Abstract
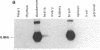
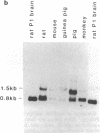
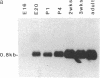
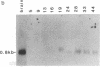
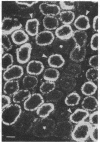
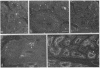
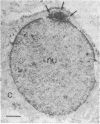
Expression of the gene encoding the neurotransmitter/neuromodulator cholecystokinin (CCK) was demonstrated in testis of several different species. Two testicular CCK mRNA transcripts of different sizes were detected, and studies on the ontogeny of CCK gene expression indicated that the gene was expressed in male germ cells. In situ hybridization revealed CCK mRNA-expressing cells in the peripheral parts of the seminiferous tubules. Biochemical identification showed that the majority of prepro-CCK products in the testis represented pro-CCK. Immunofluorescence studies revealed CCK-like peptides primarily in spermatocytes and spermatids of mouse, rat, and monkey. Immuno electron microscopy of monkey testis demonstrated CCK immunoreactivity in the acrosomal granule of spermatids. Hence, an interesting possibility is that CCK peptides can be released during the acrosome reaction and thus may be of importance in the fertilization process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barden N., Mérand Y., Rouleau D., Moore S., Dockray G. J., Dupont A. Regional distributions of somatostatin and cholecystokinin-like immunoreactivities in rat and bovine brain. Peptides. 1981 Fall;2(3):299–302. doi: 10.1016/s0196-9781(81)80123-4. [DOI] [PubMed] [Google Scholar]
- COONS A. H. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- Chen C. L., Chang C. C., Krieger D. T., Bardin C. W. Expression and regulation of proopiomelanocortin-like gene in the ovary and placenta: comparison with the testis. Endocrinology. 1986 Jun;118(6):2382–2389. doi: 10.1210/endo-118-6-2382. [DOI] [PubMed] [Google Scholar]
- Chen C. L., Madigan M. B. Regulation of testicular proopiomelanocortin gene expression. Endocrinology. 1987 Aug;121(2):590–596. doi: 10.1210/endo-121-2-590. [DOI] [PubMed] [Google Scholar]
- Chen C. L., Mather J. P., Morris P. L., Bardin C. W. Expression of pro-opiomelanocortin-like gene in the testis and epididymis. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5672–5675. doi: 10.1073/pnas.81.18.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes R. J., Lorenz L. J., Haun R. S., Roos B. A., Collier K. J., Dixon J. E. Cloning and sequence analysis of a cDNA encoding rat preprocholecystokinin. Proc Natl Acad Sci U S A. 1984 Feb;81(3):726–730. doi: 10.1073/pnas.81.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray G. J., Desmond H., Gayton R. J., Jonsson A. C., Raybould H., Sharkey K. A., Varro A., Williams R. G. Cholecystokinin and gastrin forms in the nervous system. Ann N Y Acad Sci. 1985;448:32–43. doi: 10.1111/j.1749-6632.1985.tb29904.x. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Immunochemical evidence of cholecystokinin-like peptides in brain. Nature. 1976 Dec 9;264(5586):568–570. doi: 10.1038/264568a0. [DOI] [PubMed] [Google Scholar]
- Douglass J., Cox B., Quinn B., Civelli O., Herbert E. Expression of the prodynorphin gene in male and female mammalian reproductive tissues. Endocrinology. 1987 Feb;120(2):707–713. doi: 10.1210/endo-120-2-707. [DOI] [PubMed] [Google Scholar]
- Emson P. C., Rehfeld J. F., Rossor M. N. Distribution of cholecystokinin-like peptides in the human-brain. J Neurochem. 1982 Apr;38(4):1177–1179. doi: 10.1111/j.1471-4159.1982.tb05369.x. [DOI] [PubMed] [Google Scholar]
