude of the step length after-effect, which was our measure of adaptive ability (all p > 0.18). After-effect magnitudes were also unrelated to subjects’ over ground fastest walking speeds (p =0.737).
Perceptual after-effects
During Post-adaptation, all control subjects felt that the opposite leg was moving faster, compared to during Adaptation. This perceptual after-effect is the same as was observed in the previous study (Reisman et al., 2005a). In contrast, only six out of the thirteen stroke subjects demonstrated the same perceptual after-effect. The perceptual after-effect (or lack thereof) in the stroke subjects was not correlated with the magnitude of the motor after-effect (p=0.73).
Discussion
In this study, we have demonstrated that cerebral and subcortical strokes causing a range of sensory and motor deficits did not impair a person’s ability to make immediate reactions or slower adaptations during split-belt treadmill locomotion. Importantly, we found that stroke subjects could temporarily store new interlimb relationships, demonstrating that the compromised nervous system is still capable of producing a more normal pattern. This ability was not correlated with any sensory or motor impairment measures that were collected, nor did it appear to depend on perception of the after-effects. The results of the present study are in contrast to our previous findings in people with focal cerebellar damage, who are impaired or unable to adapt interlimb coordination, despite being able to make immediate reactive changes (Morton and Bastian 2006).
Equally important is the finding that these adaptations can induce after-effects that temporarily restore symmetry in stroke survivors. This is a critical finding, given that many previous studies have shown that changes in coordination and symmetry during locomotor activities can be difficult to achieve, even with training (Den Otter et al. 2006; Kautz et al. 2005; Silver et al. 2000). The fact that our after-effects are short-lived is not surprising given that training was only 15 minutes. Yet, this demonstration suggests that the nervous system of cerebral stroke survivors is capable of flexibility which could be capitalized on with the appropriate long-term training paradigm.
Role of supraspinal inputs in locomotor adaptation
A previous study of decerebrate cats showed that they could adapt interlimb coordination when walking on a split-belt treadmill (Yaniagihara and Kondo 1996). When function of the cerebellum was altered through nitric oxide deprivation, adaptation was impaired. This suggests that cerebellar, rather than cerebral structures, are more involved in this process. The circuit in the cat likely involves cerebellar influences on brainstem structures contributing to the vestibulo- and reticulospinal pathways. Our human work has also shown that cerebellar damage disrupts interlimb adaptation (Morton and Bastian 2006). But, we hypothesized that in humans, the cerebellum could be involved through its projections to cerebral motor areas via thalamus or through its brainstem projections. In our prior study, we found that not all cerebellar lesions produced the same effect on split-belt adaptation: damage causing balance and gait impairments disrupted adaptation, whereas damage causing voluntary leg control deficits did not (Morton and Bastian 2006). Balance and gait deficits are more linked to damage of midline cerebellar structures, which project to, and receive input from, the brainstem (Chambers and Sprague 1955; Morton and Bastian 2003). This coupled with the results of the current work suggest that cerebellar projections to brainstem motor areas might be more important than projections to cerebral motor areas for this adaptive process.
Although cerebral involvement in the adaptive process cannot be definitively ruled out, our results show that many types of cerebral and subcortical lesions do not impair this adaptive ability. The subjects in this current study had damage to many structures, including: frontal, parietal and temporal regions of cerebral cortex, subcortical white matter (e.g. internal capsule), the basal ganglia, and the pons that resulted in a wide range of sensory and motor impairments. For example, one subject (S9) had a large parietal lesion that caused profound loss of proprioceptive sense from his paretic leg, yet he was able to adapt normally. Our original thought was that proprioceptive information from the limbs would be important for this adaptation. We surmise that adaptation still occurred since proprioceptive information reached the cerebellum through the dorsal and ventral spinocerebellar tracts, and that conscious perception of limb position might not be required for this adaptation. Another subject (S5) had damage to the pons, which might cause an adaptation deficit given the cortico-ponto-cerebellar projection through the middle cerebellar peduncle. Yet, he adapted quite normally. One interpretation of this result is that the damage did not sufficiently affect the inferior cerebellar peduncle, which carries information to and from brainstem motor regions.
