Abstract
There is increasing recognition that treatment failure in cancer may be associated with the failure to sterilize a small subpopulation of tumor cells that have been characterized as tumor stem cells. Defined as cells that are able to self-renew and also to replenish a phenotypically diverse tumor-cell population, such cells are also considered resistant to chemotherapy. These characteristics are optimal for targeting by using alpha-particle-emitting radionuclides. Because of their high-energy deposition density per track, alpha-particles are capable of targeting single cells or small clusters of cells with minimal normal organ toxicity. The DNA damage induced by alpha-particles is largely irreparable and, therefore, alpha-particle-induced damage is minimally susceptible to resistance mechanisms. In this work, theoretical modeling was performed to examine the potential of alpha-emitter targeting of such small clusters of cancer stem cells. Critical parameters influencing efficacy and toxicity were identified and their relationship elucidated. The results identify specific activity, antigen site density, and number of target cells as critical parameters for effective cell killing and demonstrate substantial efficacy gains by targeting a smaller number of stem cells, as opposed to the entire tumor-cell population.
Keywords: radioimmunotherapy, alpha-particle, 213Bi, treatment planning, modeling
INTRODUCTION
Small subpopulations of tumor cells that are able to self-renew and also to reconstitute the heterogeneous tumor-cell population have recently been identified in leukemia and in breast and brain cancer.1–3 There is also evidence that (CD44+CD24−) stem cells may be involved in the widespread metastatic dissemination of breast cancer.4,5 These findings, along with the observation that for solid tumors only a small proportion of cells are clonogenic, in vitro and in vivo,6 have led to the suggestion that treatment failure in cancer may be associated with the failure to eradicate cancer stem cells.7 Stem cells are less sensitive to antiproliferative agents; they are also less susceptible to apoptosis and to immune recognition.8
Given the properties outlined above, tumor stem cells are ideal targets for targeted alpha-particle therapy. Alpha-particle-emitting radio-nuclides have been the subject of considerable investigation as cancer therapeutics.9–12 Alpha-particles have the advantages of high potency and specificity. These advantages arise from the highly ionizing track and short path length of the emitted helium nucleus in tissue. The practical implication of this, and the distinction between alpha-particles and the more widely used beta-particle emitters for targeted radionuclide therapy, is that it is possible to sterilize individual tumor cells solely from self-irradiation with alpha-particle emitters, while this is generally not possible with beta-particle emitters given achievable antibody-specific activity, tumor cell antigen expression levels, and the need to avoid prohibitive toxicity.13,14 Alpha-particle-mediated targeting is also not susceptible to chemomodulation and to the majority of resistance mechanisms seen in chemo- or radiotherapy.15,16
In this work, a mathematical model was used to examine the treatment parameters that would most influence the alpha-particle therapy of rapidly accessible, disseminated tumor stem cells (e.g., as found in the bone marrow of breast cancer patients). The alpha-particle emitter, 213Bi (T1/2 = 45.6 minutes), is investigated. A recent report has demonstrated that most early disseminated cancer cells in the bone marrow of breast cancer patients have a breast cancer stem cell phenotype.4 Such rapidly accessible cells are ideal targets for a short-lived alpha-emitter such as 213Bi.
MATERIALS AND METHODS
Efficacy Modeling
A key parameter for evaluating the tumor cell kill is the number of decays, or Bq-s, per target cell. An estimate of this would provide information regarding the likelihood of effective cell killing for a population of single, isolated cells, meaning cells that are irradiated only from emissions on their surface as is especially relevant in targeting stem cells. This can be estimated as shown in Equations 1–3:
| (1) |
| (2) |
| (3) |
where Ncell is the number of disintegrations per cell, Ṅcell is the disintegration rate per cell, Ag0 is the number of target sites (receptors or antigens) per cell, SA0 is the initial specific activity of the targeting agent, T1, T2 are the estimated start and end times that define the time interval during which activity is target cell associated and λ is the physical decay-rate constant of the radionuclide (= ln(2)/radionuclide half-life).