- Eng J., Shiina Y., Pan Y. C., Blacher R., Chang M., Stein S., Yalow R. S. Pig brain contains cholecystokinin octapeptide and several cholecystokinin desoctapeptides. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6381–6385. doi: 10.1073/pnas.80.20.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P., Hallbök F., Ebendal T., Shooter E. M., Radeke M. J., Misko T. P., Persson H. Developmental and regional expression of beta-nerve growth factor receptor mRNA in the chick and rat. Neuron. 1988 Dec;1(10):983–996. doi: 10.1016/0896-6273(88)90155-9. [DOI] [PubMed] [Google Scholar]
- Faris P. L., Komisaruk B. R., Watkins L. R., Mayer D. J. Evidence for the neuropeptide cholecystokinin as an antagonist of opiate analgesia. Science. 1983 Jan 21;219(4582):310–312. doi: 10.1126/science.6294831. [DOI] [PubMed] [Google Scholar]
- Gerendai I., Shaha C., Gunsalus G. L., Bardin C. W. The effects of opioid receptor antagonists suggest that testicular opiates regulate Sertoli and Leydig cell function in the neonatal rat. Endocrinology. 1986 May;118(5):2039–2044. doi: 10.1210/endo-118-5-2039. [DOI] [PubMed] [Google Scholar]
- Gubler U., Chua A. O., Hoffman B. J., Collier K. J., Eng J. Cloned cDNA to cholecystokinin mRNA predicts an identical preprocholecystokinin in pig brain and gut. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4307–4310. doi: 10.1073/pnas.81.14.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenäs L., Plöen L., Ekwall H., Osman D. I., Ritzén E. M. Differentiation of the rat seminiferous tubules between 13 and 19 days of age. Int J Androl. 1981 Apr;4(2):257–264. doi: 10.1111/j.1365-2605.1981.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Hilsted L., Rehfeld J. F. Measurement of precursors for alpha-amidated hormones by radioimmunoassay of glycine-extended peptides after trypsin-carboxypeptidase B cleavage. Anal Biochem. 1986 Jan;152(1):119–126. doi: 10.1016/0003-2697(86)90129-6. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Herrera-Marschitz M., Seroogy K., Ju G., Staines W. A., Holets V., Schalling M., Ungerstedt U., Post C., Rehfeld J. F. Immunohistochemical studies on cholecystokinin (CCK)-immunoreactive neurons in the rat using sequence specific antisera and with special reference to the caudate nucleus and primary sensory neurons. J Chem Neuroanat. 1988 Jan-Feb;1(1):11–51. [PubMed] [Google Scholar]
- Innis R. B., Corrêa F. M., Uhl G. R., Schneider B., Snyder S. H. Cholecystokinin octapeptide-like immunoreactivity: histochemical localization in rat brain. Proc Natl Acad Sci U S A. 1979 Jan;76(1):521–525. doi: 10.1073/pnas.76.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S., Katsuura G., Yoshikawa K., Rehfeld J. F. Potentiation of beta-endorphin effects by cholecystokinin antiserum in rats. Can J Physiol Pharmacol. 1985 Jan;63(1):81–83. doi: 10.1139/y85-015. [DOI] [PubMed] [Google Scholar]
- Johnson L. R., Stening G. F., Grossman M. I. Effect of sulfation on the gastrointestinal actions of caerulein. Gastroenterology. 1970 Feb;58(2):208–216. [PubMed] [Google Scholar]
- Jorpes E., Mutt V. Cholecystokinin and pancreozymin, one single hormone? Acta Physiol Scand. 1966 Jan-Feb;66(1):196–202. doi: 10.1111/j.1748-1716.1966.tb03185.x. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Borland K., Jin D. F. Differential expression of opioid peptide genes by testicular germ cells and somatic cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5695–5699. doi: 10.1073/pnas.84.16.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Howells R. D., Noe M., Bailey L. C., Udenfriend S. Expression of preproenkephalin-like mRNA and its peptide products in mammalian testis and ovary. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7467–7469. doi: 10.1073/pnas.82.21.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Millette C. F. Expression of proenkephalin messenger RNA by mouse spermatogenic cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5015–5018. doi: 10.1073/pnas.83.14.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Rosenthal J. L. The proenkephalin gene is widely expressed within the male and female reproductive systems of the rat and hamster. Endocrinology. 1986 Jul;119(1):370–374. doi: 10.1210/endo-119-1-370. [DOI] [PubMed] [Google Scholar]