While the results of the present study suggest that cerebral structures are not critical for the split-belt locomotor adaptation studied here, there is evidence to suggest that they are important for other visually driven adjustments like obstacle avoidance. Recordings from pyramidal track and rubrospinal neurons in the cat demonstrate an increased discharge of these neurons when stepping over an obstacle (Drew 1993; Lavoie and Drew 2002; Widajewicz et al. 1994). These neurons appear to respond when the animal is using visual information to control the trajectory of paw and are not thought to be responding to peripheral feedback (Drew 1993; Drew et al. 1996). In addition, induced lesions of the motor cortex in cats lead to an inability to step over obstacles attached to a moving belt (Drew et al. 1996). Finally, stroke survivors were unable to adequately modify their stepping pattern to successfully avoid obstacles during treadmill walking, leading to a much higher failure rate than healthy controls (Den Otter et al. 2005). One interpretation of all of these results is that cerebral regions are less important for peripherally driven locomotor adaptations, but critical for visually guided gait modifications, though these issues require further study.
Locomotor flexibility post-stroke
An important finding of this study is that stroke survivors retain sufficient adaptability of the central nervous system to alter spatiotemporal interlimb relationships. Previous research has suggested that a major deficit in motor control following stroke is the inability to flexibly produce different motor patterns in response to changing demands. In a pedaling task, Brown and colleagues found that stroke subjects could increase muscle activity in response to increased workload demand, but produced the same abnormal muscle activation pattern (Brown et al. 1996). In a reaching task, stroke survivors could not decouple shoulder abduction and elbow extension torque when reaching to different target locations (Beer et al. 2004). In contrast, our results demonstrate that stroke survivors can adjust interlimb control during walking in response to changing demands. This is consistent with a previous study of stroke locomotion which found that when stroke subjects walked substantially faster than their normal speed, the symmetry of double support was improved (Lamontagne and Fung 2004). Our results further this finding by demonstrating that not only can stroke survivors flexibly adjust spatiotemporal gait parameters to changing demands, they can store the new pattern and produce it even when the new task demands are removed.
Locomotor adaptation and gait asymmetry post-stroke
After only fifteen minutes of split-belt treadmill walking, stroke subjects demonstrate after-effects in double support and step length that improve the symmetry of these variables. While this effect is short-lived, it is not trivial. Previous studies have shown that traditional locomotor training has little or no influence on interlimb coordination or symmetry post-stroke (Den Otter et al. 2006; Kautz et al. 2005; Silver et al. 2000). These results occur despite improvements in walking speed (Den Otter et al. 2006; Kautz et al. 2005) and have led to the suggestion that more targeted rehabilitation may be needed (Kautz et al. 2005). The results of novel treadmill training interventions on gait symmetry post-stroke have been mixed. Some previous studies using body-weight supported treadmill training or fast treadmill training have demonstrated improvements in symmetry post-stroke (Hesse et al. 1999; Lamontagne and Fung 2004), while others have shown no improvement (Hesse et al. 2001). Our study demonstrates that stroke subjects retain the ability to produce a symmetric gait pattern following short-term exposure to novel interlimb coordination demands like the split-belt treadmill. The results also illustrate that, because stroke subjects show heterogeneous patterns of asymmetry, in order to re-establish symmetric walking, the training pattern (i.e. which leg is on the fast belt during Adaptation) must be based on the subject’s initial asymmetry. Whether long-term training with a split-belt treadmill followed by over ground walking to practice the symmetric pattern can lead to more permanent changes in symmetry in stroke subjects remains to be tested. However, our results support the idea that the impaired nervous system following a stroke is at least capable of the flexibility required to produce shorter-term changes in gait symmetry.
Utilizing motor adaptations as a rehabilitation intervention
A legitimate question is whether after-effects from adaptive training are useful for rehabilitation. Other studies have utilized after-effects of a visuomotor adaptation to improve task performance in persons post-stroke (Patton et al. 2006; Rossetti et al. 1998). Rossetti and colleagues have shown that the after-effects following prism adaptation can improve the right-deviated pointing errors normally made by stroke survivors with left hemispatial neglect (Rossetti et al. 1998). Using a robot and force field to produce disturbing forces to the hand during reaching, Patton and colleagues found that the after-effects produced following training in the force field could improve pointing errors in group of stroke survivors (Patton et al. 2006). Just as in the present study, this group also found that the magnitude of the after-effects were not correlated with the degree of clinical impairment (Patton et al. 2006). This is potentially important as it suggests that regardless of the severity of sensorimotor deficits, subjects with cerebral damage following stroke retain the ability to make motor adaptations. Especially striking here is the lack of relationship between fast walking speed and the ability to make motor adaptations. Previous work has suggested that walking speed in stroke is correlated with level of motor impairment (Brandstater et al. 1983). However, our study reveals that neither the level of motor impairment or walking speed is correlated to the ability to make a locomotor adaptation. This makes the utility of this type of motor adaptation training more appealing, as it suggests that it may be successfully utilized across a broad spectrum of patients. However, in order to determine the role of split-belt locomotor adaptation in rehabilitation, further research is needed to investigate the effects of long-term training, and how these effects transfer to real-world tasks like walking over ground.