Given the short half-life of the radionuclide and the rapid targeting of disseminated tumor cells following an intravenous injection of radiolabeled antibody,17 the simplifying assumption is made that all administered activity decays occur on the target cells (T1 = 0; T2 = ∞). Using these values for T1 and T2, Equation 1 reduces to that shown in Equation 4:
| (4) |
Ag0 is typically obtained by Scatchard analysis as the maximum number of binding sites on the cell surface. The initial specific activity, SA0, is typically expressed as radioactivity per mass amount of the targeting agent (e.g., µCi or Bq per g or mg).
As noted above, this upper limit on the number of disintegrations per cell applies only for a cell in isolation. This is a worst-case approximation, since, depending upon the range of the emitted alpha-particle and the cellular geometry of the target tumor stem cells, cross-fire from adjacent cells may be expected. Once the number of cell-associated decays is known, then the absorbed dose to the nucleus (Dn) or to the whole cell from a surface (cs) and/or cytosolic (cy) distribution of the total number of decays, Ã, is given by Equation 5:
| (5) |
with S(n ← cs) and S(n ← cy) obtained from tabulated cell dose conversion factors.18,19
The estimated absorbed dose to the nucleus can then be used with an estimate of the radiosensitivity (D0) of the cell line to calculate the surviving fraction (SF) and from this the tumor control probability (TCP) for a given number of target cells (n), as shown in Equations 6 and 7:
| (6) |
| (7) |
Equation 7 is derived by assuming that the probability distribution for stem-cell survival is given by Poisson statistics and reflects the probability of 0 cells surviving when the average number surviving is n · SF. If, for a given number of initial target cells, n, the surviving fraction is such that, on average, only single cells are expected to survive, then the probability of no cell surviving is 0.37. If the therapeutic efficacy of the approach is such that 10 times n cells could be reduced to 1 (i.e., efficacy is improved by a factor of 10 giving, n · SF = 0.1), then the TCP would be 0.9.
Recently, the nonuniformity in antigen expression was identified as having an important impact on the tumor-control probability.20 The effect of accounting for a variable antigen distribution, compared to a single value for Ag0, was examined by assuming Ag0 to follow a normal distribution with a mean density of 1 or 2 × 105. Ten percent (10%) and 50% standard deviations were simulated. For each cell, n(i), of n (n from 10 to 10,000) cells, the number of antigens on the cell surface, Ag(i), was randomly sampled from the normal distribution by using the Box-Muller algorithm.21 For each cell number, n, 100 runs were executed. Number of disintegrations per cell, N(i), and surviving fraction of each cell, SF(i), were computed following the algorithm outlined above. Tumor control probability (TCP) for n target cells becomes that shown in Equation 8:
| (8) |
Toxicity Modeling
The approach outlined above may also be used to provide approximate potential hematologic toxicity. If the analysis is confined to saturable receptor/ligand systems (e.g., antibody/antigen), the first step is to estimate the number of free antibody molecules (i.e., not bound to tumor-cell-associated antigen) in the circulation (Ab). Given an estimate of total target cell number (n), the target sites per cell (Ag0), and knowing the amount of antibody administered (Ab0), it is possible to approximate Ab by using the molecular weight (MW) of antibody and Avogadro’s number (AV), as shown in Equation 9:
| (9) |
By equating this to the initial amount in circulation, multiplying by the initial specific activity and assuming an effective clearance rate from circulation (λeff), the total number of decays that are in the circulation (Ãcirc) can be obtained from Equation 10:
| (10) |
Note that the mass amount of antibody administered is almost always greater than that required to saturate the antigen sites on a small population of tumor cells. Although in theory, Equation 9 could give a negative result, in practice this will not be the case.
Once the total number of radionuclide decays in the circulation has been determined, a decay concentration may be obtained by dividing by the distribution volume of the antibody (Vd). Assuming that the extracellular fluid space of the marrow is in equilibrium with this volume,22 we can get the total number of decays in the red marrow (ÃRM), which is the dose-limiting organ in almost all cases of targeted radionuclide therapy, as shown in Equation 1123
| (11) |
with RMECFF as the red marrow extracellular fluid fraction and VRM as the red marrow volume.
Equation 11 is derived from the assumption that the antibody does not specifically target cellular components of the bone or red marrow.22 The distribution volume, Vd, will depend upon the properties and molecular weight of the targeting agent; for an intact antibody, this is typically the plasma and the extracellular fluid space of the reticuloendothelial organs, liver, spleen, and marrow.24
A first-order estimate of the absorbed dose to the red marrow (DRM) may then be obtained using Equation 12:
| (12) |
with Δα as the energy emitted by the alpha-emitter in the form of alpha-particles, φα as the fraction of Δα that is absorbed by the red marrow, and MRM as the red marrow mass.