- Kline D., Simoncini L., Mandel G., Maue R. A., Kado R. T., Jaffe L. A. Fertilization events induced by neurotransmitters after injection of mRNA in Xenopus eggs. Science. 1988 Jul 22;241(4864):464–467. doi: 10.1126/science.3134693. [DOI] [PubMed] [Google Scholar]
- Lorén I., Alumets J., Håkanson R., Sundler F. Distribution of gastrin and CCK-like peptides in rat brain. An immunocytochemical study. Histochemistry. 1979 Feb 21;59(4):249–257. doi: 10.1007/BF00689607. [DOI] [PubMed] [Google Scholar]
- Moriarty T. M., Gillo B., Sealfon S., Landau E. M. Activation of ionic currents in Xenopus oocytes by corticotropin-releasing peptides. Brain Res. 1988 Nov;464(3):201–205. doi: 10.1016/0169-328x(88)90026-5. [DOI] [PubMed] [Google Scholar]
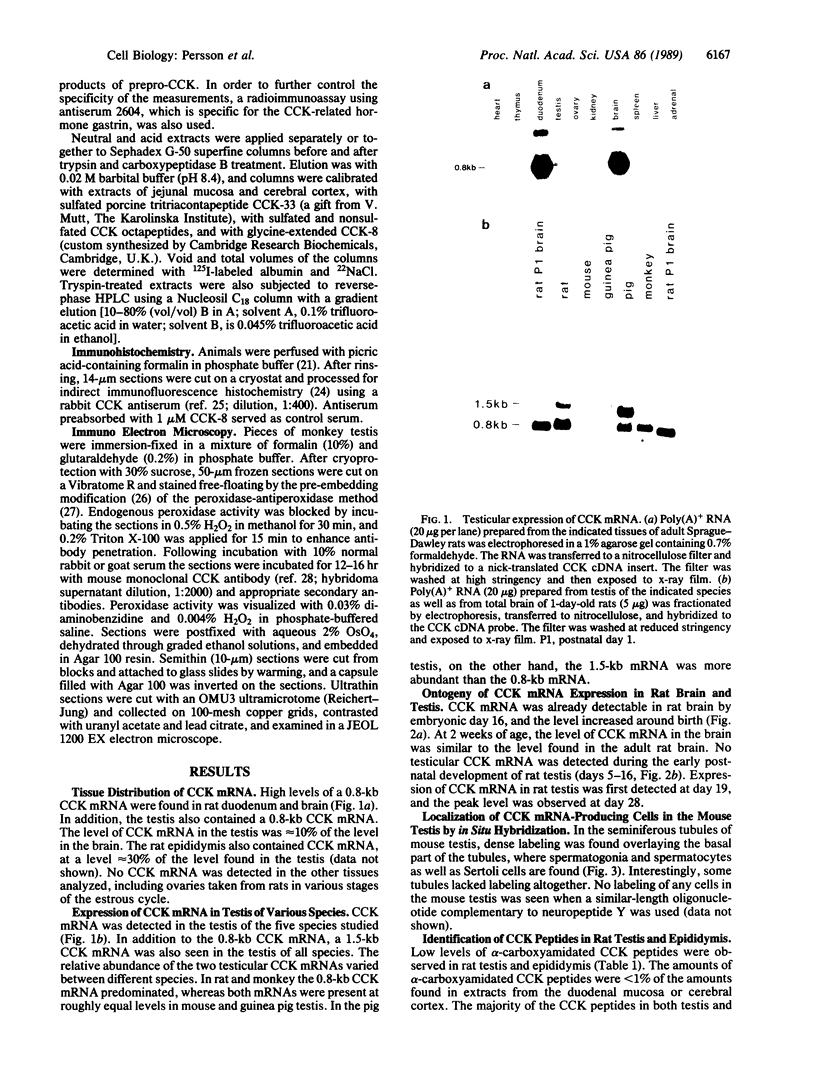
- Persson H., Ericsson A., Schalling M., Rehfeld J. F., Hökfelt T. Detection of cholecystokinin in spermatogenic cells. Acta Physiol Scand. 1988 Dec;134(4):565–566. doi: 10.1111/j.1748-1716.1998.tb08534.x. [DOI] [PubMed] [Google Scholar]
- Pintar J. E., Schachter B. S., Herman A. B., Durgerian S., Krieger D. T. Characterization and localization of proopiomelanocortin messenger RNA in the adult rat testis. Science. 1984 Aug 10;225(4662):632–634. doi: 10.1126/science.6740329. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Hansen H. F., Marley P. D., Stengaard-Pedersen K. Molecular forms of cholecystokinin in the brain and the relationship to neuronal gastrins. Ann N Y Acad Sci. 1985;448:11–23. doi: 10.1111/j.1749-6632.1985.tb29902.x. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F. Immunochemical studies on cholecystokinin. I. Development of sequence-specific radioimmunoassays for porcine triacontatriapeptide cholecystokinin. J Biol Chem. 1978 Jun 10;253(11):4016–4021. [PubMed] [Google Scholar]
- Schalling M., Dagerlind A., Brené S., Hallman H., Djurfeldt M., Persson H., Terenius L., Goldstein M., Schlesinger D., Hökfelt T. Coexistence and gene expression of phenylethanolamine N-methyltransferase, tyrosine hydroxylase, and neuropeptide tyrosine in the rat and bovine adrenal gland: effects of reserpine. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8306–8310. doi: 10.1073/pnas.85.21.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Turner P. R., Sheetz M. P., Jaffe L. A. Fertilization increases the polyphosphoinositide content of sea urchin eggs. Nature. 1984 Aug 2;310(5976):414–415. doi: 10.1038/310414a0. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen J. J., Lotstra F., De Mey J., Gilles C. Immunohistochemical localization of cholecystokinin- and gastrin-like peptides in the brain and hypophysis of the rat. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1190–1194. doi: 10.1073/pnas.77.2.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhaeghen J. J., Signeau J. C., Gepts W. New peptide in the vertebrate CNS reacting with antigastrin antibodies. Nature. 1975 Oct 16;257(5527):604–605. doi: 10.1038/257604a0. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. The biology and chemistry of fertilization. Science. 1987 Jan 30;235(4788):553–560. doi: 10.1126/science.3027891. [DOI] [PubMed] [Google Scholar]
- Whittemore S. R., Ebendal T., Lärkfors L., Olson L., Seiger A., Strömberg I., Persson H. Development and regional expression of beta nerve growth factor messenger RNA and protein in the rat central nervous system. Proc Natl Acad Sci U S A. 1986 Feb;83(3):817–821. doi: 10.1073/pnas.83.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]