Abstract
There are currently seven approved therapies for chronic hepatitis B infection, an increase from just three agents five years ago. This review will focus on the pharmacology, potency, and adverse events associated with immunomodulatory agents and nucleos(t)ide analogs, with an emphasis on targets of therapy within the hepatitis B lifecycle. We will also offer guidelines for the use of available anti-HBV agents and review the emerging challenges in hepatitis B management, including HBV drug resistance, its management, and the potential role of combination therapy.
Keywords: Hepatitis B, Therapy, Pharmacology, Side Effects, Drug Resistance
Hepatitis B virus (HBV) infection affects ∼350 million people globally and is a leading cause of end stage liver disease, hepatocellular carcinoma, and mortality 1. New therapeutic agents have increased the options for HBV treatment, but since current agents often require lifelong administration, optimizing initial therapy is essential. This review will focus on the pharmacology and adverse events of anti-HBV drugs and offer guidelines for their use.
Life Cycle of Hepatitis B
Knowledge of the HBV life cycle is important for understanding therapeutic approaches to HBV.2 HBV is an enveloped, partially double- stranded DNA virus with four overlapping reading frames: the precore/core gene, the polymerase gene, the L-, M-, and S-gene which codes for the 3 envelope proteins; and the X gene. (Figure 1). HBV enters the hepatocyte through an unidentified receptor, is uncoated in the cytoplasm, and the DNA is transported to the nucleus. Here, the relaxed circular partially double-stranded DNA is converted to covalently closed circular DNA (cccDNA), a stable episomal form that becomes the template for viral mRNA transcription. In the cytoplasm, the pregenomic RNA (pgRNA) is translated into the core protein and the viral polymerase while the subgenomic RNA is translated to the three envelope proteins and the X protein. pgRNA is reverse transcribed to DNA by the HBV polymerase, the site of action of the oral anti-HBV therapeutics. The DNA can be either reimported into the nucleus to form additional cccDNA or can be enveloped for secretion. Since the available anti-HBV therapeutic agents do not work directly against the cccDNA, eradication of HBV is difficult.
Figure 1.
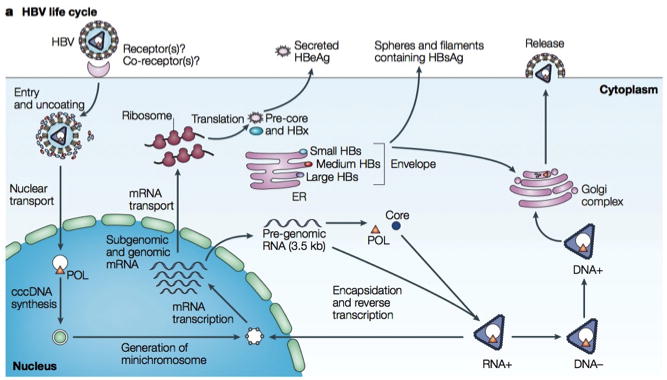
Life cycle of hepatitis B virus (HBV). Reprinted from Rehermann and Nascimbeni [3], with permission from the Nature Publishing Group. cccDNA, covalently closed circular DNA; ER, endoplasmic reticulum; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBx, HBVX protein; mRNA, messenger RNA; POL, polymerase.
Currently approved therapies
Standard interferon-α/Pegylated interferon-α
Interferon-α enhances the innate immune response by binding to the type 1 interferon receptor resulting in activation of the Jak-Stat pathway3 and up-regulation of multiple interferon-stimulated genes, which limit viral dissemination. With the addition of polyethylene glycol, pegylated interferon-α has a longer half-life than interferon-α. Although there are two formulations of pegylated interferon-α, -2a and -2b, only the former is approved in the US for CHB treatment.
The dose of pegylated interferon α-2a is 180 mcg given subcutaneously once per week. The Cmax occurs 72 -96 hours after administration with levels sustained up to 168 hours. It is cleared both by the kidney and liver but should be used with caution in patients with creatinine clearance (CrCl)< 50 mL/min, with dose adjustment required in hemodialysis (Table A). It should also be used with caution in patients on theophylline, whose level it increases. Adverse events in >25% of patients include pyrexia, myalgias, and headache, which can be ameliorated by pre-treatment with non-steroidal anti-inflammatory agents. Other adverse events include fatigue, arthralgias, alopecia, diarrhea, anorexia, insomnia, hypo-or hyperthyroidism, irritability and depression. Pegylated interferon-α is contraindicated in patients with untreated or severe depression or with decompensated cirrhosis 4.
Table A.