Note that in Equation 12, the photon- and electron-dose contributions are assumed to be negligible relative to the alpha-particle contribution. It is important to note that the methodology outlined above is a first-order approximation appropriate for feasibility analysis.
Parameter Values
An antigen cell-surface expression of 1 or 2 × 105 sites/cell was assumed; these values are, conservatively, lower by approximately a factor of 10 than the expression of HER2/neu on HER2/neu-expressing breast cancer cells.25,26 A specific activity of 440 MBq 213Bi/mg antibody was chosen to be consistent with that achievable clinically. 27,28 A breast cancer stem cell is modeled as two concentric spheres, with a nuclear radius of 4 µm and a cellular radius of 5 µm. The radiosensitivity is assumed to be 0.4 Gy26; the half-life of 213Bi is 45.6 minutes. The relevant parameter values are listed in Table 1.
Table 1.
Parameter Values for Model Simulations
| Parameter | Parameter value | Reference |
|---|---|---|
| Efficacy modeling | ||
| Ag0 | 1 or 2 × 105 sites/cell | 25, 26 |
| SA0 | 440 MBq/mg antibody | 27, 28 |
| RN | 4 µm | Chosen value |
| RC | 5 µm | Chosen value |
| D0 | 0.4 Gy | 26 |
| S(n ← cs) | 3.76 × 10−2 Gy/Bq-s | 19 |
| S(n ← cy) | 4.99 × 10−2 Gy/Bq-s | 19 |
| Toxicity modeling | ||
| n | 10,000 stem cells | Varied |
| Ab0 | 2.5 mg | Varied |
| Vd | 3.8 L | 24 |
| VRM | 1.5 L | (=MRM × 1 g/cc) |
| Δα | 1.33 × 10−2 Gy-kg/Bq-s | 34 |
| RMECFFF | 0.19 | 22 |
| MRM | 1.5 kg | 29 |
| ϕα | 1.0, 0.57 | Assumed, 31, 32 |
| λeff | 2.53 × 10−4 s−1 | (=ln(2)/213Bi T1/2) |
RESULTS
Using the molecular weight of intact antibody (= 150,000 daltons) and Avogadro’s number (6.023 × 1023), and replacing for λ (= ln(2)/T1/2), the first factor in Equation 4 gives the number of decays (or equivalently, the number of 213Bi atoms) per molecule.
Multiplying by the number of sites per cell, we get:
If we assume that antigen-bound antibody internalizes rapidly and that half of these decays occur in the cytosol and the remainder on the cell surface, then, using Equation 5, we get an absorbed dose to the nucleus of:
Using Equation 6, the surviving fraction is:
Using Equations 4–7, the tumor-control probability for different target cell numbers and antigen densities can be calculated. Results are depicted in Table 2. The analysis reveals the exquisite sensitivity of tumor control to the number of cell-associated decays. Note that the 2-fold increase in antigen-site density is equivalent to increasing the specific activity by a factor of 2. If variability in antigen site density across the cell population is considered, the results will depend upon the extent of the variability. A 10% standard deviation about the mean value gives results similar to those obtained, assuming no variation. If a 50% standard deviation is assumed, the tumor control probability is substantially reduced for all cell numbers considered. The number of cell-associated decays is one of the most important parameters in targeted alpha-emitter therapy.
Table 2.
Tumor Control Probability (TCP) for Different Target Cells and Two Different Cell-Surface Antigen Densities
| TCP | ||||
|---|---|---|---|---|
| n (cell number) | 10 | 100 | 1000 | 10,000 |
| 105 sites/cell | 0.92 | 0.43 | 2.2 × 10−4 | 2.2 × 10−37 |
| 105 sites/cell ± 10%a | 0.90 | 0.37 | 5.3 × 10−5 | 1.6 × 10−43 |
| 105 sites/cell ± 50% | 0.47 | 1.4 × 10−3 | 3.7 × 10−32 | 0.00 |
| 2 × 105 sites/cell | 1.00 | 0.99 | 0.93 | 0.49 |
| 2 × 105 sites/cell ± 10% | 1.00 | 0.99 | 0.89 | 0.30 |
| 2 × 105 sites/cell ± 50% | 0.66 | 3.4 × 10−2 | 2.3 × 10−25 | 0.00 |
Percent standard deviation for normally distributed antigen density.