Acknowledgements
We wish to thank S. Morton and J. Choi for their contribution to analyses and interpretation, and A. Torrie, R. Bunoski and N. Zamora for their expertise with data collection. This work was supported by NIH K01HD050582 and NIH R01HD048740.
References
- Armstrong DM. Supraspinal contributions to the initiation and control of locomotion in the cat. Prog Neurobiol. 1986;26:273–361. doi: 10.1016/0301-0082(86)90021-3. [DOI] [PubMed] [Google Scholar]
- Beer RF, Dewald JP, Dawson ML, Rymer WZ. Target-dependent differences between free and constrained arm movements in chronic hemiparesis. Exp Brain Res. 2004;156:458–470. doi: 10.1007/s00221-003-1807-8. [DOI] [PubMed] [Google Scholar]
- Brandstater ME, de Bruin H, Gowland C, Clark BM. Hemiplegic gait: analysis of temporal variables. Arch Phys Med Rehabil. 1983;64:583–587. [PubMed] [Google Scholar]
- Brown DA, Kautz SA, Dairaghi CA. Muscle activity patterns altered during pedaling at different body orientations. J Biomech. 1996;29:1349–1356. doi: 10.1016/0021-9290(96)00038-3. [DOI] [PubMed] [Google Scholar]
- Chambers WW, Sprague JM. Functional localization in the cerebellum. I. Organization in longitudinal cortico-nuclear zones and their contribution to the control of posture, both extrapyramidal and pyramidal. J Comp Neurol. 1955;103:105–129. doi: 10.1002/cne.901030107. [DOI] [PubMed] [Google Scholar]
- Chen G, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture. 2005a;22:51–56. doi: 10.1016/j.gaitpost.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Courtine G, Schieppati M. Tuning of a basic coordination pattern constructs straight-ahead and curved walking in humans. J Neurophysiol. 2004;91:1524–1535. doi: 10.1152/jn.00817.2003. [DOI] [PubMed] [Google Scholar]
- Den Otter AR, Geurts AC, de Haart M, Mulder T, Duysens J. Step characteristics during obstacle avoidance in hemiplegic stroke. Exp Brain Res. 2005;161:180–192. doi: 10.1007/s00221-004-2057-0. [DOI] [PubMed] [Google Scholar]
- Den Otter AR, Geurts AC, Mulder T, Duysens J. Gait recovery is not associated with changes in the temporal patterning of muscle activity during treadmill walking in patients with post-stroke hemiparesis. Clin Neurophysiol. 2006;117:4–15. doi: 10.1016/j.clinph.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Dietz V, Muller R, Colombo G. Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain. 2002;125:2626–2634. doi: 10.1093/brain/awf273. [DOI] [PubMed] [Google Scholar]
- Drew T. Motor cortical activity during voluntary gait modifications in the cat. I. Cells related to the forelimbs. J Neurophysiol. 1993;70:179–199. doi: 10.1152/jn.1993.70.1.179. [DOI] [PubMed] [Google Scholar]
- Drew T, Jiang W, Kably B, Lavoie S. Role of the motor cortex in the control of visually triggered gait modifications. Can J Physiol Pharmacol. 1996;74:426–442. [PubMed] [Google Scholar]
- Earhart GM, Fletcher WA, Horak FB, Block EW, Weber KD, Suchowersky O, Jones G Melvill. Does the cerebellum play a role in podokinetic adaptation? Exp Brain Res. 2002;146:538–542. doi: 10.1007/s00221-002-1238-y. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Halbertsma J, Rossignol S. The locomotion of the low spinal cat. II. Interlimb coordination. Acta Physiol Scand. 1980;108:283–295. doi: 10.1111/j.1748-1716.1980.tb06534.x. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Hesse S, Konrad M, Uhlenbrock D. Treadmill walking with partial body weight support versus floor walking in hemiparetic subjects. Arch Phys Med Rehabil. 1999;80:421–427. doi: 10.1016/s0003-9993(99)90279-4. [DOI] [PubMed] [Google Scholar]
- Hesse S, Werner C, Paul T, Bardeleben A, Chaler J. Influence of walking speed on lower limb muscle activity and energy consumption during treadmill walking of hemiparetic patients. Arch Phys Med Rehabil. 2001;82:1547–1550. doi: 10.1053/apmr.2001.26607. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Tang PF, Jan MH. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch Phys Med Rehabil. 2003;84:1185–1193. doi: 10.1016/s0003-9993(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Kautz SA, Duncan PW, Perera S, Neptune RR, Studenski SA. Coordination of hemiparetic locomotion after stroke rehabilitation. Neurorehabil Neural Repair. 2005;19:250–258. doi: 10.1177/1545968305279279. [DOI] [PubMed] [Google Scholar]