Dose Adjustments for Renal Insufficiency
| Drug and Creatinine Clearance (mL/min) | Recommended Dose |
|---|---|
| Pegylated interferon α-2a | |
| ≥50 | 180 mcg sc q week |
| ESRD (hemodialysis patients) | 135 mcg sc q week |
| Lamivudine* | |
| ≥50 | 100 mg po qd |
| 30-49 | 100 mg first dose, then 50 mg qd |
| 15-29 | 35 mg first dose, then 25 mg qd |
| 5-14 | 35 mg first dose, then 15 mg qd |
| <5 | 35 mg first dose, then 10 mg qd |
| Emtricitabine* | |
| ≥50 | 200 mg q24 |
| 30-49 | 200 mg q48 |
| 15-29 | 200 mg q72 |
| <15 or on HD | 200 mg q96 (after dialysis |
| Telbivudine* | |
| ≥50 | 600 mg qd |
| 30-49 | 600 mg q 48 hrs |
| <30 (without dialysis) | 600 mg q 72 hrs |
| ESRD (dialysis patients) | 600 mg q 96 hrs after HD |
| Adefovir | |
| ≥50 | 10 mg qd |
| 20-49 | 10 mg q other day |
| 10-19 | 10 mg q third day |
| Hemodialysis patients | 10 mg q week after dialysis |
| Tenofovir | |
| ≥50 | 300 mg q24 hrs |
| 30-49 | 300 mg q48 hrs |
| 10-29 | 300 mg q72-96 hrs |
| <10 with dialysis | 300 mg q week or post 12 hrs of dialysis |
| <10 without dialysis | No recommendation available |
| Entecavir* | |
| ≥50 | 1 mg qd |
| 30-49 | 0.5 mg qd or 1 mg q48 |
| 10-29 | 1 mg q72 |
| <10 or HD or CAPD | 1 mg q7 days (after dialysis) |
Lamivudine, emtricitabine, telbivudine, and entecavir are all available in oral solution. Oral solution dosing can be found in the package inserts.
Adapted from Lok 2009
In HBeAg-positive subjects, pegylated interferon-α was superior to standard interferon-α 5. The recommended 48 weeks of pegylated interferon-α results in HBV DNA loss in 25% and 63% of patients with HBeAg-positive and -negative CHB, respectively (Table B)6.
Table B.
Comparisons of Antiviral Agent Efficacy
| Placebo/Control Groups from Studies | Pegylated IFN 48 wk |
Adefovir 48 wk |
Lamivudine 48-52 wk |
Telbivudine 52 wk |
Entecavir 48 wk |
Tenofovir 48 wk |
|
|---|---|---|---|---|---|---|---|
| Loss of serum HBV DNA* | |||||||
| HBeAg + | 0%–17% | 25% | 21% | 40%–44% | 60% | 67% | 76% |
| HBeAg - | 0%–20% | 63% | 51% | 63-73% | 88% | 90% | 93% |
| Loss of HBeAg | 6%–12% | 30%/34% | 24% | 17%–32% | 26% | 22% | -- |
| HBeAg seroconversion | 4%–6% | 27%/32%† | 12% | 16%–21% | 22% | 21% | 21% |
| Loss of HBsAg | |||||||
| HBeAg + | 0%–1% | 3% | 0 | 1% | 0% | 2% | 3.2% |
| Normalization of ALT | |||||||
| HBeAg + | 7%–24% | 39% | 48% | 41%–75% | 77% | 68% | 68% |
| HBeAg - | 10%–29% | 38% | 72% | 60-79% | 74% | 78% | 76% |
| Histologic Improvement | |||||||
| HBeAg + | n/a | 38%‡ | 53% | 49%–56% | 65% | 72% | 72% |
| HBeAg - | 33% | 48% | 72% | 60%–66% | 67% | 70% | 72% |
| Durability of Response | |||||||
| HBeAg + | Na | 90% | 50%–80% | 80% | 69% | -- | |
| HBeAg - | 20% | ∼5% | <10% | na | 3% | -- | |
Some lamivudine studies used hybridization or branched chain DNA assays (lower limit of detection 20,000-200,000 IU/mL).
All other studies used PCR assays (lower limit of detection approximately 50 IU/mL).
Responses at week 48 / week 72 (24 weeks after stopping treatment).
Biopsy performed at week 72 (24 weeks after stopping treatment).
Modified from Lok 2009
Nucleos(t)ide Analogues
These oral agents can be grouped by structure and function into three groups; the L-nucleosides, acyclic phosphonates, and other.
L-nucleosides
The L-nucleosides include lamivudine, emtricitabine, and telbivudine. Lamivudine and emtricitabine are cytidine analogues while telbivudine is a thymidine analogue. They are phosphorylated intracellularly to 5′-triphosphate active metabolites. They inhibit HBV DNA polymerase by competing with natural substrates for incorporation into viral DNA, with resulting chain termination7-9. As a class, adverse events include hepatic steatosis, lactic acidosis, and hepatic flares with discontinuation of drug. They do not affect the cytochrome P450 system and do not have significant drug-drug interactions. Their bioavailability is not affected by food and all are renally excreted, requiring dose adjustments for CrCl< 50 mL/min (Table A). Lamivudine and emtricitabine are active against HIV whereas the anti-HIV activity of telbivudine is controversial 10, 11.