The potential toxicity will depend upon the number of target cells. If we consider the case of a patient with 104 rapidly accessible disseminated tumor stem cells (2 × 105 sites/cell), the patient is administered 2.5 mg of 213Bi-labeled antibody. As a first-order calculation, rapid binding of all antigen sites is assumed and, given the 45-minutes half-life of 213Bi, loss only owing to physical decay is considered. Under these assumptions, the total number of antigen-bound antibody molecules is 2 × 109, a negligible fraction of the 1.0 × 1016 antibody molecules was administered. Correspondingly, the number of decays in circulation is 5.2 × 1012 Bq-s. Using Equation 11, 3.9 × 1011 decays are distributed in the red marrow. The mean absorbed dose to the marrow, obtained from Equation 12, is 0.35 Gy, for 1.3 GBq (36 mCi) of 213Bi administered.
This estimate of red marrow absorbed dose was obtained, assuming all alpha-particle energy was absorbed in the red marrow (φα = 1). If the microarchitecture of the marrow (pelvis) is considered and a cellularity of 50%, which is typical of 30–50-year-old adults is used,29,30 φα = 0.57 for 213Bi alpha-particle emissions,31,32 and the mean red marrow absorbed dose is 0.1 Gy. To relate this value to red marrow toxicity experience with beta-particle emitters or with external beam, the dose estimate should be multiplied by a relative biologic effectiveness (RBE) value. The limited human data on red marrow RBE suggests a factor between 1.5 and 1.9.32
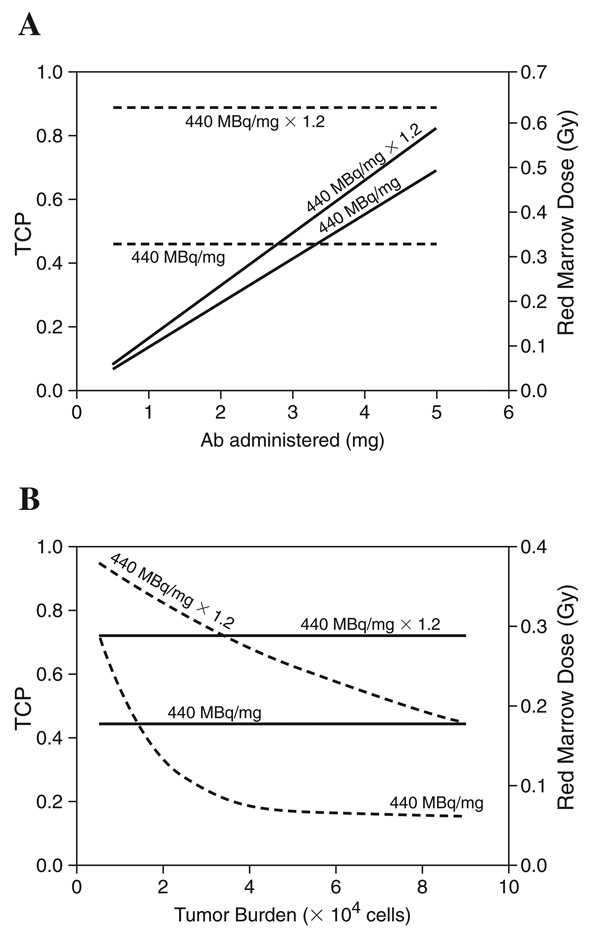
The estimated red marrow absorbed dose will depend upon the amount of radionuclide that decays in circulation, and this depends on the amount of antibody administered relative to the number of accessible antigen sites. Reducing the amount of antibody administered will also reduce the red marrow absorbed dose; a 1.25-mg antibody administration gives half the estimated mean red marrow absorbed dose. The relationship between antibody-administered specific activity and tumor burden on treatment efficacy and red marrow toxicity is summarized in Figure 1A and 1B.
Figure 1.