- Kautz SA, Patten C. Interlimb influences on paretic leg function in poststroke hemiparesis. J Neurophysiol. 2005;93:2460–2473. doi: 10.1152/jn.00963.2004. [DOI] [PubMed] [Google Scholar]
- Kautz SA, Patten C, Neptune RR. Does unilateral pedaling activate a rhythmic locomotor pattern in the nonpedaling leg in post-stroke hemiparesis? J Neurophysiol. 2006;95:3154–3163. doi: 10.1152/jn.00951.2005. [DOI] [PubMed] [Google Scholar]
- Kulagin AS, Shik ML. Interaction of symmetric extremities during controlled locomotion. Biofizika. 1970;15:164–170. [PubMed] [Google Scholar]
- Lacquaniti F, Ivanenko YP, Zago M. Kinematic control of walking. Arch Ital Biol. 2002;140:263–272. [PubMed] [Google Scholar]
- Lam T, Anderschitz M, Dietz V. Contribution of feedback and feedforward strategies to locomotor adaptations. J Neurophysiol. 2006;95:766–773. doi: 10.1152/jn.00473.2005. [DOI] [PubMed] [Google Scholar]
- Lamontagne A, Fung J. Faster is better: implications for speed-intensive gait training after stroke. Stroke. 2004;35:2543–2548. doi: 10.1161/01.STR.0000144685.88760.d7. [DOI] [PubMed] [Google Scholar]
- Lavoie S, Drew T. Discharge characteristics of neurons in the red nucleus during voluntary gait modifications: a comparison with the motor cortex. J Neurophysiol. 2002;88:1791–1814. doi: 10.1152/jn.2002.88.4.1791. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci. 2006;26:9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Relative contributions of balance and voluntary leg-coordination deficits to cerebellar gait ataxia. J Neurophysiol. 2003;89:1844–1856. doi: 10.1152/jn.00787.2002. [DOI] [PubMed] [Google Scholar]
- Olney SJ, Griffin MP, McBride ID. Temporal, kinematic, and kinetic variables related to gait speed in subjects with hemiplegia: a regression approach. Phys Ther. 1994;74:872–885. doi: 10.1093/ptj/74.9.872. [DOI] [PubMed] [Google Scholar]
- Patton JL, Stoykov ME, Kovic M, Mussa-Ivaldi FA. Evaluation of robotic training forces that either enhance or reduce error in chronic hemiparetic stroke survivors. Exp Brain Res. 2006;168:368–383. doi: 10.1007/s00221-005-0097-8. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Block H, Bastian AJ. Inter-limb coordination during locomotion: What can be adapted and stored? J Neurophysiol. 2005a;94:2403–2415. doi: 10.1152/jn.00089.2005. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Bastian AJ. Split-belt treadmill walking adaptation in post-stroke hemiparesis. 2005 Abstract Viewer/Itinerary Planner Society for Neuroscience. 2005b Program No: 756.4. [Google Scholar]
- Rossetti Y, Rode G, Pisella L, Farne A, Li L, Boisson D, Perenin MT. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature. 1998;395:166–169. doi: 10.1038/25988. [DOI] [PubMed] [Google Scholar]
- Roth EJ, Merbitz C, Mroczek K, Dugan SA, Suh WW. Hemiplegic gait. Relationships between walking speed and other temporal parameters. Am J Phys Med Rehabil. 1997;76:128–133. doi: 10.1097/00002060-199703000-00008. [DOI] [PubMed] [Google Scholar]
- Silver KH, Macko RF, Forrester LW, Goldberg AP, Smith GV. Effects of aerobic treadmill training on gait velocity, cadence, and gait symmetry in chronic hemiparetic stroke: a preliminary report. Neurorehabil Neural Repair. 2000;14:65–71. doi: 10.1177/154596830001400108. [DOI] [PubMed] [Google Scholar]
- Widajewicz W, Kably B, Drew T. Motor cortical activity during voluntary gait modifications in the cat. II. Cells related to the hindlimbs. J Neurophysiol. 1994;72:2070–2089. doi: 10.1152/jn.1994.72.5.2070. [DOI] [PubMed] [Google Scholar]
- Yanagihara D, Kondo I. Nitric oxide plays a key role in adaptive control of locomotion in cat. Proc Natl Acad Sci U S A. 1996;93:13292–13297. doi: 10.1073/pnas.93.23.13292. [DOI] [PMC free article] [PubMed] [Google Scholar]