Lamivudine is potent but is limited by the rapid development of resistance. The 100 mg dose of lamivudine results in a peak plasma concentration of 1.28 mcg/mL ± 0.56 mcg/mL that occurs between 0.5 and 2 hours after administration. The mean half-life is 5-7 hours8.
In patients with CHB, lamivudine was associated with histologic improvement, HBeAg seroconversion, and ALT normalization in 56%, 16%, and 72% of patients, respectively. 12.
Emtricitabine, given 200 mg orally, is not FDA approved for HBV, but it has been extensively used with tenofovir in HIV/HBV coinfected patients. It reaches peak plasma concentrations of 1.8 ± 0.7 mcg/mL at 1–2 hours and has a plasma half-life of 10 hours7. It has slightly greater potency and efficacy than lamivudine but cannot be used as monotherapy due to high rates of resistance13.
Telbivudine is effective at 600 mg daily and is renally excreted unchanged. Peak plasma concentrations of 3.69 ± 1.25 mcg/mL are reached 1-4 hours after administration and it has a long intracellular half-life of 15 hours9. Unique adverse events that are uncommon include myopathy, myositis, creatine kinase elevations, and peripheral neuropathy. Although telbivudine demonstrated improved HBV DNA reductions compared to lamivudine, there was no difference in ALT normalization, HBeAg loss, or anti-HBe seroconversion14 (Table B).
Acyclic diphosphonates
The two drugs in this group are adefovir dipivoxil (adefovir) and tenofovir disoproxil fumarate (TDF) with adefovir being the least potent anti-HBV agent and TDF being one of the most potent. This potency difference is due to the achievable drug levels of these two agents at their recommended doses. They are analogues of adenosine monophosphate that undergo intracellular phosphorylation to their active metabolite, which inhibits the HBV polymerase by competitive inhibition with deoxyadenosine 5′-triphosphate, resulting in chain termination15, 16.
The major adverse effect of this class is nephrotoxicity. Adefovir was first associated with proximal renal tubular dysfunction and Fanconi's syndrome in HIV infection at doses of 60 and 120 mg daily.17, 18 Although significant creatinine elevations were absent at the 10 mg dose at 48 weeks in HBV infection19, renal impairment has been reported in long term follow-up15, 20. Thus, caution is advised in those with underlying renal dysfunction and patients taking concomitant nephrotoxic agents15, 16. Hepatic flares with discontinuation are noted in both. In addition to class adverse effects, decreased bone mineral density has been associated with TDF in HIV infection16. These agents do not affect the cytochrome P450 system.
The adefovir dose is 10 mg daily, which results in peak plasma concentrations of 0.018 ± .006 mcg/mL between 0.6-4 hours. It is unaffected by food and is renally excreted requiring dose adjustments for CrCl <50 ml/min15. Clinical trials with adefovir and placebo showed modest benefits in HBeAg-positive and -negative subjects19, 21
The TDF dose is 300 mg daily with adjustment recommended for patients with a CrCl <50 mL/min. (Table A). TDF is renally excreted with maximum serum concentrations ∼ 10× higher than adefovir (0.30 ± 0.09 mcg/mL) being achieved 1 hour after administration16. The serum elimination half-life is 17 hours whereas the intracellular half-life is 95 hours22. TDF oral bioavailability is increased after a high-fat meal.
In HIV co-infected subjects, there are significant drug interactions between TDF and atazanavir and didanosine 16. When administered with TDF, the Cmin of atazanavir is reduced by 40%; thus ritonavir should be given with atazanavir to increase atazanavir levels. When TDF and didanosine are coadminstered, the area under the curve (AUC) of didanosine increases from 14% -60% therefore, patients should not receive didanosine and TDF.
In randomized trials compared to adefovir, subjects receiving TDF had higher percentages of HBV DNA<400 copies/ml 23. In HBeAg+ subjects, the biochemical response was higher with TDF, but HBeAg seroconversion, histologic response, and durability of HBeAg seroconversion were similar between adefovir and TDF23.
Other
Currently, the only agent in this group is entecavir, a guanosine analog that is one of the most potent anti-HBV agents. Its mechanism of action is unique because it inhibits the three functions of the HBV DNA polymerase: priming of the HBV DNA polymerase, reverse transcription of the negative strand, and synthesis of the positive strand HBV DNA24.
The recommended dose is 0.5 mg for nucleoside naïve patients and 1.0 mg for patients with prior lamivudine use with dose adjustment for patients with a CrCl <50 mL/min. (Table A) Entecavir is predominantly cleared by the kidney with peak plasma concentrations of .0082 mcg/mL for the 1.0 mg dose, occurring between 0.5-1.5 hours after ingestion24. Despite low plasma concentrations entecavir is potent because of a long intracellular half-life resulting in the significant accumulation of intracellular entecavir tri-phosphate25. It should be taken on an empty stomach.