(A) The impact of changing antibody administered and specific activity on tumor control probability (TCP) and red marrow absorbed dose. The solid lines correspond to red marrow dose (right axis). The dashed lines to TCP (left axis). The simulation was performed for 104 tumor cells and an antigen density of 2 × 105 sites/cell. (B) The impact of tumor burden and specific activity on TCP and red marrow absorbed dose. The solid lines correspond to red marrow dose (right axis), the dashed lines to TCP (left axis). The simulation was performed for a 2.5-mg antibody administered and a tumor-cell antigen density of 2 × 105. The red marrow absorbed dose plotted in both figures was obtained by using φ = 1 for alpha-particles. To account for the effect of cellularity, the values shown on the red marrow absorbed dose axis should be multiplied by 0.28.
Figure 1A shows that for a given tumor burden, increasing the amount of administered antibody and, therefore, the administered activity increases the red marrow absorbed dose without increasing therapeutic efficacy. This is because beyond a certain administered amount, the tumor sites are saturated, and additional antibody will not bind tumor cells but rather increase the activity in circulation. Increasing the specific activity and, therefore, the number of alphas delivered to each tumor cell, however, increases efficacy considerably at a minimum increase in potential toxicity. Figure 1 shows that a 20% increase in specific activity raises the tumor control probability (TCP) from 0.46 to 0.89, while the increase in red marrow absorbed dose is less than 30%. Figure 1B demonstrates that TCP increases as the tumor stem cell burden drops. The reduction in tumor burden does not increase the red marrow dose when the administered antibody is kept constant. This is because the tumor-bound antibody is already a very small fraction of the total administered.
Figure 1A and 1B illustrates the relationships between parameters and highlight those parameters having the greatest impact on efficacy and toxicity, rather than on specifically predicting success or failure of a particular strategy. Nevertheless, the results suggest that increasing the specific activity, or equivalently, targeting a cell-surface antigen that is highly expressed, will have the greatest impact on tumor control. On the other hand, increasing administered activity is likely to increase toxicity without improving tumor control. It is important to note that these conclusions are likely to be specific to a short-lived alpha-emitter, such as 213Bi. This is because, in the case of rapidly accessible tumor stem cells, the short half-life reduces the impact of antibody pharmacokinetics on the results obtained.
DISCUSSION
Cancer stem cells have been reported for brain, breast, and hematologic malignancies. The available evidence suggests that cancer stem cells are resistant to chemo- and radiotherapy. The double- stranded DNA damage induced by direct alpha-particle traversal of the nucleus is minimally repaired and, therefore, such damage is less susceptible to resistance mechanisms. Although this remains to be determined, it is highly unlikely that cancer stem cells will be resistant to alpha-particle-induced damage.
The results highlight the potential advantages in targeting stem cells. As shown by the analysis, the tumor-control probability is heavily influenced by the number of target cells. Because stem cells are thought to be a small fraction of the overall tumor-cell population, there is a large advantage in targeting only the stem-cell population. The results also reveal the sensitivity to antigen-site density, a factor of 2 increase in antigen-site density (or equivalently, occupancy fraction or specific activity) leads to a highly disproportionate increase in TCP.
It is important to note that the calculations described above reflect the worst case of an isolated cell that is subject only to self-irradiation and the best case that all of the target sites on the cell surface will be occupied by the delivery molecule. Whether these conditions are met will depend upon the geometry of the cancer, for example, in the targeting of leukemia stem cells, the assumptions are a reasonable representation of what might happen. In the targeting of metastatic tumor stem cells within tumor clusters, the results will be a balance between the fraction of sites occupied and the cross-fire from one cell to another. If the cells are clustered together, then the assumption of 100% target antigen occupancy is optimistic; on the other hand, clustering will increase cross-fire and the assumption of only self-irradiation is pessimistic. In the toxicity calculations, cross-reactivity with normal tissues was not included. Cross-reactivity with marrow cellular components would preclude the use of this strategy, since direct targeting of marrow would be expected to lead to prohibitive marrow toxicity. Cross-reactivity with other normal organs is less of a concern, since the 24–48-hour time required for antibody extravasation into intact normal organs would lead to negligible alpha-particle irradiation of such organs, since the extravasation time is far greater than the 45.6-minutes half-life of 213Bi. It is also important to note that a microdosimetric calculation was not performed. In most cases, given a sufficient number of cell-associated decays, microdosimetry will not be necessary and the use of cellular S values is acceptable.