In general, side effects are mild and include headaches, diarrhea, arthralgias, and insomnia. However, a recent report documented lactic acidosis in 5 of 16 cirrhotic patients treated with entecavir. All five patients had MELD scores ≥ 20.26
In randomized trials compared to lamivudine, HBeAg+ and HBeAg-negative subjects receiving entecavir had improved histologic responses, higher percentages of HBV DNA suppression, and higher percentages of patients with either ALT normalization or improvement 27-28. In HBeAg+ subjects, there was no difference in HBeAg seroconversion rates 28.
Entecavir is active against HIV and, when given as monotherapy, can result in the HIV lamivudine resistance mutation, M184V; thus limiting HIV therapeutic options29. As with tenofovir, lamivudine, and emtricitabine, patients receiving entecavir should be tested for HIV infection. Entecavir should not be used in HIV/HBV coinfected patients with uncontrolled HIV viremia.
Potency and Resistance
Potency and the genetic barrier to resistance are the two most important considerations in deciding which agent(s) to use. The ideal drug is one that is potent and has a high barrier to resistance. Although potency is difficult to quantify, some have used a semiquantitative scale based on rapidity of viral load suppression (Figure 2).
Figure 2.
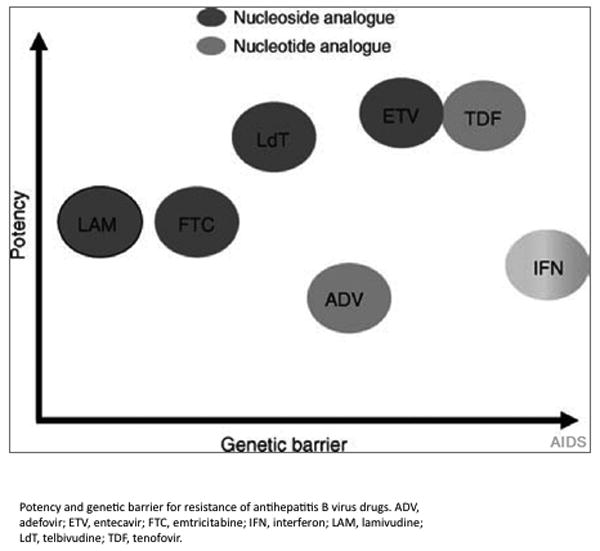
Potency and emergence of resistance. Reprinted from Soriano et al [55], with permission from Wolters Kluwer Health. ADV. adefovir; ETV, entecavir; FTC, emtricitabine; IFN, interferon; LAM, lamivudine; LdT, telbivudine; TDF, tenofovir.
The genetic barrier to resistance determines how quickly resistance develops and is qualitatively determined by the number of mutations required for resistance and the ease with which those mutations occur. Lamivudine has the lowest barrier to resistance, which develops with one mutation (rtM204V)30. Entecavir has a high barrier to resistance since at least three mutations are required31. Figure 2 illustrates the relative potency versus the relative barrier to resistance of each of the nucleos(t)ide analogues, which shows TDF and entecavir with the most favorable characteristics.
It is easiest to understand drug-resistant HBV based on the nucleos(t)ide groups above. The L-nucleosides share the primary resistance mutation, rtM204V/I. Thus, if drug-resistant HBV to one of these drugs emerges, then the virus is resistant to all others in the group. Since the rtM204V/I occurs easily, resistance rates are highest with these drugs. After four years of lamivudine monotherapy, 70%32 and 90%33 of patients with HBV monoinfection and HIV/HBV coinfection, respectively, develop the rtM204V/I. For emtricitabine, the rates of resistance in HBV monoinfection are 18% at 96 weeks, 34 and for telbivudine, they are 25% after 96 weeks in HBeAg+ patients 35.
Once the rtM204V/I emerges, compensatory mutations can develop including rtV173L and/or rtL180M, which can enhance replication fitness36. Due to overlapping reading frames, HBV Pol mutations also lead to changes in HBsAg, which may potentially lead to serious consequences. For example, the rtM204V+ rtV173L + rtL180M triple polymerase mutant leads to envelope changes that behave as a vaccine escape mutant in vitro37.
In the acyclic phosphonates, the primary adefovir resistance mutation is rtN236T while rtA181V/T has also been described. In one study, either mutation occurred in 20% of HBeAg + patients after a median of five years 38. Although viruses with rtN236T are not resistant to TDF, they have a slower response to TDF than do wild type viruses 22. Primary TDF resistance mutations have not been well defined. One study reported rtA194T as a TDF resistance mutation;39 however, this pattern was not confirmed in another study22 and was not associated with non-response to TDF in another study40. Thus, longer-term studies of patients on TDF are needed to define TDF-resistant HBV.
Resistance to entecavir requires a baseline rtM204V/I and rtL180M mutation plus either rtT184S/A/I/L, rtS202G/C, or rtM250L31. In nucleoside-naïve patients, entecavir resistance is ≤1% at 5 yrs 41, 42 while in patients with a pre-existing rtM204V/I, entecavir resistance is 51% after 5 yrs 41.
Treatment of chronic hepatitis B
The therapeutic goal is to decrease the risk of cirrhosis and hepatocellular carcinoma. Suppression of HBV replication and HBeAg seroconversion are surrogate markers of this goal. Criteria for initiation of therapy from various guidelines use HBV DNA along with an assessment of liver disease (Table C).
Table C.
Comparisons of Indications for HBV Therapy
| Patients for Whom Treatment Indicated | AASLD Guidelinesa 2009 | US guidelinesb 2008 | EASLc 2009 |
|---|---|---|---|
| HBeAg –Positive Disease | HBV DNA > 20,000 IU/ML and ALT > 2 ULN | HBV DNA >20,000 IU/Ml and elevated ALT (ULN for men 30, women 19) | HBV DNA >2000 IU/mL and/or elevated ALT and suggestive liver biopsy* |
| HBeAg- Negative Disease | HBV DNA >2000 IU/mL and ALT >2 ULN | HBV DNA >2000 IU/Ml and elevated ALT (ULN for men 30, women 19) | HBV DNA >2000 IU/mL and/or elevated ALT and suggestive liver biopsy* |
American Association for the Study of Liver Diseases (AASLD)
US Guidelines: American Gastroenterological Association (AGA)
A suggestive liver biopsy would demonstrate moderate to severe active necroinflammation and/or fibrosis.
Non-invasive markers, when validated in HBV infection, may also be utilized.
Recommendations for therapy
In treatment-naïve patients, TDF or entecavir are the preferred choices since they are potent with high genetic barriers to resistance. In patients with or at risk for renal insufficiency, entecavir is preferred. Pegylated interferon-α may be considered in patients who are noncirrhotic, have low HBV DNA, and elevated ALT. Although telbivudine is a potent agent, its resistance rate precludes its use as first-line therapy. It could be considered as a second-line agent with careful monitoring of HBV DNA levels to minimize the risk of developing resistance. Lamivudine and emtricitabine should not be used as monotherapy given the high rates of resistance. Because of its low potency adefovir is not recommended as single agent therapy.
Special Populations
HIV/HBV coinfection
Several guidelines recommend the use of combination therapy with TDF/FTC or TDF/3TC since those drugs are also included as first-line anti-HIV agents43. Entecavir should not be used unless HIV viremia is suppressed (see above). Pegylated interferon-α has not been tested in HIV-HBV co-infection but studies of standard interferon-α prior to HAART therapy demonstrated poor efficacy 44; thus pegylated interferon-α is a 2nd line option.
HBV/HCV coinfection
The recommended treatment is pegylated interferon and ribavirin as per HCV guidelines. If, after pegylated interferon discontinuation, HBV DNA is still detectable or rebounds, these patients should be subsequently treated with HBV nucleos(t)ide analogues45.
Chemotherapy/Immunosuppression
All patients receiving immunosuppression or chemotherapy, including anti-TNF-alpha agents, should be screened for HBsAg and anti-HBc. Those who are HBsAg+ should have HBV DNA determined. If criteria are met for HBV treatment, then treatment should be initiated. Those with HBV <2000 IU/ml should receive therapy during and for six months after chemotherapy completion. Those with DNA>2000 IU/ml should receive therapy until standard treatment endpoints are met. If treatment criteria are not met and HBV DNA is undetectable, then prophylaxis to prevent reactivation with lamivudine or telbivudine for short course immunosuppressive therapy (<12 months) or with tenofovir or entecavir for longer immunosuppressive therapy is recommended. Patients with anti-HBc alone or anti-HBc and anti-HBs should be monitored closely for HBV DNA elevation and treated if HBV viremia occurs6, 45, 46.
Combination therapy
Combination therapy has not consistently been associated with increased virologic suppression but decreased resistance has been demonstrated. In HBV monoinfection, adefovir with either lamivudine or emtricitabine was associated with greater HBV suppression 47, 48, but other combinations have not demonstrated this 49-50. In HIV-HBV coinfected patients naïve to therapy, TDF-lamivudine combination was superior to lamivudine monotherapy, but it was not superior to TDF monotherapy51. Similarly, while combination therapy reduces the incidence of resistance to drugs with low barriers of resistance 52, it is unknown whether this will occur with TDF or entecavir combinations as resistance rates are already low with these agents. Currently, combination therapy is recommended in HIV coinfection6, 43, 45, 46, 53, 54, in patients with drug resistance6, 45, 46, and in patients with decompensated cirrhosis 46.