The theoretical analysis is predicated on identifying a target antigen that is specific to, or preferentially expressed on, cancer stem cells. As noted above, cancer stem-cell-related markers have been identified and have been used to identify populations of putative cancer stem cells. Since most of these markers are not exclusively expressed on cancer stem cells, subsequent confirmation is required by assaying the ability of the cell population to induce cancer xenografts with a small number of injected cells. Efforts to identify specific targetable antigens have begun only recently.33
CONCLUSIONS
A theoretical analysis has been performed to examine the possibility of targeting cancer stem cells using targeted alpha-particle therapy. The analysis suggests that this would be a viable strategy for a small population of cells that express a target distinct from other tumor cells or hematopoietic tissues.
ACKNOWLEDGMENTS
This work was supported by NIH/NCI grant R01 CA113797. HS is supported by a Multidisciplinary Postdoctoral Fellowship from the Department of Defense (grant no. BC044176).
REFERENCES
- 1.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 2.Al Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 4.Balic M, Lin H, Young L, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12:5615. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 5.Wicha MS. Cancer stem cells and metastasis: Lethal seeds—commentary. Clin Cancer Res. 2006;12:5606. doi: 10.1158/1078-0432.CCR-06-1537. [DOI] [PubMed] [Google Scholar]
- 6.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 7.Al Hajj M, Becker MW, Wichal M, et al. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43. doi: 10.1016/j.gde.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Costello RT, Mallet F, Gaugler B, et al. Human acute myeloid leukemia CD34(+)/CD38(−) progenitor cells have decreased sensitivity to chemotherapy and fas-induced apoptosis, reduced immunogenicity, and impaired dendritic cell transformation capacities. Cancer Res. 2000;60:4403. [PubMed] [Google Scholar]
- 9.Bloomer WD, McLaughlin WH, Neirinckx RD, et al. Astatine-211—tellurium radiocolloid cures experimental malignant ascites. Science. 1981;212:340. doi: 10.1126/science.7209534. [DOI] [PubMed] [Google Scholar]
- 10.Macklis RM, Kinsey BM, Kassis AI, et al. Radioimmunotherapy with alpha-particle emitting immunoconjugates. Science. 1988;240:1024. doi: 10.1126/science.2897133. [DOI] [PubMed] [Google Scholar]
- 11.Zalutsky MR, Garg PK, Friedman HS, et al. Labeling monoclonal antibodies and F(ab′)2 fragments with the alpha-particle-emitting nuclide astatine-211: Preservation of immunoreactivity and in vivo localizing capacity. Proc Natl Acad Sci USA. 1989;86:7149. doi: 10.1073/pnas.86.18.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDevitt MR, Ma D, Lai LT, et al. Tumor therapy with targeted atomic nanogenerators. Science. 2001;294:1537. doi: 10.1126/science.1064126. [DOI] [PubMed] [Google Scholar]
- 13.Sgouros G. Radioimmunotherapy of micrometastases. In: Riva P, editor. Cancer Radioimmunotherapy—Present and Future. Newark, NJ: Harwood Academic; 1998. p. 191. [Google Scholar]
- 14.McDevitt MR, Sgouros G, Finn RD, et al. Radioimmunotherapy with alpha-emitting nuclides. Eur J Nucl Med. 1998;25:1341. doi: 10.1007/s002590050306. [DOI] [PubMed] [Google Scholar]
- 15.Barendsen GW, Walter HMD. Effects of different ionizing radiations on human cells in tissue culture. 4. Modification of radiation damage. Radiat Res. 1964;21:314. [PubMed] [Google Scholar]
- 16.Friesen C, Glatting G, Koop BC, et al. Breaking chemoresistance and radioresistance with [213Bi]anti-CD45 antibodies in leukemia cells. Cancer Res. 2007;67:1950. doi: 10.1158/0008-5472.CAN-06-3569. [DOI] [PubMed] [Google Scholar]