Suppression with lamivudine monotherapy
Despite high resistance rates, some patients remain virologically suppressed on lamivudine monotherapy. Data to guide optimal management of these patients do not exist. Some recommend changing to a more potent agent6, such as tenofovir, which is preferred over entecavir in this situation since entecavir and lamivudine share resistance mutations. Others recommend basing the decision on the duration of lamivudine where those with two or more years of lamivudine who suppressed within 6-12 months are continued with careful evaluation for transaminitis and HBV DNA reactivation55. All others, change to tenofovir.
Management of HBV Drug Resistance
Lamivudine Resistance
The options include changing to TDF, adding TDF, or changing to TDF/emtricitabine. Some advocate the latter two from extension of adefovir studies that show 0-2% adefovir resistance 56, 57 when added to a failing lamivudine regimen compared to 21% (3/14) adefovir resistance when lamivudine is replaced by adefovir 57. Entecavir is not recommended since rates of entecavir resistance are high with pre-existing lamivudine resistance41; however, if TDF cannot be used, then it is a second-line option with careful HBV DNA monitoring.
Adefovir resistance
A change to combination TDF/lamivudine or TDF/emtricitabine should be considered. Although TDF monotherapy has been used 58, 59, in vitro evidence suggests a 3-4 fold decreased activity of TDF in this setting 22.
Entecavir resistance
Both adefovir and TDF retain activity against entecavir-resistant virus with TDF being preferred due to its higher potency. There are as yet no clinical trial data to further guide management 6.
Duration of Therapy and Follow-up
In HBeAg-positive patients, many consider cessation of therapy 6-126, 45, 46 months after eAg seroconversion. In cirrhotics, for whom rebound hepatitis can be severe, many experts continue therapy indefinitely. In HBeAg-negative patients, duration of therapy with the currently available agents should be lifelong given the high incidence of rebound viremia and transaminitis with therapy cessation60.
With the nucleos(t)ide analogs, HBV DNA should be measured at 12 and 24 weeks. If virologic suppression is achieved then HBV DNA can be monitored every 24 weeks thereafter 46. In patients with HBeAg+ CHB, HBeAg and anti-HBe should be monitored every 6 months. In addition, monitoring for hepatocellular carcinoma should occur every 6 months in high risk patients 6.
Summary
Over the last several years, several new agents have been added to the armamentarium of drugs against hepatitis B infection. Currently the optimal agents for first line therapy are entecavir, TDF, and potentially pegylated interferon in some situations. Several challenges in this field remain including the inability to eradicate a latent reservoir of HBV, emerging drug resistance, and the need to define the role of optimal combination antiviral therapy.
Acknowledgments
Financial Support: National Institutes of Health (AI071820 and AI060449 to C.L.T. and KAI066983A to DB), UCLA Center for AIDS Research, and Johns Hopkins University Center for AIDS Research.
Footnotes
Potential Conflicts of Interest: All authors: no conflicts
Contributor Information
Debika Bhattacharya, David Geffen School of Medicine at the University of California, Los Angeles; Los Angeles, CA.
Chloe L. Thio, Johns Hopkins University School of Medicine, Baltimore, MD.
References
- 1.Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486–500. doi: 10.1056/NEJMra0801644. [DOI] [PubMed] [Google Scholar]
- 2.Urban S, Schulze A, Dandri M, Petersen J. The replication cycle of hepatitis B virus. J Hepatol. 2010;52:282–4. doi: 10.1016/j.jhep.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–21. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 4.Pegasys. [package insert]. Nutley, NJ 2002;Hoffmann-La Roche.
- 5.Cooksley WGE, Piratvisuth T, Lee SD, et al. Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepat. 2003;10:298–305. doi: 10.1046/j.1365-2893.2003.00450.x. [DOI] [PubMed] [Google Scholar]
- 6.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–2. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 7.Emtriva. [package insert]. Foster City, CA 2003;Gilead Sciences, Inc.
- 8.Epivir-HBV. [package insert]. Research Triangle Park, NC 1998;GlaxoSmithKline.
- 9.Tyzeka. [package insert]. East Hanover, NJ 2006;Novartis.
- 10.Lin K, Karwowska S, Lam E, Limoli K, Evans TG, Avila C. Telbivudine exhibits no inhibitory activity against HIV-1 clinical isolates in vitro. Antimicrob Agents Chemother. doi: 10.1128/AAC.01703-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milazzo L, Caramma I, Lai A, et al. Telbivudine in the treatment of chronic hepatitis B: experience in HIV type-1-infected patients naive for antiretroviral therapy. Antivir Ther. 2009;14:869–72. doi: 10.3851/IMP1303. [DOI] [PubMed] [Google Scholar]
- 12.Lai CL, Chien RN, Leung NW, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61–8. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 13.Gish RG, Trinh H, Leung N, et al. Safety and antiviral activity of emtricitabine (FTC) for the treatment of chronic hepatitis B infection: a two-year study. J Hepatol. 2005;43:60–6. doi: 10.1016/j.jhep.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Lai CL, Gane E, Liaw YF, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576–88. doi: 10.1056/NEJMoa066422. [DOI] [PubMed] [Google Scholar]
- 15.Hepsera. [package insert]. Foster City, CA 2002;Gilead Sciences, Inc;.
- 16.Viread. [package insert]. Foster City, CA 2009;Gilead Sciences, Inc.
- 17.Fisher EJ, Chaloner K, Cohn DL, et al. The safety and efficacy of adefovir dipivoxil in patients with advanced HIV disease: a randomized, placebo-controlled trial. AIDS. 2001;15:1695–700. doi: 10.1097/00002030-200109070-00013. [DOI] [PubMed] [Google Scholar]
- 18.Tanji N, Tanji K, Kambham N, Markowitz GS, Bell A, D'agati VD. Adefovir nephrotoxicity: possible role of mitochondrial DNA depletion. Hum Pathol. 2001;32:734–40. doi: 10.1053/hupa.2001.25586. [DOI] [PubMed] [Google Scholar]
- 19.Marcellin P, Chang TT, Lim SG, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808–16. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- 20.Ha NB, Garcia RT, Trinh HN, et al. Renal dysfunction in chronic hepatitis B patients treated with adefovir dipivoxil. Hepatology. 2009;50:727–34. doi: 10.1002/hep.23044. [DOI] [PubMed] [Google Scholar]
- 21.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med. 2005;352:2673–81. doi: 10.1056/NEJMoa042957. [DOI] [PubMed] [Google Scholar]
- 22.Delaney WE, Ray AS, Yang H, et al. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob Agents Chemother. 2006;50:2471–7. doi: 10.1128/AAC.00138-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–55. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 24.Baraclude. [package insert]. Princeton, NJ 2005;Bristol-Myers Squibb Company.
- 25.Levine S, H D, Yamanaka G, Zhang S, Rose R, Weinheimer S, Colonno RJ. Efficacies of Entecavir against Lamivudine-Resistant Hepatitis B Virus Replication and Recombinant Polymerases In Vitro. Antimicrob Agents Chemother. 2002;46:2525–32. doi: 10.1128/AAC.46.8.2525-2532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lange CM, Bojunga J, Hofmann WP, et al. Severe lactic acidosis during treatment of chronic hepatitis B with entecavir in patients with impaired liver function. Hepatology. 2009;50:2001–6. doi: 10.1002/hep.23346. [DOI] [PubMed] [Google Scholar]
- 27.Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–20. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 28.Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–10. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 29.McMahon MA, Jilek BL, Brennan TP, et al. The HBV drug entecavir - effects on HIV-1 replication and resistance. N Engl J Med. 2007;356:2614–21. doi: 10.1056/NEJMoa067710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen MI, Deslauriers M, Andrews CW, et al. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Lamivudine Clinical Investigation Group. Hepatology (Baltimore, Md) 1998;27:1670–7. doi: 10.1002/hep.510270628. [DOI] [PubMed] [Google Scholar]
- 31.Tenney DJ, Levine SM, Rose RE, et al. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to Lamivudine. Antimicrob Agents Chemother. 2004;48:3498–507. doi: 10.1128/AAC.48.9.3498-3507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lok ASF, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714–22. doi: 10.1053/j.gastro.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 33.Benhamou Y, Bochet M, Thibault V, et al. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology (Baltimore, Md) 1999;30:1302–6. doi: 10.1002/hep.510300525. [DOI] [PubMed] [Google Scholar]
- 34.Gish RG, Trinh H, Leung N, et al. Safety and antiviral activity of emtricitabine (FTC) for the treatment of chronic hepatitis B infection: a two-year study. J Hepatol. 2005;43:60–6. doi: 10.1016/j.jhep.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Liaw YF, Gane E, Leung N, et al. 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486–95. doi: 10.1053/j.gastro.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 36.Fu L, Cheng YC. Role of additional mutations outside the YMDD motif of hepatitis B virus polymerase in L(-)SddC (3TC) resistance. Biochem Pharmacol. 1998;55:1567–72. doi: 10.1016/s0006-2952(98)00050-1. [DOI] [PubMed] [Google Scholar]
- 37.Sheldon J, Ramos B, Garcia-Samaniego J, et al. Selection of hepatitis B virus (HBV) vaccine escape mutants in HBV-infected and HBV/HIV-coinfected patients failing antiretroviral drugs with anti-HBV activity. J Acquir Immune Defic Syndr. 2007;46:279–82. doi: 10.1097/qai.0b013e318154bd89. [DOI] [PubMed] [Google Scholar]
- 38.Marcellin P, Chang TT, Lim SGL, et al. Long-term efficacy and safety of adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. Hepatology (Baltimore, Md) 2008;48:750–8. doi: 10.1002/hep.22414. [DOI] [PubMed] [Google Scholar]