- 17.Song H, Shahverdi K, Fox J, et al. Targeting HER2/neu expressing micrometastases in HER2/neu transgenic mice using alpha-particle-emitting monoclonal antibody [(BI)-B-213-7.16.4] Cancer Biother Radiopharm. 2006;21:396. [Google Scholar]
- 18.Goddu SM, Howell RL, Bouchet LG, et al. MIRD cellular S values. Reston, VA: Society of Nuclear Medicine; 1997. [Google Scholar]
- 19.Hamacher KA, Den RB, Den EI, et al. Cellular dose conversion factors for alpha-particle-emitting radionuclides of interest in radionuclide therapy. J Nucl Med. 2001;42:1216. [PubMed] [Google Scholar]
- 20.Kvinnsland Y, Stokke T, Aurlien E. Radioimmunotherapy with alpha-particle emitters: Microdosimetry of cells with a heterogeneous antigen expression and with various diameters of cells and nuclei. Radiat Res. 2001;155:288. doi: 10.1667/0033-7587(2001)155[0288:rwapem]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Press WH, Teukolsky SA, Vetterling WT, et al. Numerical Recipe in Fortran 77: The Art of Scientific Computing. Vol. 2001. Cambridge University Press; [Google Scholar]
- 22.Sgouros G. Bone marrow dosimetry for radioimmunotherapy: Theoretical considerations. J Nucl Med. 1993;34:689. [PubMed] [Google Scholar]
- 23.Junghans RP, Sgouros G, Scheinberg DA. Antibody-based immunotherapies of cancer. In: Chabner BA, Longo DL, editors. Cancer Chemotherapy and Biotherapy. Philadelphia, PA: Lippincott-Raven; 1996. p. 655. [Google Scholar]
- 24.Sgouros G, Graham MC, Divgi CR, et al. Modeling and dosimetry of monoclonal antibody M195 (anti-CD33) in acute myelogenous leukemia. J Nucl Med. 1993;34:422. [PubMed] [Google Scholar]
- 25.Shepard HM, Lewis GD, Sarup JC, et al. Monoclonal antibody therapy of human cancer: Taking the HER2 protooncogene to the clinic. J Clin Immunol. 1991;11:117. doi: 10.1007/BF00918679. [DOI] [PubMed] [Google Scholar]
- 26.Ballangrud AM, Yang WH, Palm S, et al. Alpha-particle emitting atomic generator (actinium-225)-labeled trastuzumab (Herceptin) targeting of breast cancer spheroids: Efficacy versus HER2/neu expression. Clin Cancer Res. 2004;10:4489. doi: 10.1158/1078-0432.CCR-03-0800. [DOI] [PubMed] [Google Scholar]
- 27.Jurcic JG, Larson SM, Sgouros G, et al. Targeted alpha particle immunotherapy for myeloid leukemia. Blood. 2002;100:1233. [PubMed] [Google Scholar]
- 28.McDevitt MR, Finn RD, Ma D, et al. Preparation of alpha-emitting 213Bi-labeled antibody constructs for clinical use. J Nucl Med. 1999;40:1722. [PubMed] [Google Scholar]
- 29.Snyder WS, Cook MJ, Nasset ES, et al. Report of the task group on reference man, ICRP publication 23. Elmsford, NY: International Commission on Radiological Protection; 1975. [Google Scholar]
- 30.Aladhadh AN, Cavill I. Assessment of cellularity in bone-marrow fragments. J Clin Pathol. 1983;36:176. doi: 10.1136/jcp.36.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watchman CJ, Jokisch DW, Patton PW, et al. Absorbed fractions for alpha-particles in tissues of trabecular bone: Considerations of marrow cellularity within the ICRP reference male. J Nucl Med. 2005;46:1171. [PubMed] [Google Scholar]
- 32.Sgouros G, Bolch WE, Shah A, et al. Relative biological effectiveness for efficacy and toxicity in leukemia patients of the alpha-particle emitter, bismuth-213. Cancer Biother Radiopharm. 2006;21:397. [Google Scholar]
- 33.Schulenburg A, Ulrich-Pur H, Thurnher D, et al. Neoplastic stem cells: A novel therapeutic target in clinical oncology. Cancer. 2006;107:2512. doi: 10.1002/cncr.22277. [DOI] [PubMed] [Google Scholar]
- 34.Sgouros G, Ballangrud AM, Jurcic JG, et al. Pharmacokinetics and dosimetry of an alpha-particle-emitter labeled antibody: 213Bi-HuM195 (anti-CD33) in patients with leukemia. J Nucl Med. 1999;40:1935. [PubMed] [Google Scholar]