- 39.Sheldon J, Camino N, Rodés B, et al. Selection of hepatitis B virus polymerase mutations in HIV-coinfected patients treated with tenofovir. Antivir Ther (Lond) 2005;10:727–34. [PubMed] [Google Scholar]
- 40.Fung S, M T, Sherman M, Popovic V. Tenofovir (TDF) Is Effective In Lamivudine (LAM)-Resistant Chronic Hepatitis B Patients Who Harbour rtA194T At Baseline. 60th Annual Meeting of the American Association for the Study of Liver Diseases; Boston, MA. 2009. [Google Scholar]
- 41.Tenney DJ, Rose RE, Baldick CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology (Baltimore, Md) 2009;49:1503–14. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 42.Chang TT, Lai CL, Kew Yoon S, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology (Baltimore, Md) 2010;51:422–30. doi: 10.1002/hep.23327. [DOI] [PubMed] [Google Scholar]
- 43.Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents – December 1, 2009. 2009. pp. 1–168. [Google Scholar]
- 44.Di Martino V, Thevenot T, Colin JF, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology. 2002;123:1812–22. doi: 10.1053/gast.2002.37061. [DOI] [PubMed] [Google Scholar]
- 45.EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–42. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Keeffe EB, Dieterich DT, Han SH, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6:1315–41. doi: 10.1016/j.cgh.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 47.Sung JJY, Lai JY, Zeuzem S, et al. Lamivudine compared with lamivudine and adefovir dipivoxil for the treatment of HBeAg-positive chronic hepatitis B. J Hepatol. 2008;48:728–35. doi: 10.1016/j.jhep.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 48.Hui CK, Zhang HY, Bowden S, et al. 96 weeks combination of adefovir dipivoxil plus emtricitabine vs. adefovir dipivoxil monotherapy in the treatment of chronic hepatitis B. J Hepatol. 2008;48:714–20. doi: 10.1016/j.jhep.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 49.Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–95. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 50.Lai CL, Leung N, Teo EK, et al. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology. 2005;129:528–36. doi: 10.1016/j.gastro.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 51.Matthews GV, Avihingsanon A, Lewin SR, et al. A randomized trial of combination hepatitis B therapy in HIV/HBV coinfected antiretroviral naïve individuals in Thailand. Hepatology. 2008;48:1062–9. doi: 10.1002/hep.22462. [DOI] [PubMed] [Google Scholar]
- 52.Dore GJ, Cooper DA, Pozniak AL, et al. Efficacy of tenofovir disoproxil fumarate in antiretroviral therapy-naive and -experienced patients coinfected with HIV-1 and hepatitis B virus. J Infect Dis. 2004;189:1185–92. doi: 10.1086/380398. [DOI] [PubMed] [Google Scholar]
- 53.Rockstroh JK, Bhagani S, Benhamou Y, et al. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. HIV Med. 2008;9:82–8. doi: 10.1111/j.1468-1293.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 54.Soriano V, Puoti M, Peters M, et al. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV-Hepatitis B Virus International Panel. AIDS. 2008;22:1399–410. doi: 10.1097/QAD.0b013e3282f8b46f. [DOI] [PubMed] [Google Scholar]
- 55.Lok A. Overview of the management of chronic hepatitis B and case examples. In: Basow DS, editor. UpToDate. Waltham, MA: 2010. [Google Scholar]
- 56.Yatsuji H, Suzuki F, Sezaki H, et al. Low risk of adefovir resistance in lamivudine-resistant chronic hepatitis B patients treated with adefovir plus lamivudine combination therapy: two-year follow-up. J Hepatol. 2008;48:923–31. doi: 10.1016/j.jhep.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 57.Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology (Baltimore, Md) 2007;45:307–13. doi: 10.1002/hep.21534. [DOI] [PubMed] [Google Scholar]
- 58.van Bömmel F, Zöllner B, Sarrazin C, et al. Tenofovir for patients with lamivudine-resistant hepatitis B virus (HBV) infection and high HBV DNA level during adefovir therapy. Hepatology (Baltimore, Md) 2006;44:318–25. doi: 10.1002/hep.21253. [DOI] [PubMed] [Google Scholar]
- 59.Schildgen O, Sirma H, Funk A, et al. Variant of hepatitis B virus with primary resistance to adefovir. N Engl J Med. 2006;354:1807–12. doi: 10.1056/NEJMoa051214. [DOI] [PubMed] [Google Scholar]
- 60.Fung SK, Wong F, Hussain M, Lok AS. Sustained response after a 2-year course of lamivudine treatment of hepatitis B e antigen-negative chronic hepatitis B. J Viral Hepat. 2004;11:432–8. doi: 10.1111/j.1365-2893.2004.00556.x. [DOI] [PubMed] [Google Scholar]


